Direct Measure of the Local Concentration of Pyrenyl Groups in Pyrene-Labeled Dendrons Derived from the Rate of Fluorescence Collisional Quenching
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ethyl 4-(1-pyrene)butyrate (PyBE)
2.3. UV–Vis Spectroscopy
2.4. Steady-State Fluorometer
2.5. Time-Resolved Fluorometer
2.6. Model-Free Analysis (MFA) of the Fluorescence Decays
2.7. Birks Scheme Analysis of the Fluorescence Decays
3. Results and Discussion
4. Conclusions
Supplementary Materials
 ) toluene, (
) toluene, ( ) THF, (
) THF, ( ) DMF, and (
) DMF, and ( ) DMSO; Table S1: Parameters retrieved from the global Birks scheme analysis of both the monomer and excimer decays of ethyl 4-(1-pyrene)butyrate in degassed toluene (Tol), N,N-dimethylformamide (DMF), dimethylsulfoxide (DMSO), and tetrahydrofuran (THF). Analysis program: globirks32bg; Table S2: Parameters retrieved from the MFA (analysis program: sumegs10bg) of both the monomer and excimer decays of ethyl 4-(1-pyrene)butyrate in degassed tetrahydrofuran (THF), degassed toluene (Tol), degassed dimethylformamide (DMF), and degassed dimethylsulfoxide (DMSO); Table S3: Parameters retrieved from the MFA (analysis programs: sumegs14bg or sumegs33bg-4) of the monomer decays of the Pyx-G(N) dendrons in degassed toluene (Tol), degassed dimethylformamide (DMF), and degassed dimethylsulfoxide (DMSO); Table S4: Parameters retrieved from the MFA of the excimer decays of the Pyx-G(N) dendrons in degassed toluene (Tol), degassed dimethylformamide (DMF), and degassed dimethylsulfoxide (DMSO); Table S5: Molar fractions obtained from the MFA of the Pyx-G(N) dendrons in degassed toluene (Tol), degassed dimethylformamide (DMF), and degassed dimethylsulfoxide (DMSO).
) DMSO; Table S1: Parameters retrieved from the global Birks scheme analysis of both the monomer and excimer decays of ethyl 4-(1-pyrene)butyrate in degassed toluene (Tol), N,N-dimethylformamide (DMF), dimethylsulfoxide (DMSO), and tetrahydrofuran (THF). Analysis program: globirks32bg; Table S2: Parameters retrieved from the MFA (analysis program: sumegs10bg) of both the monomer and excimer decays of ethyl 4-(1-pyrene)butyrate in degassed tetrahydrofuran (THF), degassed toluene (Tol), degassed dimethylformamide (DMF), and degassed dimethylsulfoxide (DMSO); Table S3: Parameters retrieved from the MFA (analysis programs: sumegs14bg or sumegs33bg-4) of the monomer decays of the Pyx-G(N) dendrons in degassed toluene (Tol), degassed dimethylformamide (DMF), and degassed dimethylsulfoxide (DMSO); Table S4: Parameters retrieved from the MFA of the excimer decays of the Pyx-G(N) dendrons in degassed toluene (Tol), degassed dimethylformamide (DMF), and degassed dimethylsulfoxide (DMSO); Table S5: Molar fractions obtained from the MFA of the Pyx-G(N) dendrons in degassed toluene (Tol), degassed dimethylformamide (DMF), and degassed dimethylsulfoxide (DMSO).Author Contributions
Funding
Conflicts of Interest
References
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. (Eds.) In Polymer Handbook, 4th ed.; Wiley: New York, NY, USA, 1999; pp. 1–83. [Google Scholar]
- Lakowicz, J.R. In Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; pp. 277–330. [Google Scholar]
