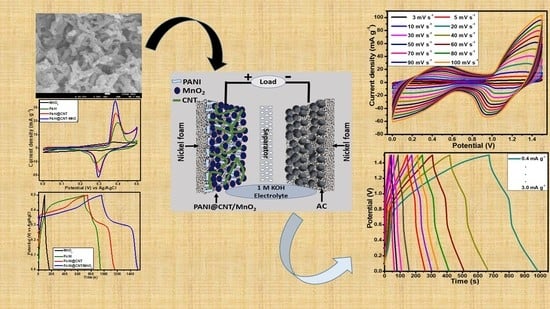
Hydrothermally Assisted Synthesis of Porous Polyaniline@Carbon Nanotubes–Manganese Dioxide Ternary Composite for Potential Application in Supercapattery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MnO2, PANI, PANI@CNT and PANI@CNT/MnO2
2.3. Characterization
2.4. Electrodes Development and Electrochemical Analysis
3. Results and Discussion
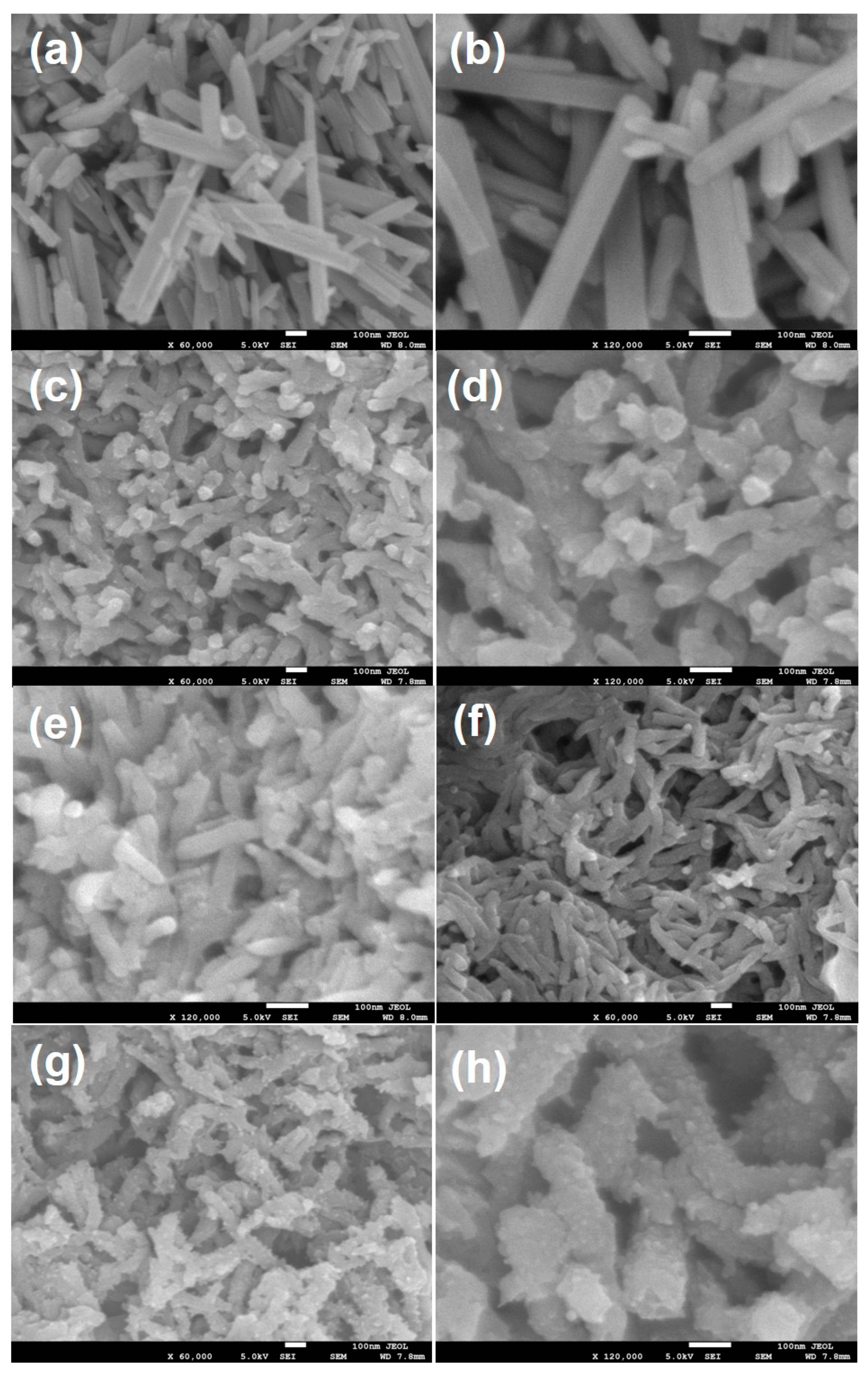
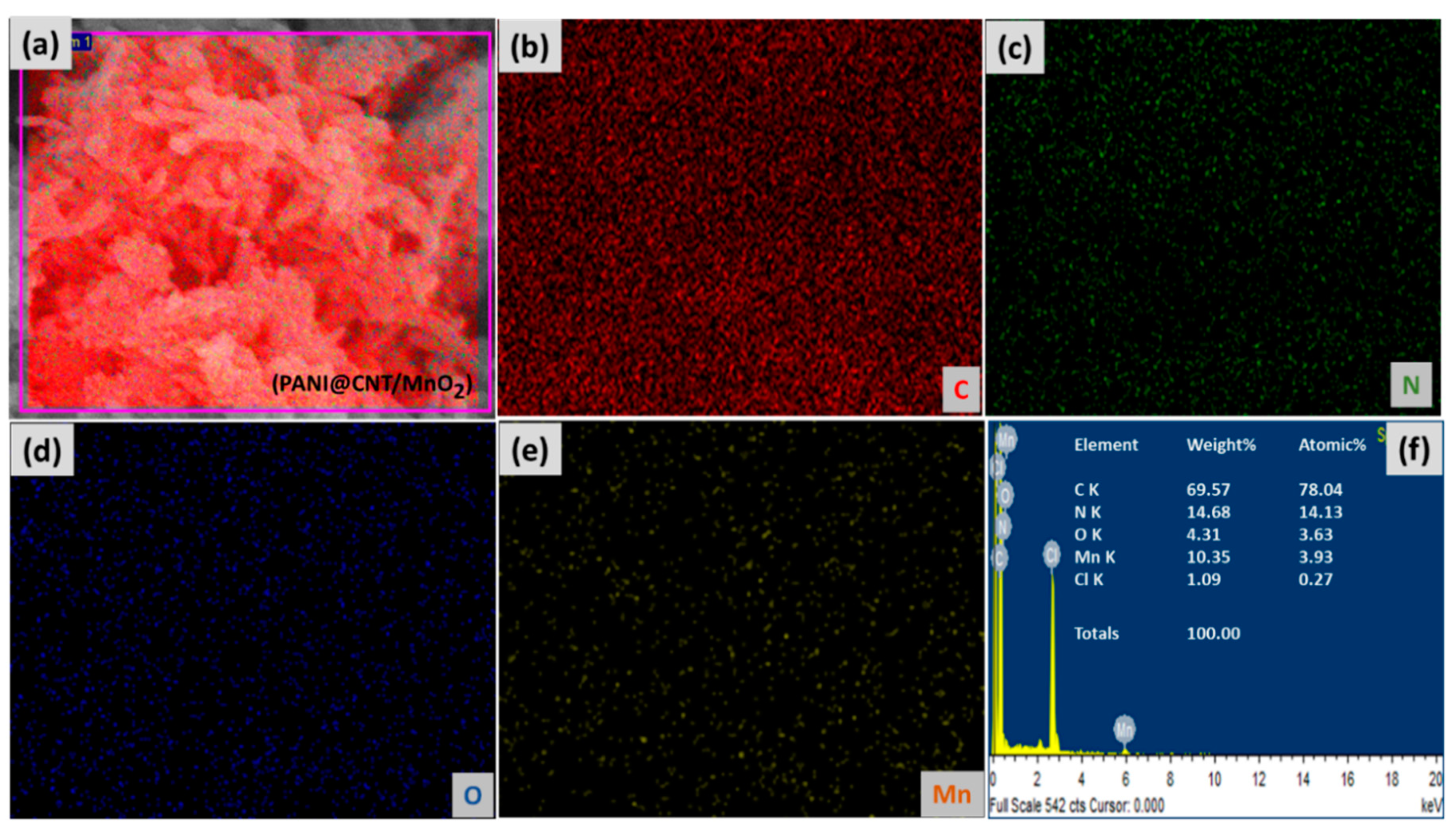
3.1. Morphological Characterizations
3.2. X-ray Diffraction and Raman Studies
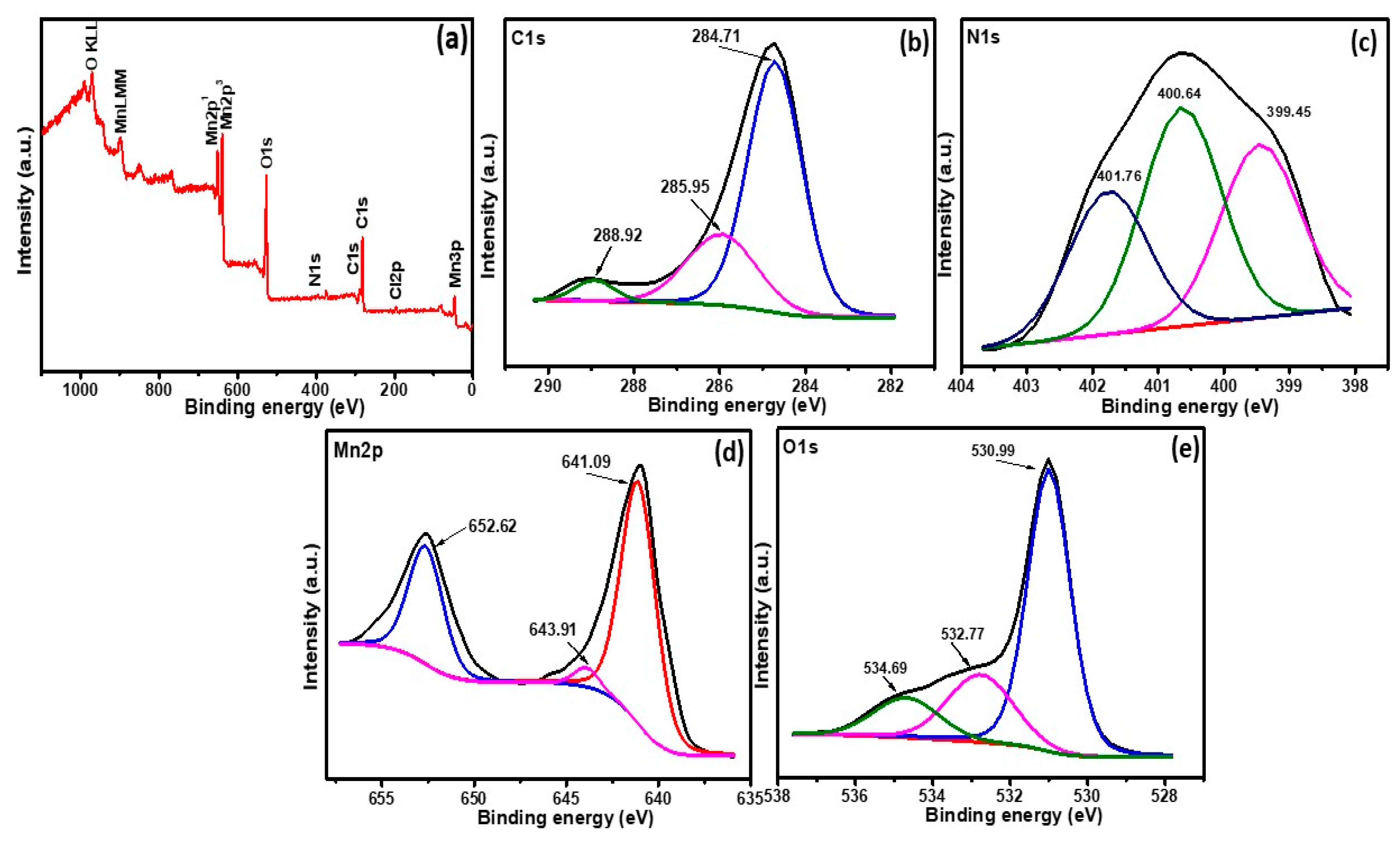
3.3. X-ray Photoelectron Spectroscopy Studies
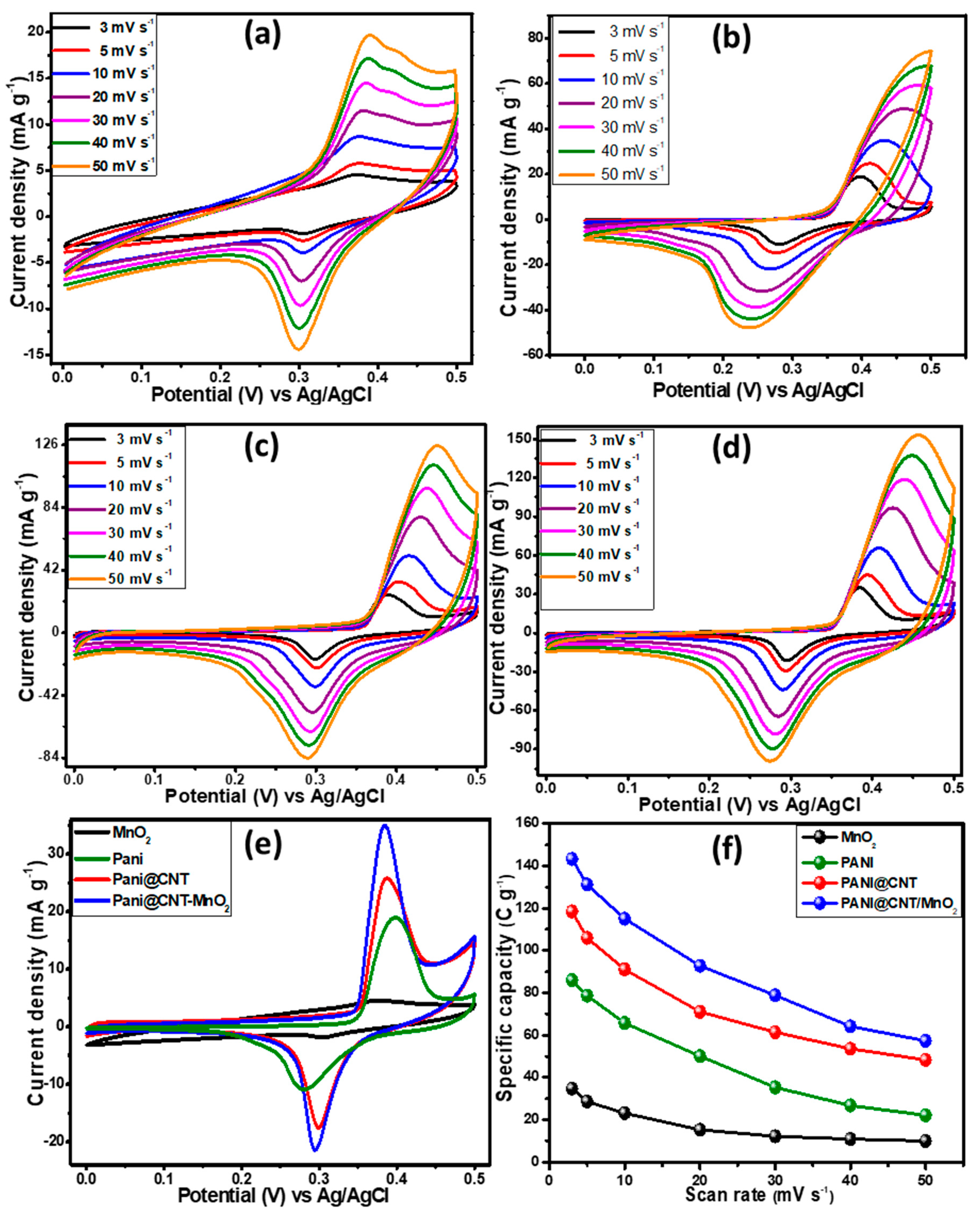
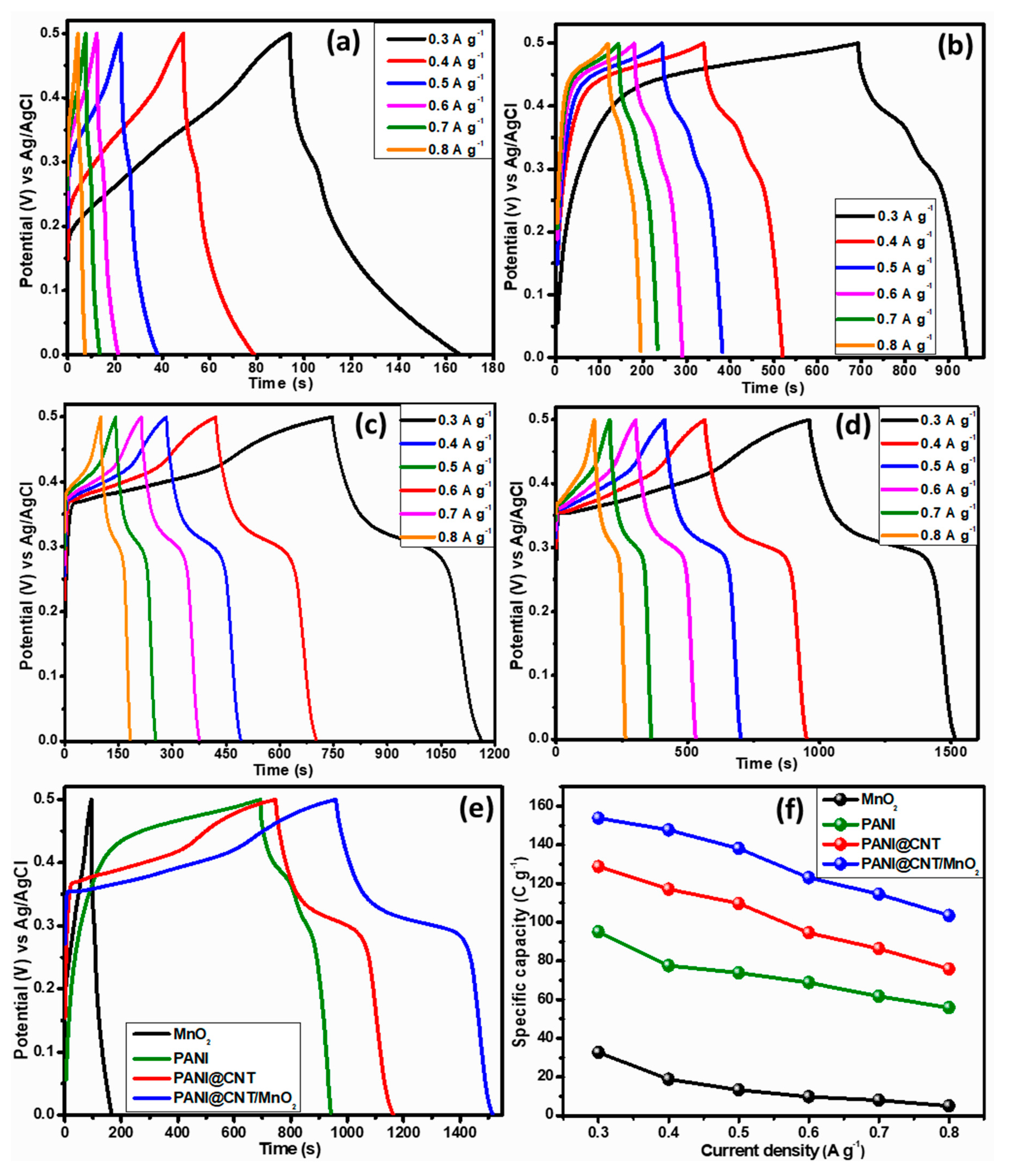
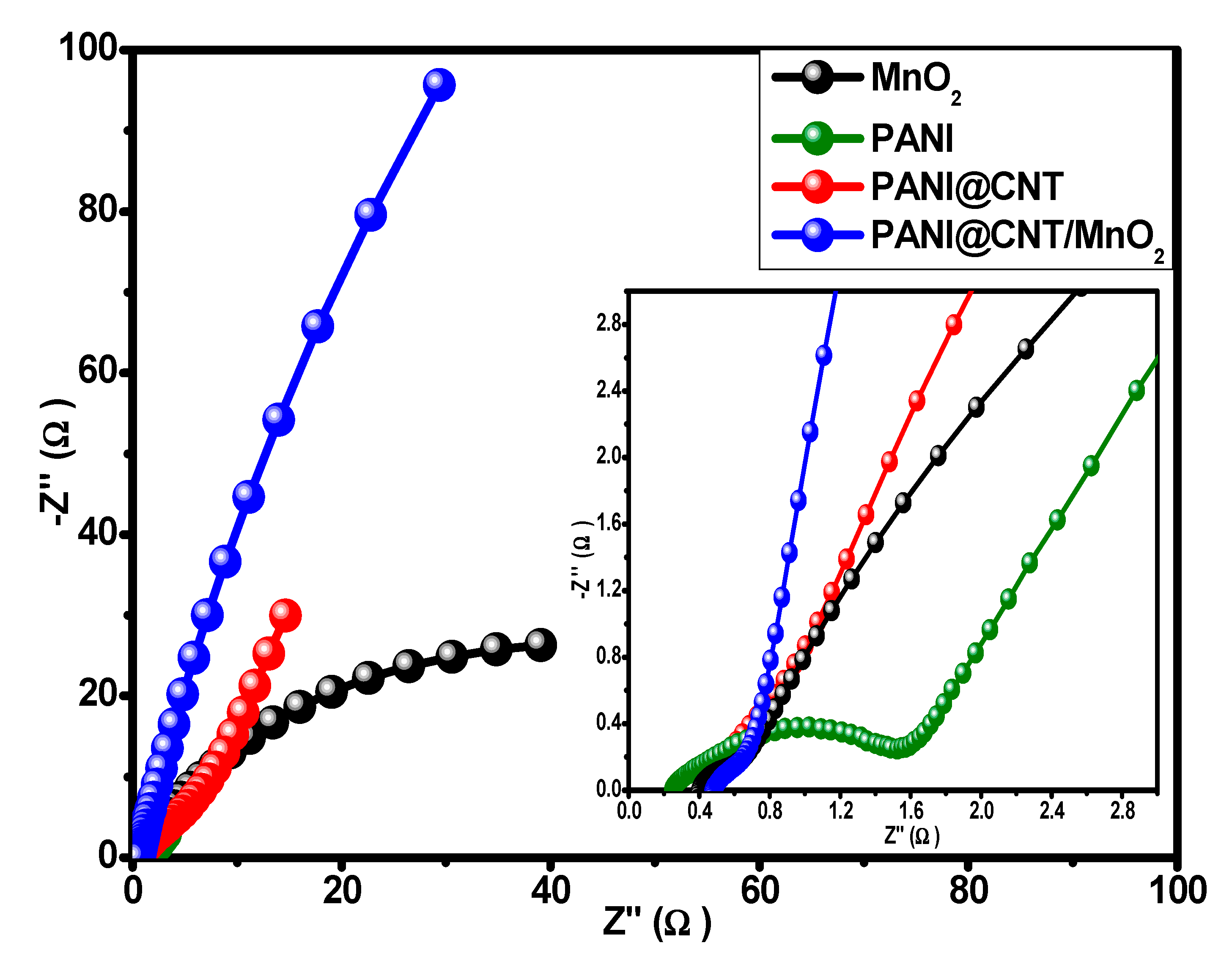
3.4. Three Electrodes Electrochemical Studies of MnO2, PANI, PANI@CNT and PANI@CNT/MnO2
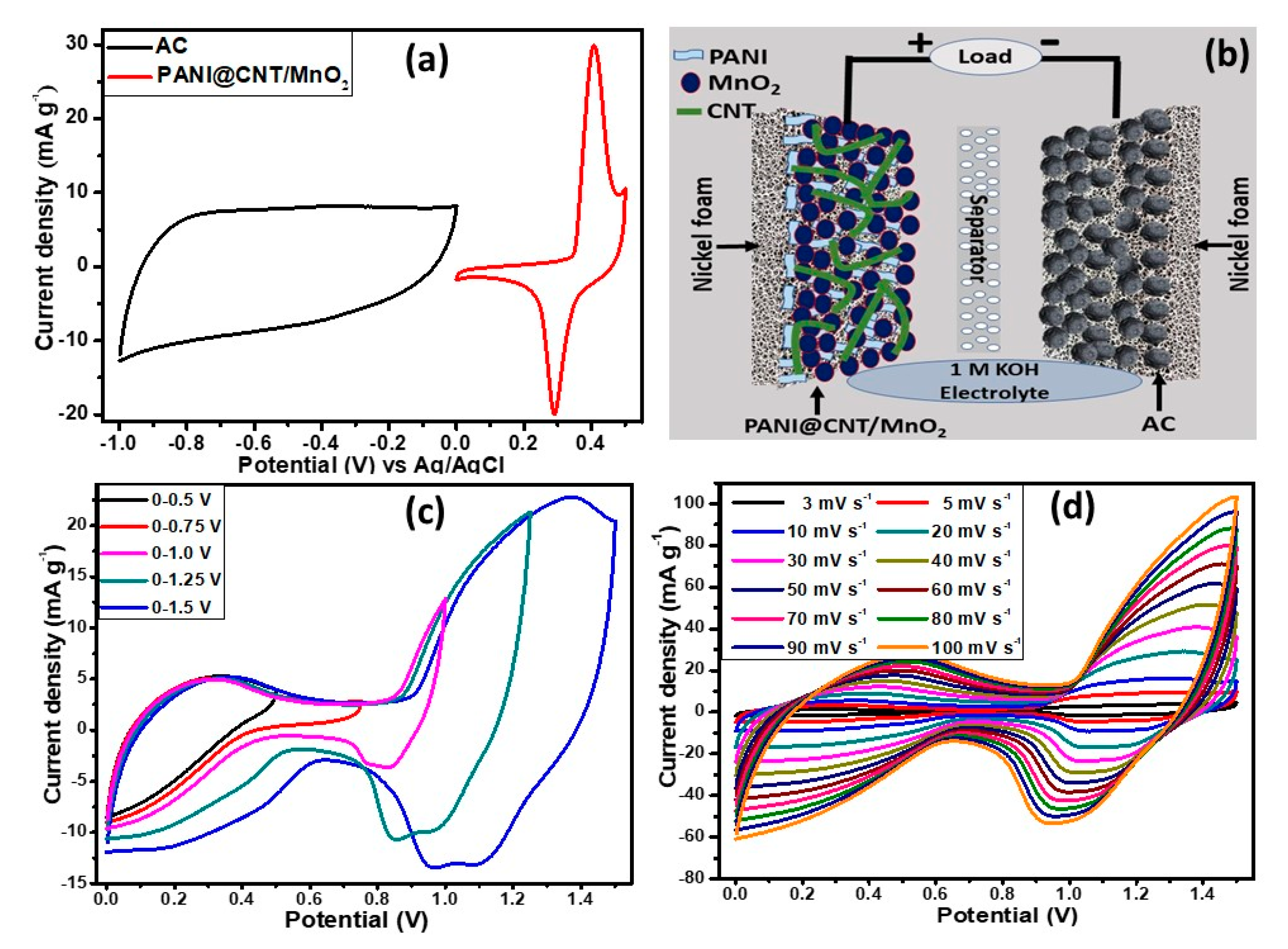
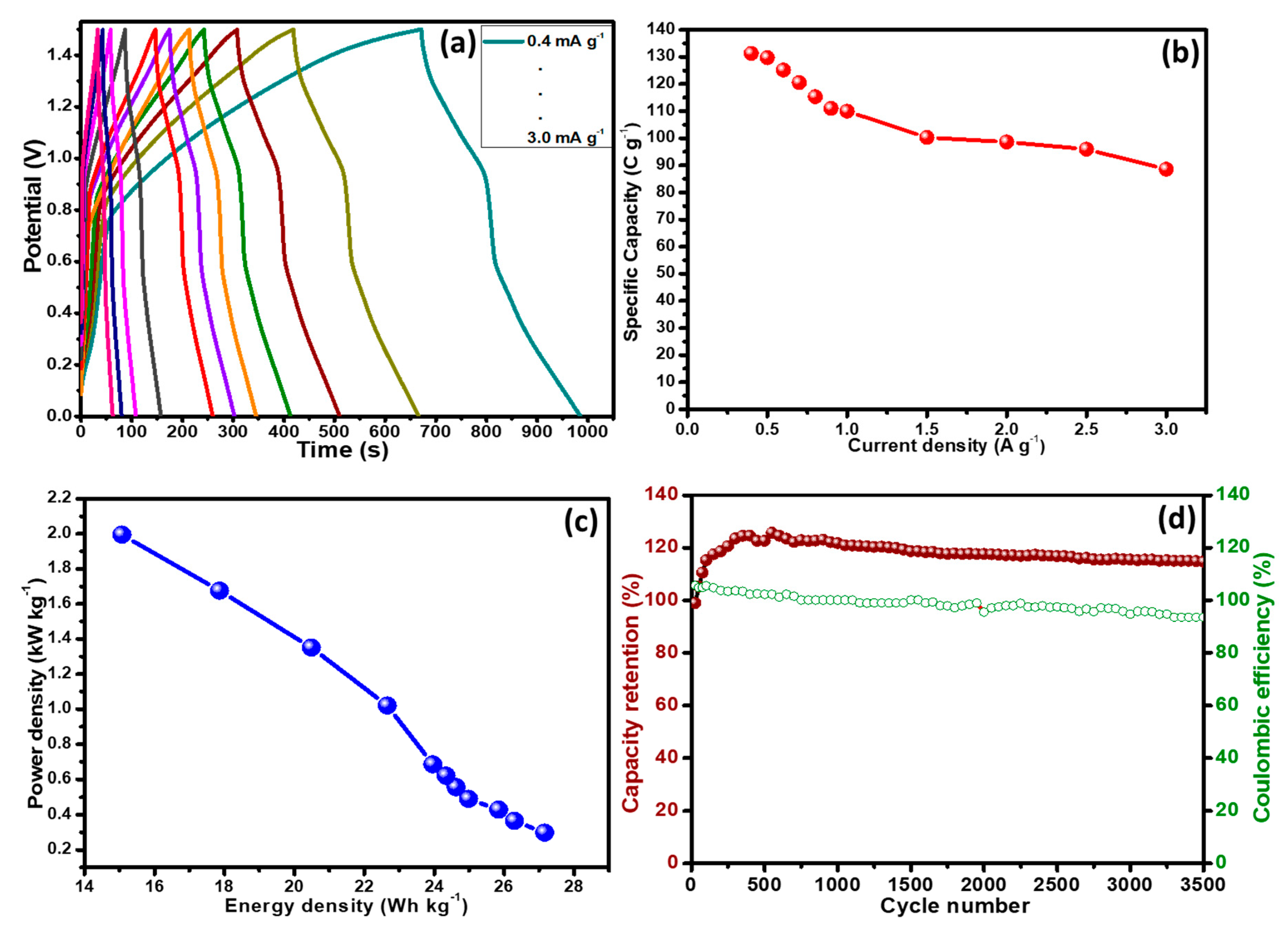
3.5. Fabrication and Characterization of Two Electrodes Assembly (PANI@CNT/MnO2//AC)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whiting, K.; Carmona, L.G.; Sousa, T. A review of the use of exergy to evaluate the sustainability of fossil fuels and non-fuel mineral depletion. Renew. Sustain. Energy Rev. 2017, 76, 202–211. [Google Scholar] [CrossRef]
- Bello, I.T.; Oladipo, A.O.; Adedokun, O.; Dhlamini, S.M. Recent advances on the preparation and electrochemical analysis of MoS2-based materials for supercapacitor applications: A mini-review. Mater. Today Commun. 2020, 25, 101664. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Khan, A.; Numan, A.; Alzaid, M.; Iqbal, J. Facile sonochemical synthesis of strontium phosphate based materials for potential application in supercapattery devices. Int. J. Hydrog. Energy 2020, 45, 32331–32342. [Google Scholar] [CrossRef]
- Alshahrie, A.; Ansari, M.O. High Performance Supercapacitor Applications and DC Electrical Conductivity Retention on Surfactant Immobilized Macroporous Ternary Polypyrrole/Graphitic-C3N4@Graphene Nanocomposite. Electron. Mater. Lett. 2019, 15, 238–246. [Google Scholar] [CrossRef]
