Biodiversity Patterns and Community Construction in Subtropical Forests Driven by Species Phylogenetic Environments
Abstract
1. Introduction
2. Results
2.1. Distribution Characteristics of Species and Phylogenetic Diversity in Different Forest Types
2.2. The Relationship Between Species Diversity and Phylogenetic Diversity of Different Forest Types and the Impact of Environmental Factors on Diversity
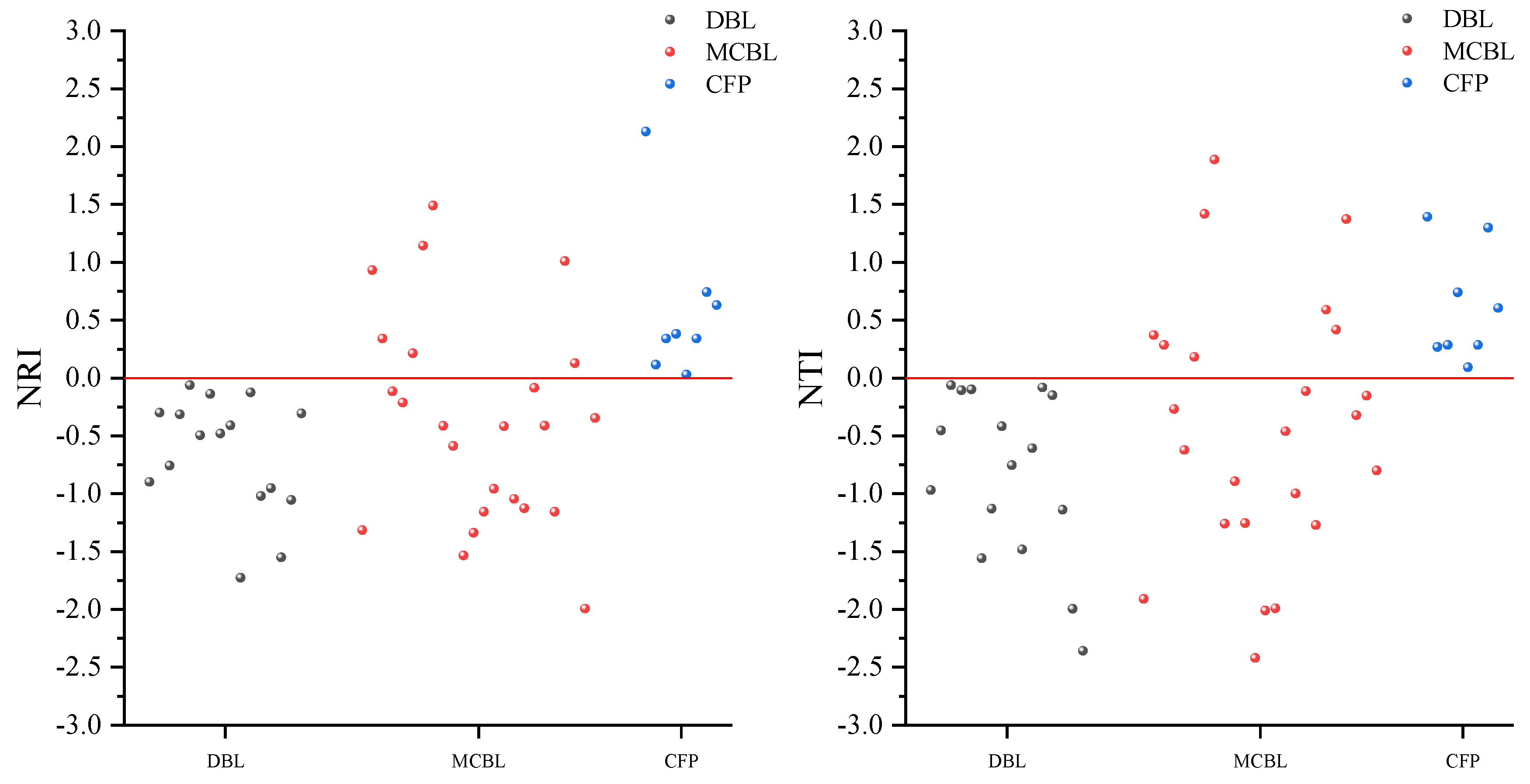
2.3. Community Construction and Driving Mechanisms of Different Forest Types
3. Discussion
3.1. Distribution Characteristics of Species and Phylogenetic Diversity
3.2. Characteristics of Community Construction in Different Forest Types
3.3. The Impact of Environment on Diversity and the Driving Mechanism of Communities
4. Materials and Methods
4.1. Overview of the Study Area
4.2. Experimental Design and Data Acquisition
4.2.1. Sample Plot Setting and Vegetation Survey
4.2.2. Forest Type Classification
4.3. Soil Factor Investigation
4.3.1. Soil Sampling
4.3.2. Soil Determination
4.4. Species Diversity Calculation
4.5. Phylogenetic Diversity Calculation
4.6. Calculation of Phylogenetic Structure Index
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; Matthews, J.B.R.; Berger, S.; et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; Volume 2, p. 2391. [Google Scholar]
- He, Z.H.; Yang, H.; Wu, H.Y.; Chen, F.J.; Tian, B. Simulation of species distribution for styracaceae in east asia under climate change. J. Southwest For. Univ. 2025, 45, 124–131. [Google Scholar]
- Fox, J.W. Partitioning the effects of species loss on community variability using multi-level selection theory. Oikos 2010, 119, 1823–1833. [Google Scholar] [CrossRef]
- Hector, A.; Hautier, Y.; Saner, P.; Wacker, L.; Bagchi, R.; Joshi, J.; Scherer-Lorenzen, M.; Spehn, E.M.; Bazeley-White, E.; Weilenmann, M.; et al. General stabilizing effects of plant diversity on grassland productivity at multiple sites through population asynchrony and overyielding. Ecology 2010, 91, 2213–2220. [Google Scholar] [CrossRef]
- Tavankar, F.; Picchio, R.; Venanzi, R.; Latterini, F.; Nikooy, M. Restoring soil features and biodiversity indicators in managed forests to the levels of protected forests: After one silvicultural rotation period. Forests 2025, 16, 274. [Google Scholar] [CrossRef]
- Rafael, M.; Gianluigi, O.; Giandiego, C.; Roberto, C.; Stefano, C. Biogeographic deconstruction of phylogenetic and functional diversity provides insights into the formation of regional assemblages. Ecography 2022, 2022, e06140. [Google Scholar] [CrossRef]
- Zheng, J.M.; Zheng, Y.S. Exploring the species and phylogenetic diversity, phylogenetic structure of mixed communities along the coastal gradient. A case study in a subtropical island, China. Appl. Ecol. Environ. Res. 2022, 20, 3129–3141. [Google Scholar] [CrossRef]
- Veldkornet, A.D. The Influence of Macroclimatic Drivers on the Macrophyte Phylogenetic Diversity in South African Estuaries. Diversity 2023, 15, 986. [Google Scholar] [CrossRef]