- Duhamel, J. Global Analysis of Fluorescence Decays to Probe the Internal Dynamics of Fluorescently Labeled Macromolecules. Langmuir 2013, 30, 2307–2324. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, J. Internal Dynamics of Dendritic Molecules Probed by Pyrene Excimer Formation. Polymers 2012, 4, 211–239. [Google Scholar] [CrossRef]
- Duhamel, J. New Insights in the Study of Pyrene Excimer Fluorescence to Characterize Macromolecules and their Supramolecular Assemblies in Solution. Langmuir 2012, 28, 6527–6538. [Google Scholar] [CrossRef]
- Siu, H.; Duhamel, J. Comparison of the Association Level of a Hydrophobically Modified Associative Polymer Obtained from an Analysis Based on Two Different Models. J. Phys. Chem. B 2005, 109, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.B. Photophysics of Aromatic Molecules; Wiley: London, UK, 1970. [Google Scholar]
- Winnik, M.A. End-to-end cyclization of polymer chains. Accounts Chem. Res. 1985, 18, 73–79. [Google Scholar] [CrossRef]
- Chen, S.; Duhamel, J.; Winnik, M.A. Probing End-to-End Cyclization Beyond Willemski and Fixmann. J. Phys. Chem. B 2011, 115, 3289–3302. [Google Scholar] [CrossRef]
- Cuniberti, C.; Perico, A. Intramolecular excimer formation in polymers. Eur. Polym. J. 1980, 16, 887–893. [Google Scholar] [CrossRef]
- Yip, J.; Duhamel, J.; Bahun, G.J.; Adronov, A. A Study of the Dynamics of the Branch Ends of a Series of Pyrene-Labeled Dendrimers Based on Pyrene Excimer Formation. J. Phys. Chem. B 2010, 114, 10254–10265. [Google Scholar] [CrossRef]
- Modrakowski, C.; Flores, S.C.; Beinhoff, M.; Schlüter, A.D. Synthesis of Pyrene Containing Building Blocks for Dendrimer Synthesis. Synthese 2001, 2001, 2143–2155. [Google Scholar] [CrossRef]
- Figueira-Duarte, T.M.; Simon, S.C.; Wagner, M.; Druzhinin, S.I.; Zachariasse, K.A.; Müllen, K. Polypyrene Dendrimers. Angew. Chem. Int. Ed. 2008, 47, 10175–10178. [Google Scholar] [CrossRef]
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-Based Materials for Organic Electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.M.; Fox, M.A. Fluorophore-labeled dendrons as light harvesting antennae. J. Am. Chem. Soc. 1996, 118, 4354–4360. [Google Scholar] [CrossRef]
- Giovanella, U.; Mróz, W.; Foggi, P.; Fabbrizzi, P.; Cicchi, S.; Botta, C. Multi-Colour Electroluminescence of Dendronic Antennae Containing Pyrenes as Light Harvesters. ChemPhysChem 2009, 11, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.R.J.; Thompson, A.L.; Sivakumar, A.V.; Bardeen, C.J.; Thayumanavan, S. Energy and Electron Transfer in Bifunctional Non-Conjugated Dendrimers. J. Am. Chem. Soc. 2005, 127, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Vanjinathan, M.; Lin, H.-C.; Nasar, A.S. Synthesis, Characterization and Photophysical Properties of DCM-Based Light-Harvesting Dendrimers. Macromol. Chem. Phys. 2011, 212, 849–859. [Google Scholar] [CrossRef]
- Gu, T.; Whitesell, J.K.; Fox, M.A. Intramolecular Charge Transfer in 1:1 Cu(II)/Pyrenylcyclam Dendrimer Complexes. J. Phys. Chem. B 2006, 110, 25149–25152. [Google Scholar] [CrossRef]
- Morales-Espinoza, E.G.; Lijanova, I.V.; Morales-Saavedra, O.G.; Torres-Zuñiga, V.; Hernández-Ortega, S.; Garcia, M.M. Synthesis of Porphyrin-Dendrimers with a Pyrene in the Periphery and Their Cubic Nonlinear Optical Properties. Molecules 2011, 16, 6950–6968. [Google Scholar] [CrossRef]
- Baker, L.A.; Crooks, R.M. Photophysical Properties of Pyrene-Functionalized Poly (propylene imine) Dendrimers. Macromolecules 2000, 33, 9034–9039. [Google Scholar] [CrossRef]