- Ansari, S.A.; Ansari, M.O.; Cho, M.H. Facile and scale up synthesis of red phosphorus-graphitic carbon nitride heterostructures for energy and environment applications. Sci. Rep. 2016, 6, 27713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, J.; Numan, A.; Jafer, R.; Bashir, S.; Jilani, A.; Mohammad, S.; Khalid, M.; Ramesh, K.; Ramesh, S. Ternary nanocomposite of cobalt oxide nanograins and silver nanoparticles grown on reduced graphene oxide conducting platform for high-performance supercapattery electrode material. J. Alloys Compd. 2020, 821, 153452. [Google Scholar] [CrossRef]
- Xiong, C.; Li, M.; Nie, S.; Dang, W.; Zhao, W.; Dai, L.; Ni, Y. Non-carbonized porous lignin-free wood as an effective scaffold to fabricate lignin-free Wood@ Polyaniline supercapacitor material for renewable energy storage application. J. Power Sources 2020, 471, 228448. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, M.O.; Cho, M.H. Route to high surface area, mesoporosity of polyaniline–titanium dioxide nanocomposites via one pot synthesis for energy storage applications. Ind. Eng. Chem. Res. 2016, 55, 116–124. [Google Scholar] [CrossRef]
- Iqbal, J.; Numan, A.; Ansari, M.O.; Jagadish, P.R.; Jafer, R.; Bashir, S.; Mohamad, S.; Ramesh, K.; Ramesh, S. Facile synthesis of ternary nanocomposite of polypyrrole incorporated with cobalt oxide and silver nanoparticles for high performance supercapattery. Electrochim. Acta 2020, 348, 136313. [Google Scholar] [CrossRef]
- Dhawale, D.; Salunkhe, R.; Jamadade, V.; Dubal, D.; Pawar, S.; Lokhande, C. Hydrophilic polyaniline nanofibrous architecture using electrosynthesis method for supercapacitor application. Curr. Appl. Phys. 2010, 10, 904–909. [Google Scholar] [CrossRef]
- Liang, M.; Xin, H. Microwave to Terahertz: Characterization of Carbon-Based Nanomaterials. IEEE Microw. Mag. 2014, 15, 40–51. [Google Scholar] [CrossRef]
- Bianco, A.; Chen, Y.; Frackowiak, E.; Holzinger, M.; Koratkar, N.; Meunier, V.; Mikhailovsky, S.; Strano, M.; Tascon, J.M.D.; Terrones, M. Carbon science perspective in 2020: Current research and future challenges. Carbon 2020, 161, 373–391. [Google Scholar] [CrossRef]
