Chemical Hydrogels Bearing Thiazolium Groups with a Broad Spectrum of Antimicrobial Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hydrogels
2.3. Characterization Techniques
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klouda, L. Thermoresponsive hydrogels in biomedical applications A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Palza, H.; Zapata, P.A.; Angulo-Pineda, C. Electroactive Smart Polymers for Biomedical Applications. Materials 2019, 12, 277. [Google Scholar]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Wang, X.; Peng, J.; Xu, Y.; Chang, J. Multifunctional Hydrogels Prepared by Dual Ion Cross-Linking for Chronic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 16054–16062. [Google Scholar] [CrossRef]
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic antimicrobial polymers: Recent advances in molecular design. Polym. Chem. 2018, 9, 2407–2427. [Google Scholar] [CrossRef]
- Kumar, A.; Boyer, C.; Nebhani, L.; Wong, E.H.H. Highly Bactericidal Macroporous Antimicrobial Polymeric Gel for Point-of-Use Water Disinfection. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Harvey, A.C.; Madsen, J.; Douglas, C.W.I.; MacNeil, S.; Armes, S.P. Antimicrobial Graft Copolymer Gels. Biomacromolecules 2016, 17, 2710–2718. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, M. Antimicrobial and antiviral hydrogels. Soft Matter 2011, 7, 8725–8736. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A. Antimicrobial Polymeric Gels; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081021798. [Google Scholar]
- Muñoz-Bonilla, A.; Fernández-García, M. The roadmap of antimicrobial polymeric materials in macromolecular nanotechnology. Eur. Polym. J. 2015, 65. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Poly(ionic liquid)s as antimicrobial materials. Eur. Polym. J. 2018, 105. [Google Scholar] [CrossRef]
- Alvarez-Paino, M.; Munoz-Bonilla, A.; Fernandez-Garcia, M.; Álvarez-Paino, M.; Muñoz-Bonilla, A.; Fernández-García, M. Antimicrobial Polymers in the Nano-World. Nanomaterials 2017, 7, 48. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-Based Polymers with Antimicrobial Properties towards Sustainable Development. Materials 2019, 12, 641. [Google Scholar] [CrossRef]
- Kuroda, K.; Caputo, G.A.; DeGrado, W.F. The Role of Hydrophobicity in the Antimicrobial and Hemolytic Activities of Polymethacrylate Derivatives. Chem. A Eur. J. 2009, 15, 1123–1133. [Google Scholar] [CrossRef]
- Palermo, E.F.; Sovadinova, I.; Kuroda, K. Structural determinants of antimicrobial activity and biocompatibility in membrane-disrupting methacrylamide random copolymers. Biomacromolecules 2009, 10, 3098–3107. [Google Scholar] [CrossRef]
- Palermo, E.F.; Kuroda, K. Chemical Structure of Cationic Groups in Amphiphilic Polymethacrylates Modulates the Antimicrobial and Hemolytic Activities. Biomacromolecules 2009, 10, 1416–1428. [Google Scholar] [CrossRef]
- Palermo, E.F.; Kuroda, K. Structural determinants of antimicrobial activity in polymers which mimic host defense peptides. Appl. Microbiol. Biotechnol. 2010, 87, 1605–1615. [Google Scholar] [CrossRef]
- Takahashi, H.; Palermo, E.F.; Yasuhara, K.; Caputo, G.A.; Kuroda, K. Molecular design, structures, and activity of antimicrobial peptide-mimetic polymers. Macromol. Biosci. 2013, 13, 1285–1299. [Google Scholar] [CrossRef]
- Pavlukhina, S.; Lu, Y.; Patimetha, A.; Libera, M.; Sukhishvili, S. Polymer Multilayers with pH-Triggered Release of Antibacterial Agents. Biomacromolecules 2010, 11, 3448–3456. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.A.; Figuly, G.D.; Chapman, J.S.; Hunt, T.W.; Glunt, C.D.; Rivenbark, J.A.; Chenault, H.K. Antimicrobial hydrogels formed by crosslinking polyallylamine with aldaric acid derivatives. J. Appl. Polym. Sci. 2011, 119, 3244–3252. [Google Scholar] [CrossRef]