- Maestre, F.T.; Soliveres, S.; Gotelli, N.J.; Quero, J.L.; Berdugo, M. Response to Comment on “Plant Species Richness and Ecosystem Multifunctionality in Global Drylands”. Science 2012, 337, 155. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Srivastava, D.S.; Emmett Duffy, J.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef]
- Newman, J.A.; Varner, G.; Linquist, S. Defending Biodiversity: Environmental Science and Ethics; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Valiente-Banuet, A.; Verdú, M. Facilitation can increase the phylogenetic diversity of plant communities. Ecol. Lett. 2007, 10, 1029–1036. [Google Scholar] [CrossRef]
- Maguire, G.V.; Bordenave, D.C.; Nieva, S.A.; María ELlames Ruiz, O.A. Soil bacterial and fungal community structure of a rice monoculture and rice-pasture rotation systems. Appl. Soil Ecol. 2020, 151, 103535. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Stegen, J.C.; Kim, M.; Dong, K.; Adams, J.M.; Lee, Y.K. Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. ISME J. 2018, 12, 1072–1083. [Google Scholar] [CrossRef]
- Cadotte, W.M.; Dinnage, R.; Tilman, D. Phylogenetic diversity promotes ecosystem stability. Ecology 2012, 93, S223–S233. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Cavender-Bares, J.; Tilman, D.; Oakley, T.H.; Freckleton, R.P. Using phylogenetic, functional and trait diversity to understand patterns of plant community productivity. PLoS ONE 2009, 4, e5695. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.O.; Ackerly, D.D.; Kembel, S.W. Phylocom: Software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 2008, 24, 2098–2100. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.H.; Shi, W.; Tang, Y.S.; Zheng, W.Y.; Cao, K.F. Comparison of the species diversity and phylogenetic structure of tree communities in karst and non-karst mountains in Guangxi. Acta Ecol. Sin. 2018, 38, 8708–8716. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Zeng, D.; Si, X.F. The application of phylogenetic methods in community ecology. Bio-101 2021, e1010670. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, S.; Hu, G.; Mwachala, G.; Yan, X.; Wang, Q. Species richness and phylogenetic diversity of seed plants across vegetation zones of Mount Kenya, East Africa. Ecol. Evol. 2018, 8, 8930–8939. [Google Scholar] [CrossRef]
- Li, M.J.; He, Z.S.; Jiang, L.; Gu, X.G.; Jin, M.R.; Chen, B.; Liu, J.F. Distribution pattern and driving factors of species diversity and phylogenetic diversity along altitudinal gradient on the south slope of Daiyun Mountain. Acta Ecol. Sin. 2021, 41, 1148–1157. [Google Scholar] [CrossRef]
- Huang, J.X.; Zheng, F.Y.; Mi, X.C. Influence of environmental factors on phylogenetic structure at multiple spatial scales in an evergreen broad-leaved forest of China. Chin. J. Plant Ecol. 2010, 34, 309–315. [Google Scholar]