- Parveen, N.; Mahato, N.; Ansari, M.O.; Cho, M.H. Enhanced electrochemical behavior and hydrophobicity of crystalline polyaniline@ graphene nanocomposite synthesized at elevated temperature. Compos. Part B Eng. 2016, 87, 281–290. [Google Scholar] [CrossRef]
- Deng, M.; Yang, B.; Hu, Y. Polyaniline deposition to enhance the specific capacitance of carbon nanotubes for supercapacitors. J. Mater. Sci. 2005, 40, 5021–5023. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, Y. Pseudo Capacitive Studies of Polyaniline-Carbon Nanotube Composites as Electrode Material for Supercapacitor. Anal. Lett. 2012, 45, 2075–2085. [Google Scholar] [CrossRef]
- Wu, D.; Xie, X.; Zhang, Y.; Zhang, D.; Du, W.; Zhang, X.; Wang, B. MnO2/Carbon Composites for Supercapacitor: Synthesis and Electrochemical Performance. Front. Mater. 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Yang, C.; Chen, P.; Yuan, D.; Guo, K. MnO2-decorated hierarchical porous carbon composites for high-performance asymmetric supercapacitors. J. Power Sources 2019, 425, 1–9. [Google Scholar] [CrossRef]
- Wang, J.-G.; Kang, F.; Wei, B. Engineering of MnO2-based nanocomposites for high-performance supercapacitors. Prog. Mater. Sci. 2015, 74, 51–124. [Google Scholar] [CrossRef]
- Li, L.; Hu, Z.A.; An, N.; Yang, Y.Y.; Li, Z.M.; Wu, H.Y. Facile Synthesis of MnO2/CNTs Composite for Supercapacitor Electrodes with Long Cycle Stability. J. Phys. Chem. C 2014, 118, 22865–22872. [Google Scholar] [CrossRef]
- Liu, T.; Shao, G.; Ji, M.; Wang, G. Polyaniline/MnO2 composite with high performance as supercapacitor electrode via pulse electrodeposition. Polym. Compos. 2015, 36, 113–120. [Google Scholar] [CrossRef]
- Wang, J.-G.; Yang, Y.; Huang, Z.-H.; Kang, F. Shape-controlled synthesis of hierarchical hollow urchin-shape α-MnO2 nanostructures and their electrochemical properties. Mater. Chem. Phys. 2013, 140, 643–650. [Google Scholar] [CrossRef]