- Hamzé, A.; Rubi, E.; Arnal, P.; Boisbrun, M.; Carcel, C.; Salom-Roig, X.; Maynadier, M.; Wein, S.; Vial, H.; Le Calas, M. Brief Articles Mono-and Bis-Thiazolium Salts Have Potent Antimalarial Activity. J. Med. Chem. 2005, 48, 3639–3643. [Google Scholar] [CrossRef] [PubMed]
- Caldarelli, S.A.; El Fangour, S.; Wein, S.; Van Tran Ba, C.; Périgaud, C.; Pellet, A.; Vial, H.J.; Peyrottes, S. New bis-thiazolium analogues as potential antimalarial agents: Design, synthesis, and biological evaluation. J. Med. Chem. 2013, 56, 496–509. [Google Scholar] [CrossRef]
- Shiradkar, M.; Suresh Kumar, G.V.; Dasari, V.; Tatikonda, S.; Akula, K.C.; Shah, R. Clubbed triazoles: A novel approach to antitubercular drugs. Eur. J. Med. Chem. 2007, 42, 807–816. [Google Scholar] [CrossRef]
- Wang, M.W.; Zhu, H.H.; Wang, P.Y.; Zeng, D.; Wu, Y.Y.; Liu, L.W.; Wu, Z.B.; Li, Z.; Yang, S. Synthesis of Thiazolium-Labeled 1,3,4-Oxadiazole Thioethers as Prospective Antimicrobials: In Vitro and in Vivo Bioactivity and Mechanism of Action. J. Agric. Food Chem. 2019, 67, 12696–12708. [Google Scholar] [CrossRef]
- Tejero, R.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Fernández-García, M. Antimicrobial polymethacrylates based on quaternized 1,3-thiazole and 1,2,3-triazole side-chain groups. Polym. Chem. 2015, 6. [Google Scholar] [CrossRef]
- Tejero, R.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Fernández-García, M. High efficiency antimicrobial thiazolium and triazolium side-chain polymethacrylates obtained by controlled alkylation of the corresponding azole derivatives. Biomacromolecules 2015, 16. [Google Scholar] [CrossRef]
- Tejero, R.; Gutiérrez, B.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Fernández-García, M. Copolymers of acrylonitrile with quaternizable thiazole and triazole side-chain methacrylates as potent antimicrobial and hemocompatible systems. Acta Biomater. 2015, 25. [Google Scholar] [CrossRef]
- Alvarez-Paino, M.; Juan-Rodríguez, R.; Cuervo-Rodríguez, R.; Tejero, R.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Muñoz-Bonilla, A.; Fernández-García, M. Antimicrobial films obtained from latex particles functionalized with quaternized block copolymers. Colloids Surf. B Biointerfaces 2016, 140. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; López, D.; Fernández-García, M. Providing Antibacterial Activity to Poly(2-Hydroxy Ethyl Methacrylate) by Copolymerization with a Methacrylic Thiazolium Derivative. Int. J. Mol. Sci. 2018, 19, 4120. [Google Scholar] [CrossRef] [PubMed]
- Tejero, R.; Gutiérrez, B.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.J.L.; Muñoz-Bonilla, A.; Fernández-García, M. Tailoring macromolecular structure of cationic polymers towards efficient contact active antimicrobial surfaces. Polymers 2018, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Rodríguez, R.; López-Fabal, F.; Gómez-Garcés, J.L.; Muñoz-Bonilla, A.; Fernández-García, M. Contact Active Antimicrobial Coatings Prepared by Polymer Blending. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, A.; Echeverria, C.; San Martin, M.; Cuervo-Rodriguez, R.; Fernandez-Garcia, M.; Munoz-Bonilla, A. Porous Microstructured Surfaces with pH-Triggered Antibacterial Properties. Macromol. Biosci. 2019, 19, e1900127. [Google Scholar] [CrossRef]
- Echeverría, C.; Muñoz-Bonilla, A.; Cuervo-Rodríguez, R.; López, D.; Fernández-García, M. Antibacterial PLA Fibers Containing Thiazolium Groups as Wound Dressing Materials. ACS Appl. Bio Mater. 2019, 2, 4714–4719. [Google Scholar] [CrossRef]
- ASTM E2149-01, Standard Test Method for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents Under Dynamic Contact Conditions (Withdrawn 2010); ASTM International: West Conshohocken, PA, USA, 2001; Available online: www.astm.org (accessed on 1 November 2020).