- Wang, B.-B.; Zhang, X.; Jia, X.-R.; Li, Z.-C.; Ji, Y.; Yang, L.; Wei, Y. Fluorescence and aggregation behaviour of poly(amidoamine) dendrimers peripherally modified with aromatic fluorophores: The effect of dendritic architecture. J. Am. Chem. Soc. 2004, 126, 15180–15194. [Google Scholar] [CrossRef]
- Wang, B.-B.; Zhang, X.; Yang, L.; Jia, X.-R.; Ji, Y.; Li, W.-S.; Wei, Y. Poly (amisoamine) dendrimers bearing electron-donating fluorophores: Fluorescence and electrochemical properties. Polym. Bull. 2006, 56, 63–74. [Google Scholar] [CrossRef]
- Brauge, L.; Caminade, A.-M.; Majoral, J.-P.; Slomkowski, S.; Wolszczak, M. Segmental Mobility in Phosphorus-Containing Dendrimers. Studies by Fluorescent Spectroscopy. Macromolecules 2001, 34, 5599–5606. [Google Scholar] [CrossRef]
- Brauge, L.; Veriot, G.; Franc, G.; Deloncle, R.; Caminade, A.-M.; Majoral, J.-P. Synthesis of phosphorus dendrimers bearing chromophoric end groups: Toward organic blue light-emitting diodes. Tetrahedron 2006, 62, 11891–11899. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Hameau, A.; Turrin, C.-O.; Ianchuk, M.; Delavaux-Nicot, B.; Majoral, J.-P. Fluorescent Phosphorus Dendrimers and Their Role in Supramolecular Interactions. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 860–868. [Google Scholar] [CrossRef]
- Lekha, P.K.; Prasad, E. Tunable Emission of Static Excimer in a Pyrene-Modified Polyamidoamine Dendrimer Aggregate through Positive Solvatochromism. Chem. A Eur. J. 2011, 17, 8609–8617. [Google Scholar] [CrossRef] [PubMed]
- Wilken, R.; Adams, J. End-group dynamics of fluorescently labeled dendrimers. Macromol. Rapid Commun. 1997, 18, 659–665. [Google Scholar] [CrossRef]
- McNelles, S.A.; Thoma, J.L.; Adronov, A.; Duhamel, J. Quantitative Characterization of the Molecular Dimensions of Flexible Dendritic Macromolecules in Solution by Pyrene Excimer Fluorescence. Macromolecules 2018, 51, 1586–1590. [Google Scholar] [CrossRef]
- Press, W.H.; Flanery, B.P.; Tenkolsky, S.A.; Vetterling, W.T. Numerical Receipes in Fortran: The Art of Scientific Computing; Cambridge University Press: New York, NY, USA, 1992; pp. 523–528. [Google Scholar]
- Farhangi, S.; Casier, R.; Li, L.; Thoma, J.L.; Duhamel, J. Characterization of the Long-Range Internal Dynamics of Pyrene-Labeled Macromolecules by Pyrene Excimer Fluorescence. Macromolecules 2016, 49, 9597–9604. [Google Scholar] [CrossRef]
- Farhangi, S.; Duhamel, J. Long Range Polymer Chain Dynamics Studied by Fluorescence Quenching. Macromolecules 2016, 49, 6149–6162. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 77th ed.; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Hall, T.; Whitton, G.; Casier, R.; Gauthier, M.; Duhamel, J. Arborescent Poly(l-glutamic acid)s as Standards To Study the Dense Interior of Polypeptide Mesoglobules by Pyrene Excimer Fluorescence. Macromolecules 2018, 51, 7914–7923. [Google Scholar] [CrossRef]
- Casier, R.; Duhamel, J. Effect of Like Charges on the Conformation and Internal Dynamics of Polypeptides Probed by Pyrene Excimer Fluorescence. Macromolecules 2020, 53, 5147–5157. [Google Scholar] [CrossRef]

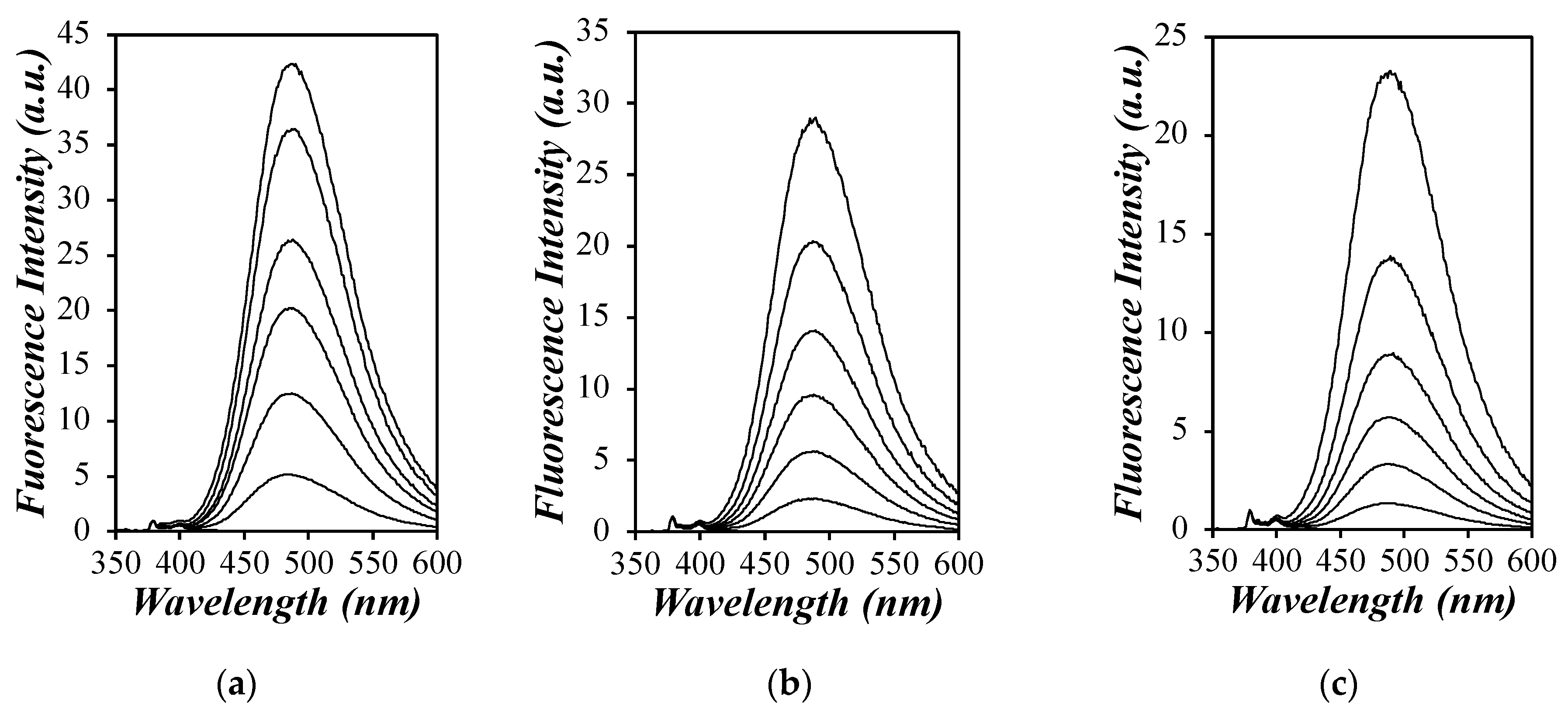
 ) toluene, (
) toluene, ( ) tetrahydrofuran (THF) [29], (
) tetrahydrofuran (THF) [29], ( ) DMF, and (
) DMF, and ( ) DMSO versus (a) generation number and (b) the [Py]loc.