- Hassanpoor, S.; Rouhi, N. Electrochemical sensor for determination of trace amounts of cadmium (II) in environmental water samples based on MnO2/RGO nanocomposite. Int. J. Environ. Anal. Chem. 2019, 1–20. [Google Scholar] [CrossRef]
- Ansari, M.O.; Khan, M.M.; Ansari, S.A.; Lee, J.; Cho, M.H. Enhanced thermoelectric behaviour and visible light activity of Ag@TiO2/polyaniline nanocomposite synthesized by biogenic-chemical route. RSC Adv. 2014, 4, 23713–23719. [Google Scholar] [CrossRef]
- Kumar, R.; Ansari, M.O.; Alshahrie, A.; Darwesh, R.; Parveen, N.; Yadav, S.K.; Barakat, M.A.; Cho, M.H. Adsorption modeling and mechanistic insight of hazardous chromium on para toluene sulfonic acid immobilized-polyaniline@CNTs nanocomposites. J. Saudi Chem. Soc. 2019, 23, 188–197. [Google Scholar] [CrossRef]
- Su, D.; Ahn, H.-J.; Wang, G. Hydrothermal synthesis of α-MnO2 and β-MnO2 nanorods as high capacity cathode materials for sodium ion batteries. J. Mater. Chem. A 2013, 1, 4845–4850. [Google Scholar] [CrossRef] [Green Version]
- Bhadra, S.; Khastgir, D. Determination of crystal structure of polyaniline and substituted polyanilines through powder X-ray diffraction analysis. Polym. Test. 2008, 27, 851–857. [Google Scholar] [CrossRef]
- Ansari, M.O.; Yadav, S.K.; Cho, J.W.; Mohammad, F. Thermal stability in terms of DC electrical conductivity retention and the efficacy of mixing technique in the preparation of nanocomposites of graphene/polyaniline over the carbon nanotubes/polyaniline. Compos. Part B Eng. 2013, 47, 155–161. [Google Scholar] [CrossRef]
- Ansari, M.O.; Ansari, S.P.; Yadav, S.K.; Anwer, T.; Cho, M.H.; Mohammad, F. Ammonia vapor sensing and electrical properties of fibrous multi-walled carbon nanotube/polyaniline nanocomposites prepared in presence of cetyl-trimethylammonium bromide. J. Ind. Eng. Chem. 2014, 20, 2010–2017. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Quan, J.; Wang, N.; Zhu, Y. Surface-enhanced Raman scattering activities of carbon nanotubes decorated with silver nanoparticles. Analyst 2016, 141, 5527–5534. [Google Scholar] [CrossRef]