- Vargün, E.; Usanmaz, A. Degradation of Poly(2-hydroxyethyl methacrylate) Obtained by Radiation in Aqueous Solution. J. Macromol. Sci. Part A 2010, 47, 882–891. [Google Scholar] [CrossRef]
- Fernández-García, M.; Torrado, M.F.F.; Martínez, G.; Sánchez-Chaves, M.; Madruga, E.L. Free radical copolymerization of 2-hydroxyethyl methacrylate with butyl methacrylate: Determination of monomer reactivity ratios and glass transition temperatures. Polymer 2000, 41, 8001–8008. [Google Scholar] [CrossRef]
- Demirelli, K.; Coşkun, M.; Kaya, E. A detailed study of thermal degradation of poly(2-hydroxyethyl methacrylate). Polym. Degrad. Stab. 2001, 72, 75–80. [Google Scholar] [CrossRef]
- Çaykara, T.; Özyürek, C.; Kantoğlu, Ö. Investigation of thermal behavior of poly(2-hydroxyethyl methacrylate-co-itaconic acid) networks. J. Appl. Polym. Sci. 2006, 103, 1602–1607. [Google Scholar] [CrossRef]
- Sanna, R.; Alzari, V.; Nuvoli, D.; Scognamillo, S.; Marceddu, S.; Mariani, A. Polymer hydrogels of 2-hydroxyethyl acrylate and acrylic acid obtained by frontal polymerization. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1515–1520. [Google Scholar] [CrossRef]
- Cuervo-Rodríguez, R.; Muñoz-Bonilla, A.; Araujo, J.; Echeverría, C.; Fernández-García, M. Influence of side chain structure on the thermal and antimicrobial properties of cationic methacrylic polymers. Eur. Polym. J. 2019, 117, 86–93. [Google Scholar] [CrossRef]

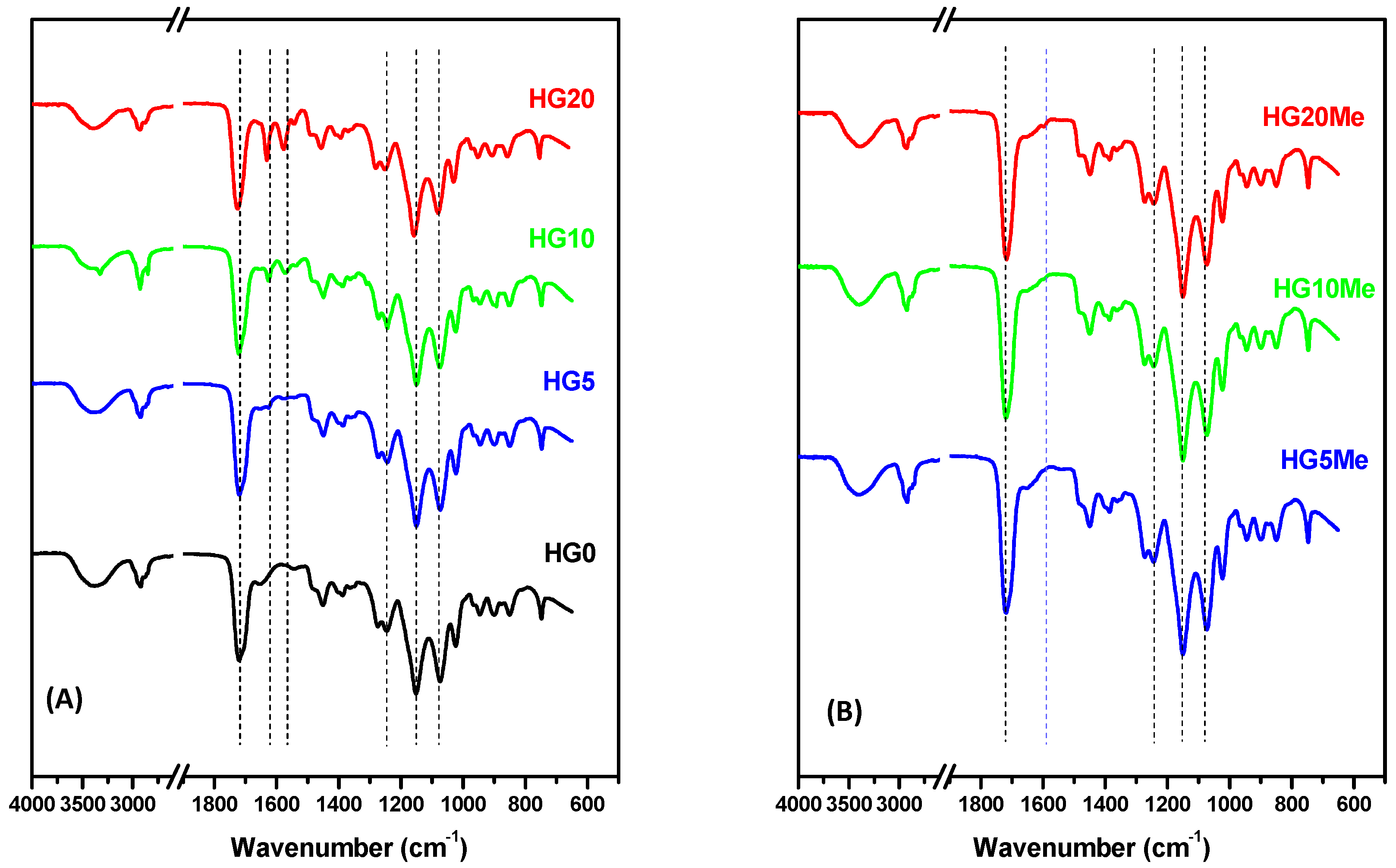
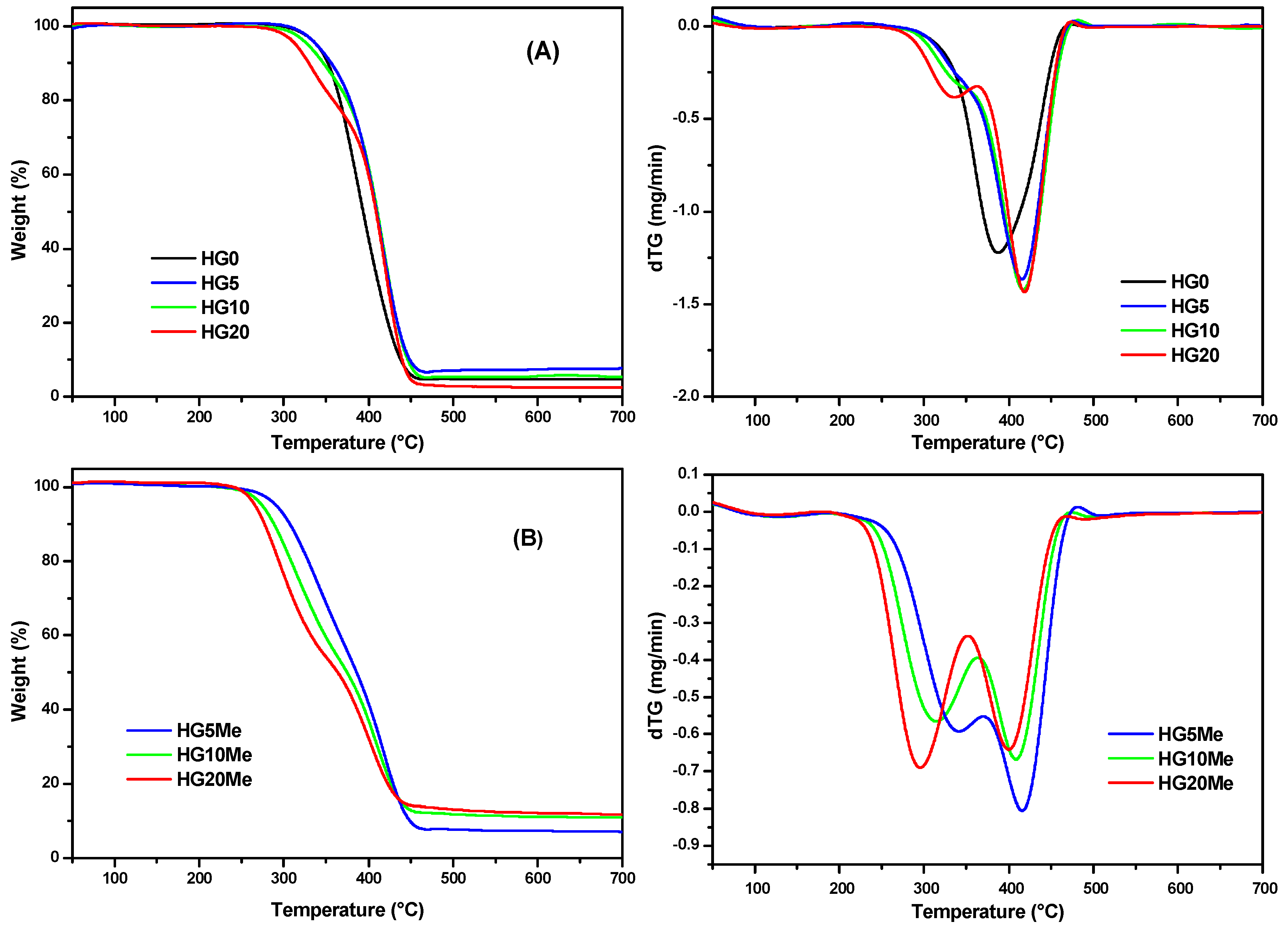

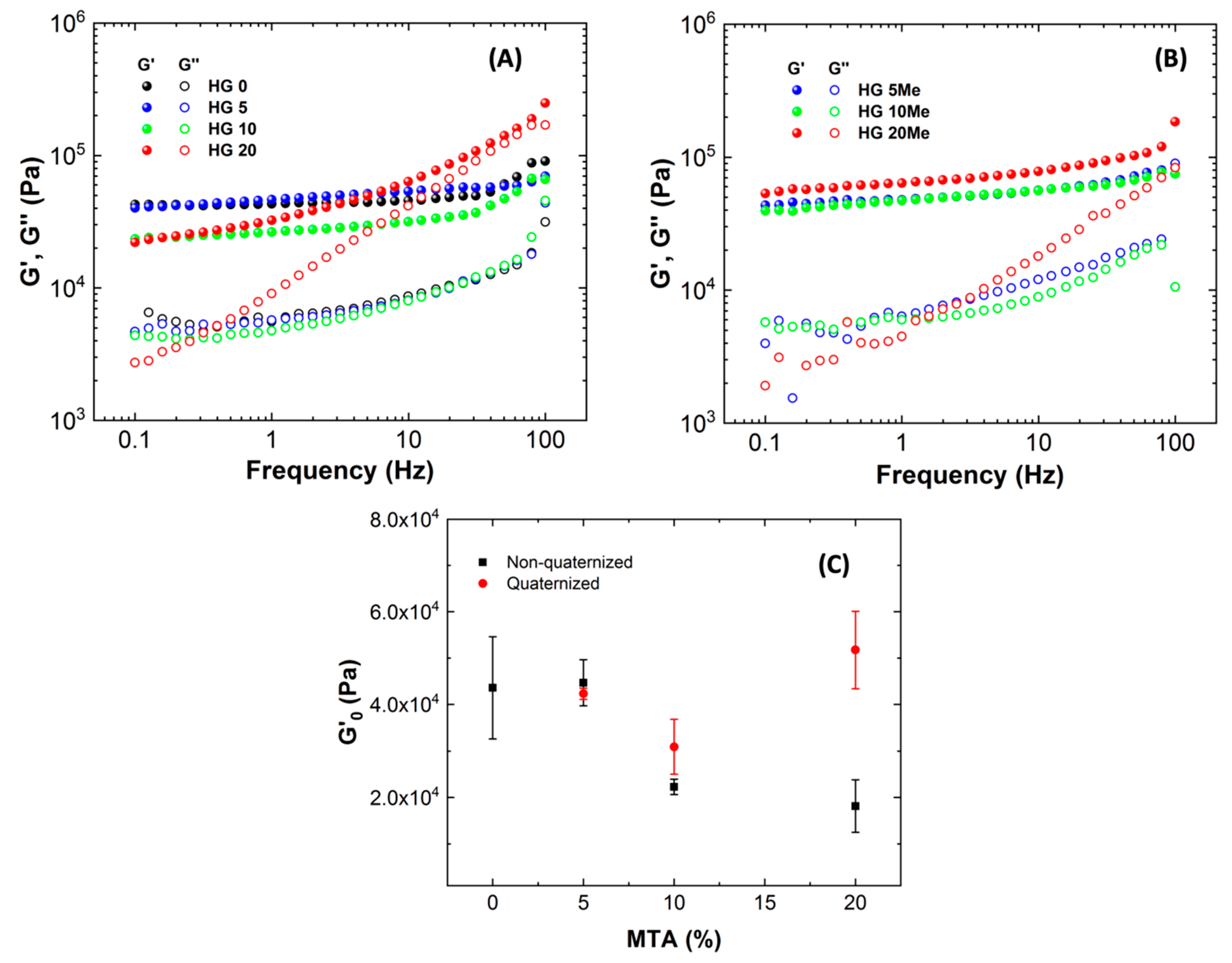

| Hydrogels | HEMA | PEGDA | ACPA | MTA |