) DMSO versus (a) generation number and (b) the [Py]loc.
 ) toluene, (
) toluene, ( ) tetrahydrofuran (THF) [29], (
) tetrahydrofuran (THF) [29], ( ) DMF, and (
) DMF, and ( ) DMSO versus (a) generation number and (b) the [Py]loc.
) DMSO versus (a) generation number and (b) the [Py]loc.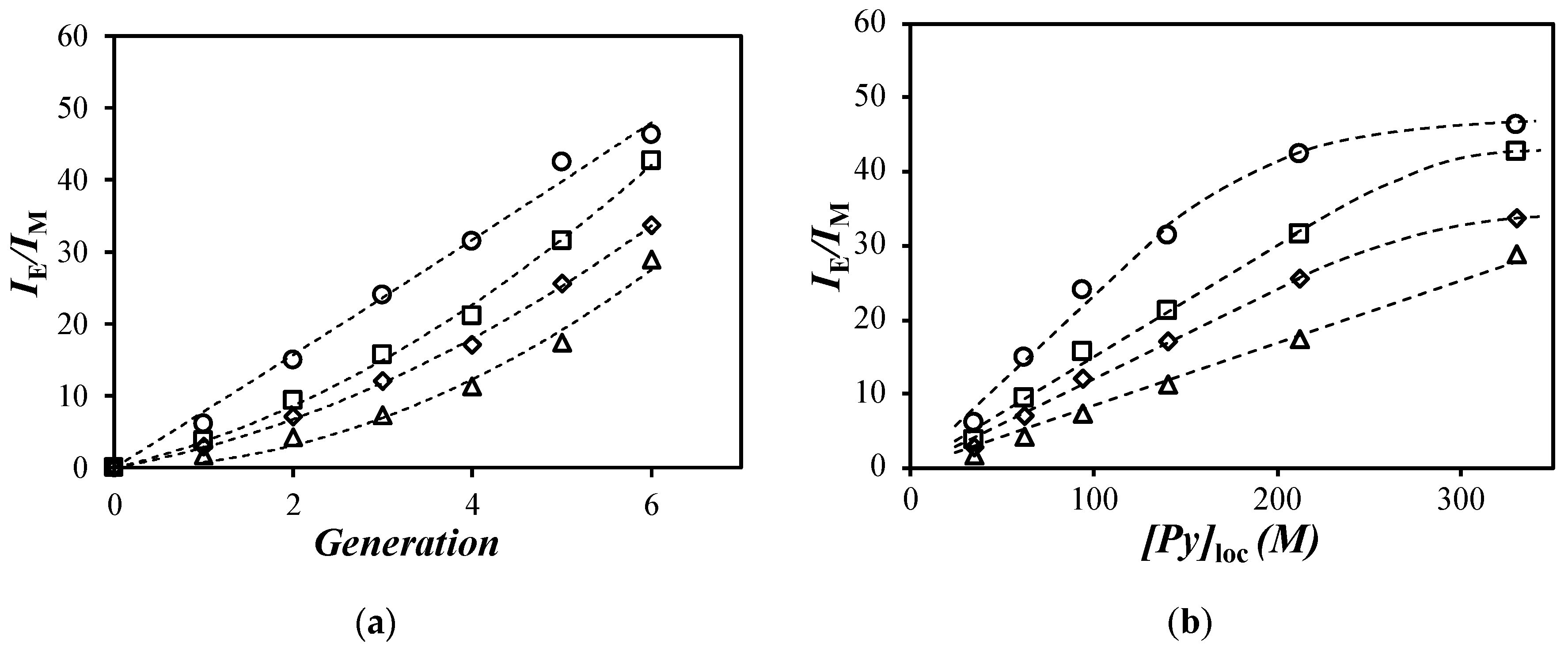
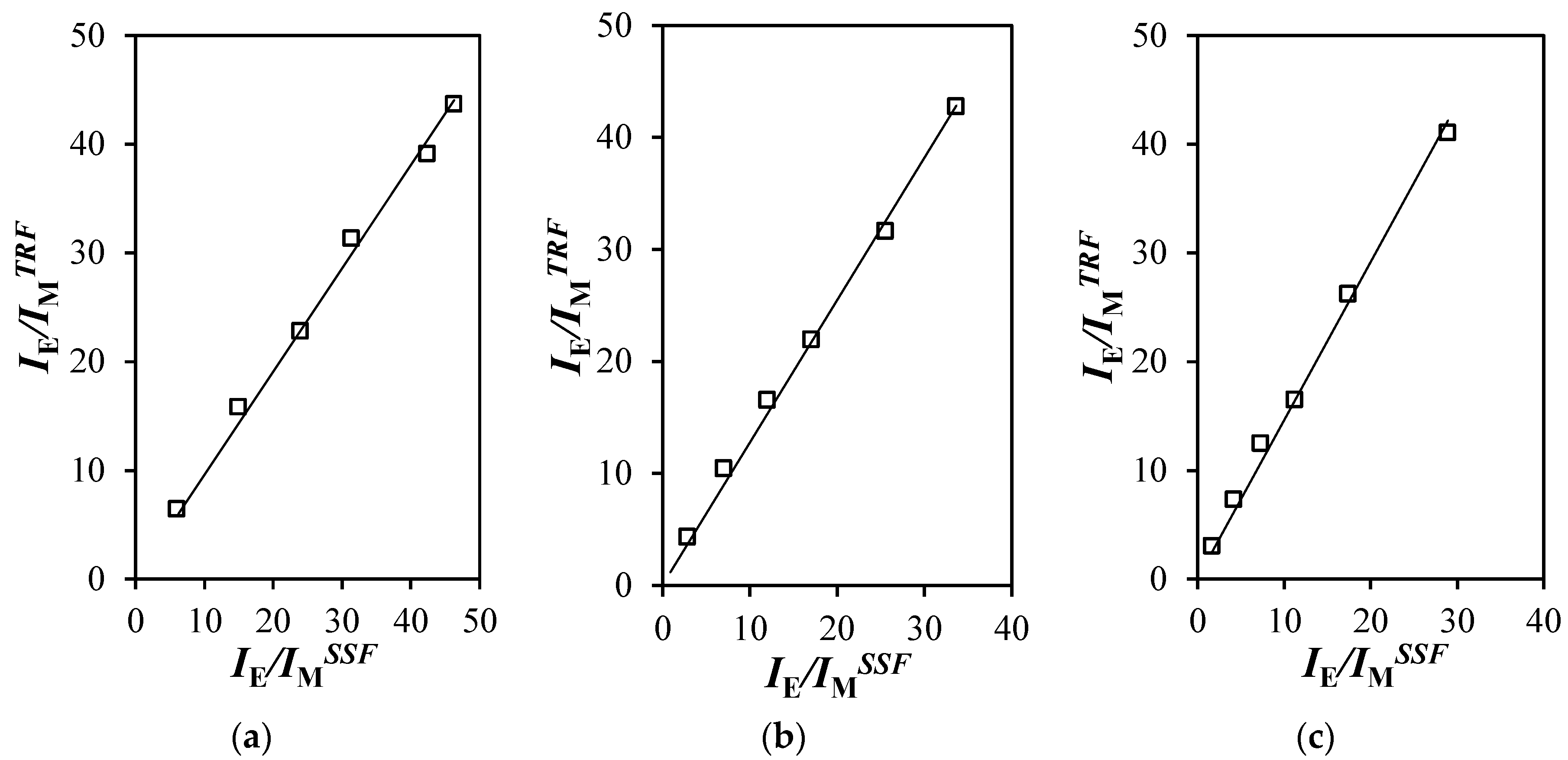
 ) toluene, (
) toluene, ( ) THF [29], (
) THF [29], ( ) DMF, and (
) DMF, and ( ) DMSO.
) DMSO.
 ) toluene, (
) toluene, ( ) THF [29], (
) THF [29], ( ) DMF, and (
) DMF, and ( ) DMSO.