- Yuan, C.-L.; Chang, C.-P.; Hong, Y.-S.; Sung, Y. Fabrication of MWNTs–PANI composite—A chemiresistive sensor material for the detection of explosive gases. Mater. Sci. Pol. 2009, 27, 509–520. [Google Scholar]
- Wang, N.; Chen, Y.; Ren, J.; Huang, X.; Chen, X.; Li, G.; Liu, D. Electrically conductive polyaniline/polyimide microfiber membrane prepared via a combination of solution blowing and subsequent in situ polymerization growth. J. Polym. Res. 2017, 24, 42. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Sun, K.; Mu, J.; Zhou, X.; Lei, Z. A novel fabrication of nitrogen-containing carbon nanospheres with high rate capability as electrode materials for supercapacitors. RSC Adv. 2015, 5, 12034–12042. [Google Scholar] [CrossRef]
- Subramanian, V.; Zhu, H.; Vajtai, R.; Ajayan, P.M.; Wei, B. Hydrothermal Synthesis and Pseudocapacitance Properties of MnO2 Nanostructures. J. Phys. Chem. B 2005, 109, 20207–20214. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Liu, X.; Li, Q.; Lin, H.; Li, Y.; Nie, M.; Qin, L. The evolution of α-MnO 2 from hollow cubes to hollow spheres and their electrochemical performance for supercapacitors. J. Mater. Sci. 2017, 52, 10915–10926. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Faisal, M.M.; Alzaid, S.R.A.M. A facile approach to investigate the charge storage mechanism of MOF/PANI based supercapattery devices. Solid State Ion. 2020, 354, 115411. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, F.; Yan, Q.; Wu, X. Investigation on electrochemical behaviors of NiCo2O4 battery-type supercapacitor electrodes: The role of an aqueous electrolyte. Inorg. Chem. Front. 2017, 4, 1642–1648. [Google Scholar] [CrossRef]
- Iqbal, J.; Li, L.; Numan, A.; Rafique, S.; Jafer, R.; Mohamad, S.; Khalid, M.; Ramesh, K.; Ramesh, S. Density functional theory simulation of cobalt oxide aggregation and facile synthesis of a cobalt oxide, gold and multiwalled carbon nanotube based ternary composite for a high performance supercapattery. New J. Chem. 2019, 43, 13183–13195. [Google Scholar] [CrossRef]
- Park, H.-W.; Kim, T.; Huh, J.; Kang, M.; Lee, J.E.; Yoon, H. Anisotropic growth control of polyaniline nanostructures and their morphology-dependent electrochemical characteristics. ACS Nano 2012, 6, 7624–7633. [Google Scholar] [CrossRef]