- Xu, W.M.; Ci, X.; Song, C.Y.; He, T.; Zhang, W.; Li, Q.; Li, J. Soil phosphorus heterogeneity promotes tree species diversity and phylogenetic clustering in a tropical seasonal rainforest. Ecol. Evol. 2016, 6, 8719–8726. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.E.; Yang, S.N.; Ye, Z.H.; Fu, W.; Pan, X.T.; Ye, X.M.; Wu, W.X. Effects of tending and thinning on understory plant diversity of Chinese fir. J. Green Sci. Technol. 2023, 25, 156–159. [Google Scholar]
- Zhu, Q. Study on Geographic Variation and Genetic Diversity of Natural Populations of Machilus pauhoi Kanchira. Ph.D. Thesis, South China Agricultural University, Guangzhou, China, 2017. [Google Scholar]
- Tilman, D.; Reich, P.B.; Knops, J.M.H. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 2006, 441, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Kluge, J.; Kessler, M. Phylogenetic diversity, trait diversity and niches: Species assembly of ferns along a tropical elevational gradient. J. Biogeogr. 2011, 38, 394–405. [Google Scholar] [CrossRef]
- Li, R.; Kraft, N.J.B.; Yu, H.; Li, H. Seed plant phylogenetic diversity and species richness in conservation planning within a global biodiversity hotspot in eastern Asia. Conserv. Biol. 2015, 29, 1552–1562. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, L.; Xie Zy Li, Y.D.; Li, J.X.; Li, M.J.; Wei, C.S.; Xing, C.; Liu, J.F.; He, Z.S. Taxonomic and phylogenetic diversity of plants in a Castanopsis kawakamiinatural forest. Biodivers. Sci. 2021, 29, 439–448. [Google Scholar] [CrossRef]
- Lu, M.M.; Huang, X.C.; Ci, X.Q.; Yang, G.P.; Li, J. Phylogenetic community structure of subtropical forests along elevational gradients in Ailao Mountains of southwest China. Biodiver. Sci. 2014, 22, 438–448. [Google Scholar]
- Silva, I.A.; Batalha, M.A. Phylogenetic overdispersion of plant species in southern Brazilian savannas. Braz. J. Biol. 2009, 69, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Cavender-Bares, J.; Ackerly, D.D.; Baum, D.A.; Bazzaz, F.A. Phylogenetic overdispersion in Floridian oak communities. Am. Nat. 2004, 163, 823–843. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Amaya, N.; Brambach, F.; Corral-Rivas, J.J.; Ehbrecht, M. Tree diversity patterns along an elevational gradient in Durango, Mexico. Front. Ecol. Evol. 2025, 13, 1562841. [Google Scholar] [CrossRef]
- Siddique, I.; Vieira, I.C.G.; Schmidt, S.; Lamb, D.; Figueiredo, C.J.R.; de Oliveira Figueiredo, R.; Blomberg, S.; Davidson, E.A. Nitrogen and phosphorus additions negatively affect tree species diversity in tropical forest regrowth trajectories. Ecology 2010, 91, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Raven, J.A.; Shaver, G.R.; Smith, S.E. Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Hou, E.; Chen, C.; McGroddy, M.E.; Wen, D. Nutrient limitation on ecosystem productivity and processes of mature and old-growth subtropical forests in China. PLoS ONE 2012, 7, e52071. [Google Scholar] [CrossRef]
- Gallego-Zamorano, J.; Huijbregts, M.A.J.; Schipper, A.M. Changes in plant species richness due to land use and nitrogen deposition across the globe. Divers. Distrib. 2022, 28, 745–755. [Google Scholar] [CrossRef]