) DMSO.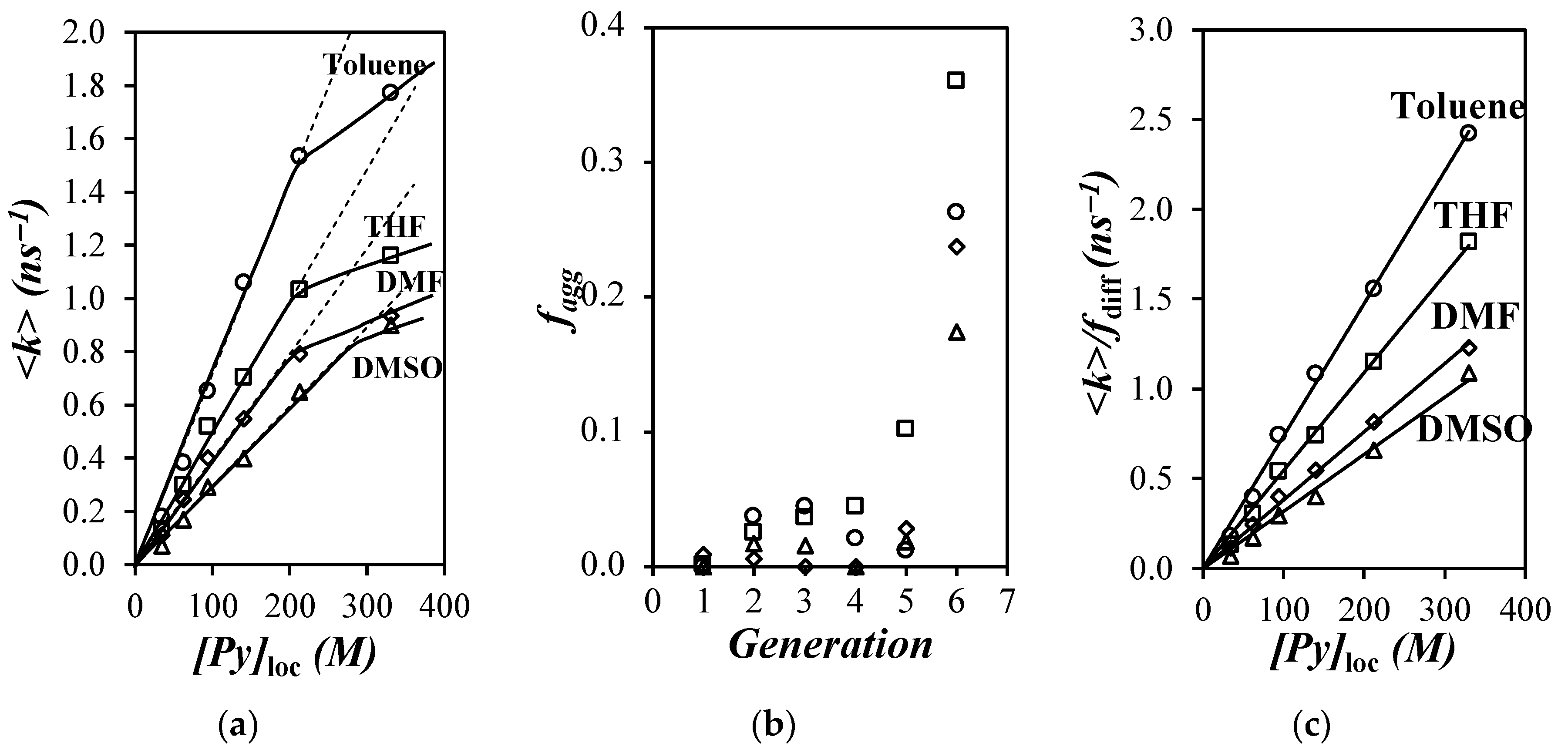
 ,
, ) toluene, (
) toluene, ( ,
, ) THF, (
) THF, ( ,
, ) DMF, and (
) DMF, and ( ,
, ) DMSO; solid and hollow symbols are for the fluorescence decay analysis according to the Birks scheme and MFA, respectively. (b) Plot of kdiff for (
) DMSO; solid and hollow symbols are for the fluorescence decay analysis according to the Birks scheme and MFA, respectively. (b) Plot of kdiff for ( ) the Pyx-G(N) dendrons and the PyBE model compound when the fluorescence decays were fitted according to (
) the Pyx-G(N) dendrons and the PyBE model compound when the fluorescence decays were fitted according to ( ) the MFA and (
) the MFA and ( ) the Birks scheme. (c) kdiff × η of PyBE versus solvent viscosity.
) the Birks scheme. (c) kdiff × η of PyBE versus solvent viscosity.
 ,
, ) toluene, (
) toluene, ( ,
, ) THF, (
) THF, ( ,
, ) DMF, and (
) DMF, and ( ,
, ) DMSO; solid and hollow symbols are for the fluorescence decay analysis according to the Birks scheme and MFA, respectively. (b) Plot of kdiff for (
) DMSO; solid and hollow symbols are for the fluorescence decay analysis according to the Birks scheme and MFA, respectively. (b) Plot of kdiff for ( ) the Pyx-G(N) dendrons and the PyBE model compound when the fluorescence decays were fitted according to (
) the Pyx-G(N) dendrons and the PyBE model compound when the fluorescence decays were fitted according to ( ) the MFA and (
) the MFA and ( ) the Birks scheme. (c) kdiff × η of PyBE versus solvent viscosity.