- Kumar, M.S.; Yasoda, K.Y.; Batabyal, S.K.; Kothurkar, N.K. Carbon-polyaniline nanocomposites as supercapacitor materials. Mater. Res. Express 2018, 5, 045505. [Google Scholar] [CrossRef]
- Omar, F.S.; Numan, A.; Bashir, S.; Duraisamy, N.; Vikneswaran, R.; Loo, Y.-L.; Ramesh, K.; Ramesh, S. Enhancing rate capability of amorphous nickel phosphate supercapattery electrode via composition with crystalline silver phosphate. Electrochim. Acta 2018, 273, 216–228. [Google Scholar] [CrossRef]
- Numan, A.; Khalid, M.; Ramesh, S.; Ramesh, K.; Shamsudin, E.; Zhan, Y.; Jagadesh, P. Facile sonochemical synthesis of 2D porous Co3O4 nanoflake for supercapattery. J. Alloys Compd. 2020, 819, 153019. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Numan, A.; Shahid, M.M.; Khanam, R.; Saidur, R.; Pandey, A.; Ramesh, S. Polyaniline-SrTiO3 nanocube based binary nanocomposite as highly stable electrode material for high performance supercapaterry. Ceram. Int. 2019, 45, 11428–11437. [Google Scholar] [CrossRef]
- Li, X.; Song, H.; Zhang, Y.; Wang, H.; Du, K.; Li, H.; Yuan, Y.; Huang, J. Enhanced electrochemical capacitance of graphene nanosheets coating with polyaniline for supercapacitors. Int. J. Electrochem Sci. 2012, 7, 5163–5171. [Google Scholar]
- Sankar, K.V.; Selvan, R.K. Fabrication of flexible fiber supercapacitor using covalently grafted CoFe2O4/reduced graphene oxide/polyaniline and its electrochemical performances. Electrochim. Acta 2016, 213, 469–481. [Google Scholar] [CrossRef]
- Shendkar, J.H.; Zate, M.; Tehare, K.; Jadhav, V.V.; Mane, R.S.; Naushad, M.; Yun, J.M.; Kim, K.H. Polyaniline-cobalt hydroxide hybrid nanostructures and their supercapacitor studies. Mater. Chem. Phys. 2016, 180, 226–236. [Google Scholar] [CrossRef]
- Ghosh, K.; Yue, C.Y.; Sk, M.M.; Jena, R.K. Development of 3D urchin-shaped coaxial manganese dioxide@ polyaniline (MnO2@ PANI) composite and self-assembled 3D pillared graphene foam for asymmetric all-solid-state flexible supercapacitor application. ACS Appl. Mater. Interfaces 2017, 9, 15350–15363. [Google Scholar] [CrossRef]