- Stevens, C.J.; David, T.I.; Storkey, J. Atmospheric nitrogen deposition in terrestrial ecosystems: Its impact on plant communities and consequences across trophic levels. Funct. Ecol. 2018, 32, 1757–1769. [Google Scholar] [CrossRef]
- Mahaut, L.; Fort, F.; Violle, C.; Grégoire, T.F. Multiple facets of diversity effects on plant productivity: Species richness, functional diversity, species identity and intraspecific competition. Funct. Ecol. 2020, 34, 287–298. [Google Scholar] [CrossRef]
- Strengbom, J.; Walheim, M.; Näsholm, T.; Ericson, L. Regional Differences in the Occurrence of Understorey Species Reflect Nitrogen Deposition in Swedish Forests. AMBIO A J. Hum. Environ. 2003, 32, 91–97. [Google Scholar] [CrossRef]
- Saiz, H.; Alados, C.L. Changes in semi-arid plant species associations along a livestock grazing gradient. PLoS ONE 2012, 7, e40551. [Google Scholar] [CrossRef]
- Curtis, J.T.; McIntosh, R.P. The interrelations of certain analytic and synthetic phytosociological characters. Ecology 1950, 31, 434–455. [Google Scholar] [CrossRef]
- Wang, Q.H.; Ren, Y.F.; Meng, L.; Li, H.; Fu, H.M.; Wang, H.F. Simultaneous determination of total nitrogen and organic carbon in soil with an elemental analyzer. Chin. J. Anal. Lab. 2013, 32, 41–45. [Google Scholar]
- Cao, X.Y.; Zhao, W.F.; Li, J.P.; Yan, W.D.; Pang, Y.F.; Sun, Y.P.; Xie, Z.C. A comprehensive evaluation soil nutrients in main typical forests central-subtropical China. Acta Ecol. Sin. 2022, 42, 3525–3535. [Google Scholar]
- Gamito, S. Caution is needed when applying Margalef diversity index. Ecol. Indic. 2010, 10, 550–551. [Google Scholar] [CrossRef]
- Su, Y.Q.; Zhang, Y.; Jia, X.R.; Xue, Y.G. Application of several diversity indexes in forest community analysis. Ecol. Sci. 2017, 36, 132–138. [Google Scholar]
- Ali, S.; Amin, A.; Akhtar, M.S.; Zaman, W. Phylogenetic Diversity in Forests: Insights into Evolutionary Patterns and Conservation Strategies. Forests 2025, 16, 1004. [Google Scholar] [CrossRef]
- R Core Developement Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2019. [Google Scholar]

| Forest Type | Margalef | Shannon | Simpson | Pielou | PD |
|---|---|---|---|---|---|
| Deciduous broad-leaved forest | 4.121 ± 1.425 a | 1.448 ± 0.632 a | 0.630 ± 0.225 a | 0.616 ± 0.166 a | 21.265 ± 7.796 a |
| Mixed coniferous and broad-leaved forest | 3.507 ± 1.158 a | 1.019 ± 0.426 a | 0.469 ± 0.175 a | 0.501 ± 0.155 ab | 18.279 ± 6.975 ab |
| Chinese fir plantation | 2.049 ± 0.570 b | 0.377 ± 0.156 b | 0.198 ± 0.121 b | 0.366 ± 0.248 ab | 10.034 ± 4.610 b |
| Forest Type | Phylogenetic Diversity Index | Species Diversity Index | AIC | R2 | p-Value |
|---|---|---|---|---|---|
| Deciduous broad-leaved forest | PD | Margalef | 70.5636 | 0.9419 | ** |
| PD | Shannon | 97.4848 | 0.6874 | ** | |
| PD | Simpson | 100.459 | 0.6235 | ** | |
| PD | Pielou | 111.1831 | 0.2641 | * | |
| Mixed coniferous and broad-leaved forest | PD | Margalef | 105.9070 | 0.9193 | ** |
| PD | Shannon | 142.2155 | 0.6337 | ** | |
| PD | Simpson | 153.6855 | 0.4093 | ** | |
| PD | Pielou | 166.2070 | 0.0047 | / | |