) the Birks scheme. (c) kdiff × η of PyBE versus solvent viscosity.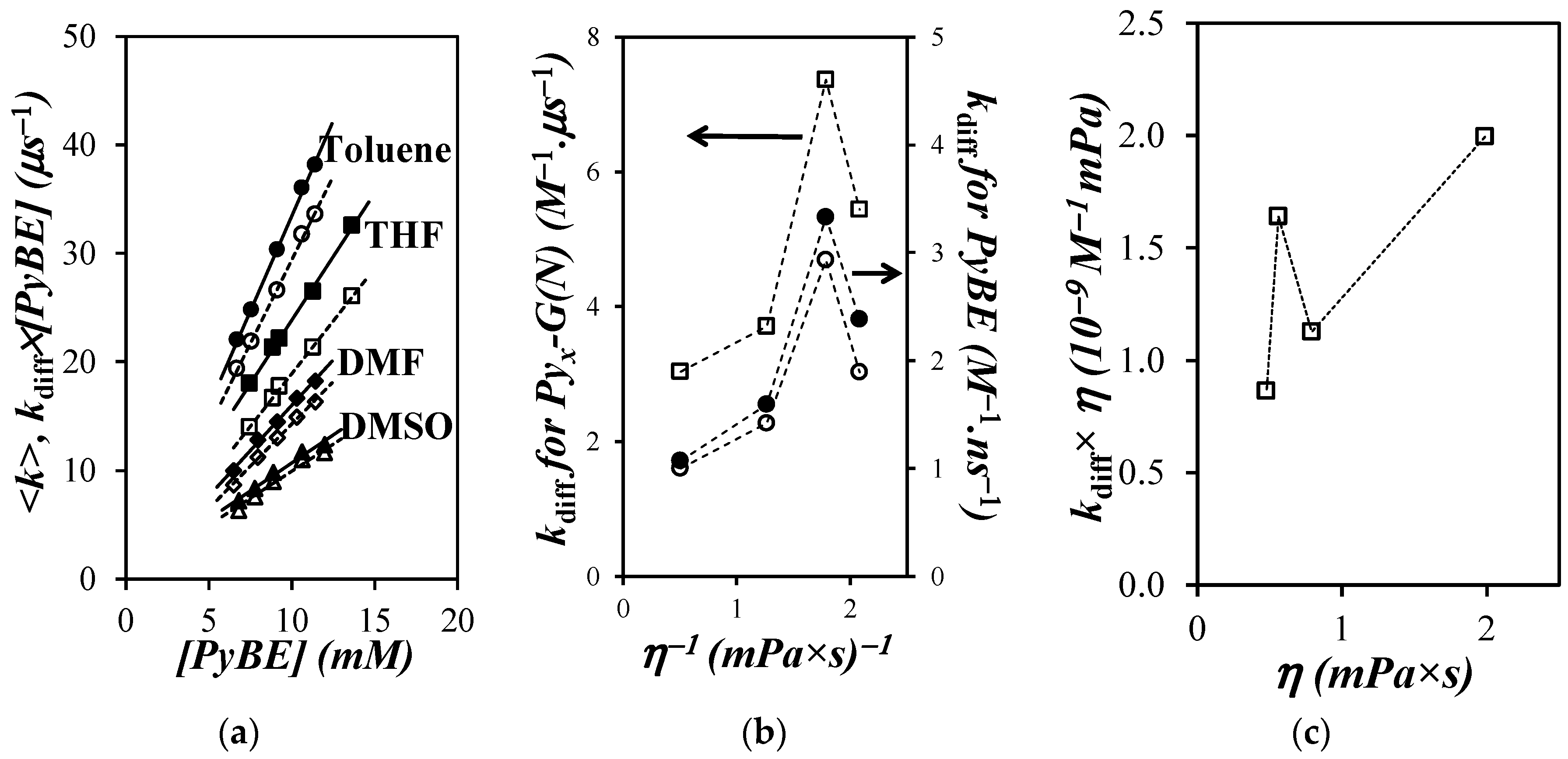
 ) toluene, (
) toluene, ( ) THF [29], (
) THF [29], ( ) DMF, and (
) DMF, and ( ) DMSO.
) DMSO.
 ) toluene, (
) toluene, ( ) THF [29], (
) THF [29], ( ) DMF, and (
) DMF, and ( ) DMSO.