- He, X.; Liu, Q.; Liu, J.; Li, R.; Zhang, H.; Chen, R.; Wang, J. High-performance all-solid-state asymmetrical supercapacitors based on petal-like NiCo2O4/Polyaniline nanosheets. Chem. Eng. J. 2017, 325, 134–143. [Google Scholar] [CrossRef]
- Das, T.; Verma, B. Polyaniline based ternary composite with enhanced electrochemical properties and its use as supercapacitor electrodes. J. Energy Storage 2019, 26, 100975. [Google Scholar] [CrossRef]
- Iqbal, M.; Faisal, M.M.; Sulman, M.; Ali, S.R.; Alzaid, M. Facile synthesis of strontium oxide/polyaniline/graphene composite for the high-performance supercapattery devices. J. Electroanal. Chem. 2020, 879, 114812. [Google Scholar] [CrossRef]
- Iqbal, J.; Numan, A.; Omaish Ansari, M.; Jafer, R.; Jagadish, P.R.; Bashir, S.; Hasan, P.M.Z.; Bilgrami, A.L.; Mohamad, S.; Ramesh, K.; et al. Cobalt Oxide Nanograins and Silver Nanoparticles Decorated Fibrous Polyaniline Nanocomposite as Battery-Type Electrode for High Performance Supercapattery. Polymers 2020, 12, 2816. [Google Scholar] [CrossRef]

| Electrode Material | Electrolyte | Specific Capacity | Energy Density | Cycle Life % Retention/Cycles | Ref. |
|---|---|---|---|---|---|
| Graphene/polyaniline nanosheets | 6 M KOH | 261.4 F g−1 at 100 mA g−1 | 61.67%/500 cycles | [43] | |
| CoFe2O4/reduced graphene oxide/polyaniline composite | 1 M KOH | 9 mF m−1 at 1 mA | 270 × 10−8 Wh cm−1 | 87%/1000 cycles | [44] |
| Cobalt hydroxide/polyaniline hybrid nanostructure | 1 M NaOH | 215 F g−1 at 10 mV s−1 | × | 60%/1000 cycles | [45] |
| CoFe2O4/reduced graphene oxide/polyaniline composite | 1 M KOH | 9 mF m−1 at 1 mA | 270 × 10−8 Wh cm−1 | 87%/1000 cycles | [44] |
| Manganese dioxide-polyaniline composite | Polyvinyl alcohol/KOH gel | 129.2 F g−1 at 0.5 A g−1 | 22.3 Wh kg−1 | 89%/5000 cycles | [46] |
| NiCo2S4/polyaniline nanosheets | Polyvinyl alcohol/KOH gel | 152.06 F g−1 at 1 A g−1 | 54.06 Wh kg−1 | 91.1%/2000 cycles | [47] |