|---|---|---|---|---|
| HG0 | 81.70 | 16.34 | 1.96 | 0 |
| HG5 | 78.49 | 15.70 | 1.88 | 3.92 |
| HG10 | 75.53 | 15.11 | 1.81 | 7.55 |
| HG20 | 70.2 | 14.01 | 1.69 | 14.1 |
| Hydrogels | T0 (°C) | Tmax1 (°C) | Tmax2 (°C) | Residue (%) |
|---|---|---|---|---|
| HG0 | 317 | 388 | 4.7 | |
| HG5 HG5Me | 317 287 | 336 * 342 | 416 416 | 7.6 7.3 |
| HG10 HG10Me | 305 269 | 335 * 314 | 418 409 | 5.5 11.1 |
| HG20 HG20Me | 305 250 | 334 296 | 418 400 | 2.5 12.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Bonilla, A.; Zagora, J.; Plachá, D.; Echeverría, C.; Chiloeches, A.; Fernández-García, M. Chemical Hydrogels Bearing Thiazolium Groups with a Broad Spectrum of Antimicrobial Behavior. Polymers 2020, 12, 2853. https://doi.org/10.3390/polym12122853
Muñoz-Bonilla A, Zagora J, Plachá D, Echeverría C, Chiloeches A, Fernández-García M. Chemical Hydrogels Bearing Thiazolium Groups with a Broad Spectrum of Antimicrobial Behavior. Polymers. 2020; 12(12):2853. https://doi.org/10.3390/polym12122853
Chicago/Turabian StyleMuñoz-Bonilla, Alexandra, Jakub Zagora, Daniela Plachá, Coro Echeverría, Alberto Chiloeches, and Marta Fernández-García. 2020. "Chemical Hydrogels Bearing Thiazolium Groups with a Broad Spectrum of Antimicrobial Behavior" Polymers 12, no. 12: 2853. https://doi.org/10.3390/polym12122853
APA StyleMuñoz-Bonilla, A., Zagora, J., Plachá, D., Echeverría, C., Chiloeches, A., & Fernández-García, M. (2020). Chemical Hydrogels Bearing Thiazolium Groups with a Broad Spectrum of Antimicrobial Behavior. Polymers, 12(12), 2853. https://doi.org/10.3390/polym12122853