) DMSO.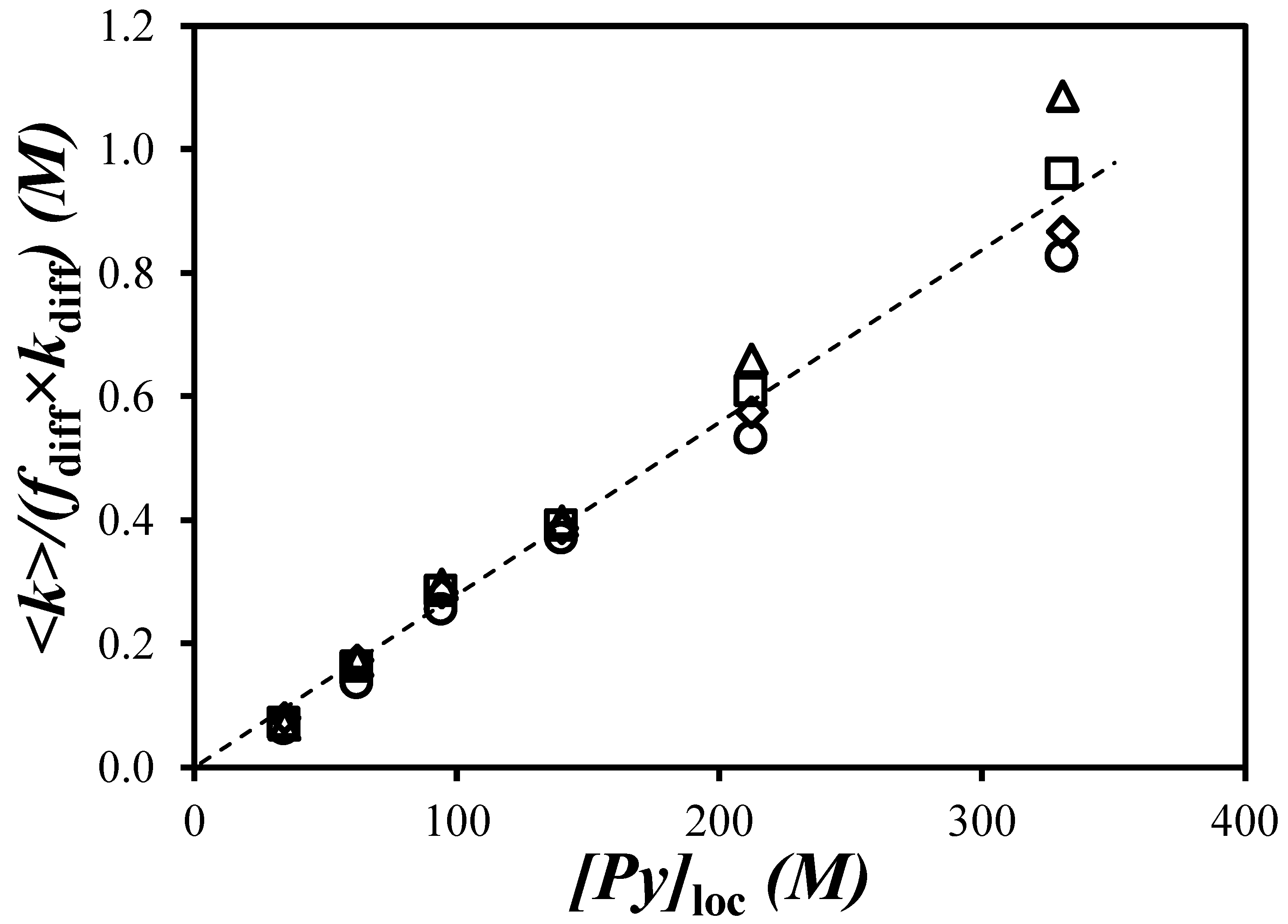
| Sample | Py2-G(1) | Py4-G(2) | Py8-G(3) | Py16-G(4) |
| Structure | 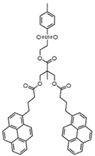 | 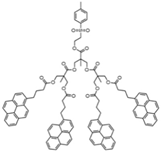 | 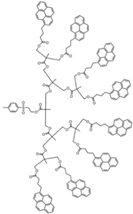 | 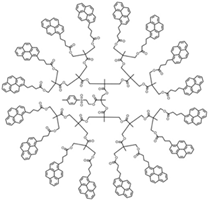 |
| [Py]loc (M) | 34 | 62 | 94 | 140 |
| Sample | Py32-G(5) | Py64-G(6) | ||
| Structure | 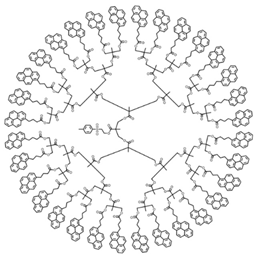 | 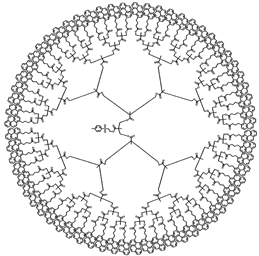 | ||
| [Py]loc (M) | 212 | 330 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thoma, J.L.; McNelles, S.A.; Adronov, A.; Duhamel, J. Direct Measure of the Local Concentration of Pyrenyl Groups in Pyrene-Labeled Dendrons Derived from the Rate of Fluorescence Collisional Quenching. Polymers 2020, 12, 2919. https://doi.org/10.3390/polym12122919
Thoma JL, McNelles SA, Adronov A, Duhamel J. Direct Measure of the Local Concentration of Pyrenyl Groups in Pyrene-Labeled Dendrons Derived from the Rate of Fluorescence Collisional Quenching. Polymers. 2020; 12(12):2919. https://doi.org/10.3390/polym12122919
Chicago/Turabian StyleThoma, Janine L., Stuart A. McNelles, Alex Adronov, and Jean Duhamel. 2020. "Direct Measure of the Local Concentration of Pyrenyl Groups in Pyrene-Labeled Dendrons Derived from the Rate of Fluorescence Collisional Quenching" Polymers 12, no. 12: 2919. https://doi.org/10.3390/polym12122919
APA StyleThoma, J. L., McNelles, S. A., Adronov, A., & Duhamel, J. (2020). Direct Measure of the Local Concentration of Pyrenyl Groups in Pyrene-Labeled Dendrons Derived from the Rate of Fluorescence Collisional Quenching. Polymers, 12(12), 2919. https://doi.org/10.3390/polym12122919