| Chinese fir plantation | PD | Margalef | 30.6551 | 0.9314 | ** |
| PD | Shannon | 51.7857 | 0.0368 | / | |
| PD | Simpson | 50.2968 | 0.2004 | / | |
| PD | Pielou | 46.8579 | 0.4798 | / |
| Different Types of Forests | Index | Soil Factors | Regression Coefficient | Standard Error | t-Values | p-Value | AIC |
|---|---|---|---|---|---|---|---|
| Deciduous broad-leaved forest | Shannon | AP | −0.251 | 0.132 | −2.650 | * | 29.171 |
| Simpson | AP | −0.149 | 0.042 | −3.551 | ** | −7.664 | |
| Pielou | AP | −0.116 | 0.029 | −3.969 | ** | −19.070 | |
| Mixed coniferous and broad-leaved forest | Shannon | TP | 3.782 | 1.463 | 2.586 | * | 25.811 |
| Shannon | pH | −0.389 | 0.165 | −2.357 | * | 26.777 | |
| Simpson | TP | 1.491 | 0.609 | 2.449 | * | −16.265 | |
| Chinese fir plantation | Margalef | AN | −0.0123 | 0.005 | −2.548 | * | 12.780 |
| PD | AN | −3.998 | 5.314 | −0.752 | / | 51.364 | |
| PD | TP | −123.77 | 49.48 | −2.501 | * | 46.371 |
| Forest Type | Species | Importance Values Index (%) |
|---|---|---|
| Deciduous broad-leaved forest | Liquidambar formosana Hance (Hamamelidaceae) | 16.14 |
| Cunninghamia lanceolata (Lamb.) Hook. (Cupressaceae) | 13.85 | |
| Pinus massoniana Lamb. (Pinaceae) | 11.84 | |
| Sassafras tzumu (Hemsl.) Hemsl. (Lauraceae) | 8.18 | |
| Cinnamomum japonicum Siebold (Lauraceae) | 7.19 | |
| Mixed coniferous and broad-leaved forest | Cunninghamia lanceolata (Lamb.) Hook. (Cupressaceae) | 35.72 |
| Pinus massoniana Lamb. (Pinaceae) | 21.05 | |
| Sassafras tzumu (Hemsl.) Hemsl. (Lauraceae) | 10.59 | |
| Liriodendron chinense (Hemsl.) Sarg. (Magnoliaceae) | 8.04 | |
| Paulownia fortune(Seem.) Hemsl. (Paulowniaceae) | 3.56 | |
| Chinese fir plantation | Cunninghamia lanceolata (Lamb.) Hook. (Cupressaceae) | 75.55 |
| Sassafras tzumu (Hemsl.) Hemsl. (Lauraceae) | 17.17 | |
| Pinus massoniana Lamb. (Pinaceae) | 2.60 | |
| Liriodendron chinense (Hemsl.) Sarg. (Magnoliaceae) | 1.05 | |
| Dalbergia hupeana Hance (Fabaceae) | 1.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Jiao, J.; Wu, C.; Shao, W.; Liu, X.; Yao, L. Biodiversity Patterns and Community Construction in Subtropical Forests Driven by Species Phylogenetic Environments. Plants 2025, 14, 2397. https://doi.org/10.3390/plants14152397
Liu P, Jiao J, Wu C, Shao W, Liu X, Yao L. Biodiversity Patterns and Community Construction in Subtropical Forests Driven by Species Phylogenetic Environments. Plants. 2025; 14(15):2397. https://doi.org/10.3390/plants14152397
Chicago/Turabian StyleLiu, Pengcheng, Jiejie Jiao, Chuping Wu, Weizhong Shao, Xuesong Liu, and Liangjin Yao. 2025. "Biodiversity Patterns and Community Construction in Subtropical Forests Driven by Species Phylogenetic Environments" Plants 14, no. 15: 2397. https://doi.org/10.3390/plants14152397
APA StyleLiu, P., Jiao, J., Wu, C., Shao, W., Liu, X., & Yao, L. (2025). Biodiversity Patterns and Community Construction in Subtropical Forests Driven by Species Phylogenetic Environments. Plants, 14(15), 2397. https://doi.org/10.3390/plants14152397