| Acetylene black-manganese cobaltite- polyaniline composite | 1 M KOH | 0.35 F cm−2 at 1 mA cm−2 | 18.203 Wh kg−1 | 90%/3000 cycles | [48] |
| Strontium oxide/graphene/polyaniline ternary composite | 1 M KOH | 151.66 C g−1 | 33.8 Wh kg−1 | 80%/3000 cycles | [49] |
| Metal organic framework (MOF)/polyaniline composites | 1 M KOH | 162.5 C g−1 at 0.4 A g−1 | 23.2 Wh kg−1 at 1 A g−1 | 146%/3000 cycles | [35] |
| Co3O4/Ag/polyaniline ternary composites | 0.1 M KOH | 262.62 C g−1 at 3 mV s−1 | 14.01 Wh kg−1 at 0.2 A g−1 | 121.03%/3500 cycles | [50] |
| polyaniline@CNT/MnO2 ternary composites | 0.1 M KOH | 131.27 C g−1 at 0.4 A g−1 | 27.17 Wh kg−1 at 0.3 A g−1 | 119%/3500 cycles | Our work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, J.; Ansari, M.O.; Numan, A.; Wageh, S.; Al-Ghamdi, A.; Alam, M.G.; Kumar, P.; Jafer, R.; Bashir, S.; Rajpar, A.H. Hydrothermally Assisted Synthesis of Porous Polyaniline@Carbon Nanotubes–Manganese Dioxide Ternary Composite for Potential Application in Supercapattery. Polymers 2020, 12, 2918. https://doi.org/10.3390/polym12122918
Iqbal J, Ansari MO, Numan A, Wageh S, Al-Ghamdi A, Alam MG, Kumar P, Jafer R, Bashir S, Rajpar AH. Hydrothermally Assisted Synthesis of Porous Polyaniline@Carbon Nanotubes–Manganese Dioxide Ternary Composite for Potential Application in Supercapattery. Polymers. 2020; 12(12):2918. https://doi.org/10.3390/polym12122918
Chicago/Turabian StyleIqbal, Javed, Mohammad Omaish Ansari, Arshid Numan, S. Wageh, Ahmed Al-Ghamdi, Mohd Gulfam Alam, Pramod Kumar, Rashida Jafer, Shahid Bashir, and A. H. Rajpar. 2020. "Hydrothermally Assisted Synthesis of Porous Polyaniline@Carbon Nanotubes–Manganese Dioxide Ternary Composite for Potential Application in Supercapattery" Polymers 12, no. 12: 2918. https://doi.org/10.3390/polym12122918
APA StyleIqbal, J., Ansari, M. O., Numan, A., Wageh, S., Al-Ghamdi, A., Alam, M. G., Kumar, P., Jafer, R., Bashir, S., & Rajpar, A. H. (2020). Hydrothermally Assisted Synthesis of Porous Polyaniline@Carbon Nanotubes–Manganese Dioxide Ternary Composite for Potential Application in Supercapattery. Polymers, 12(12), 2918. https://doi.org/10.3390/polym12122918