Physiological and Transcriptomic Mechanisms Underlying Vitamin C-Mediated Cold Stress Tolerance in Grafted Cucumber
Abstract
1. Introduction
2. Results
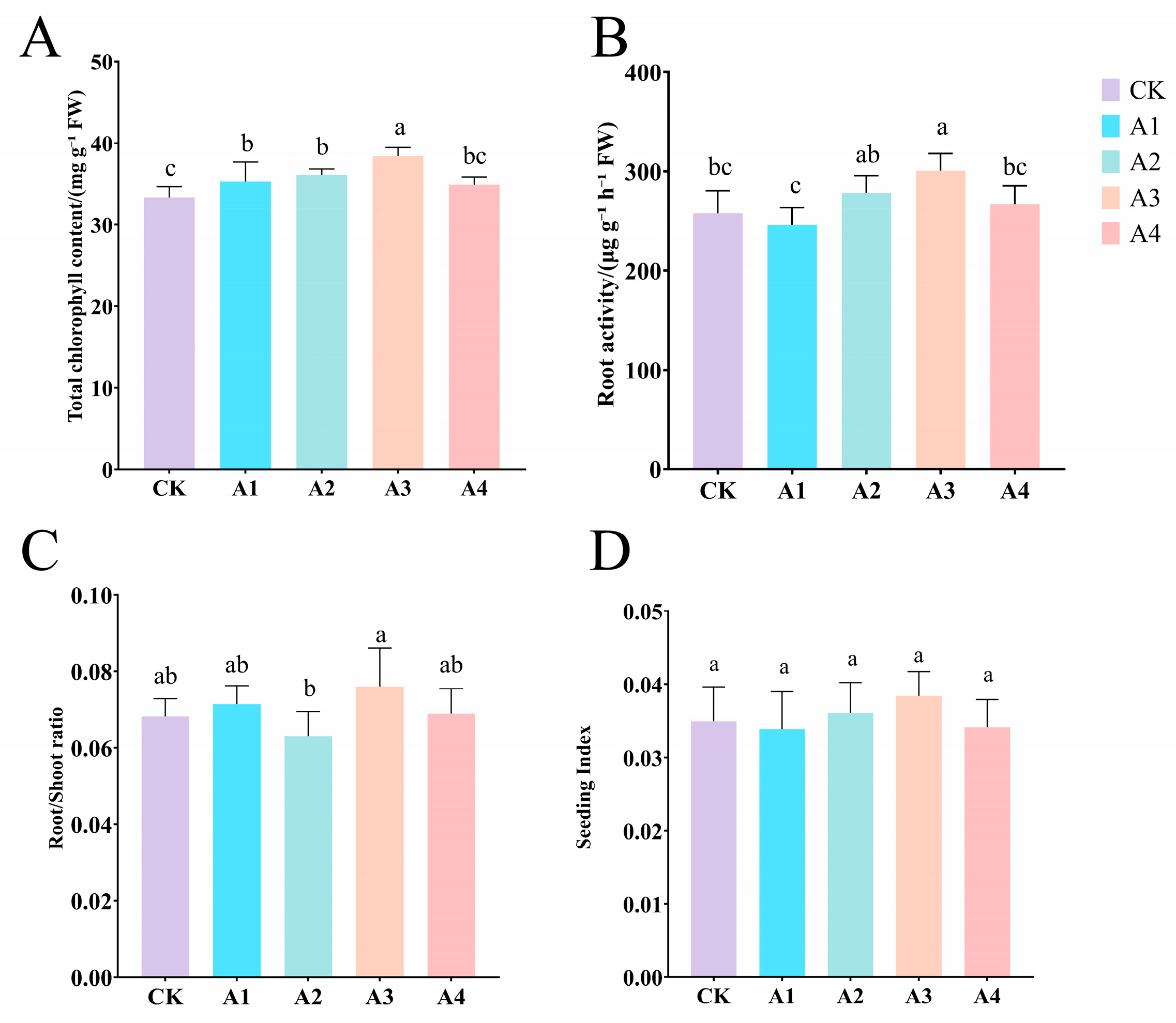
2.1. Impact of Vc on Morphological Characteristics of Grafted Cucumber Seedlings Under Low-Temperature Stress
2.2. Impact of Vc on Physiological Indicators of Grafted Cucumber Seedlings Under Low-Temperature Stress
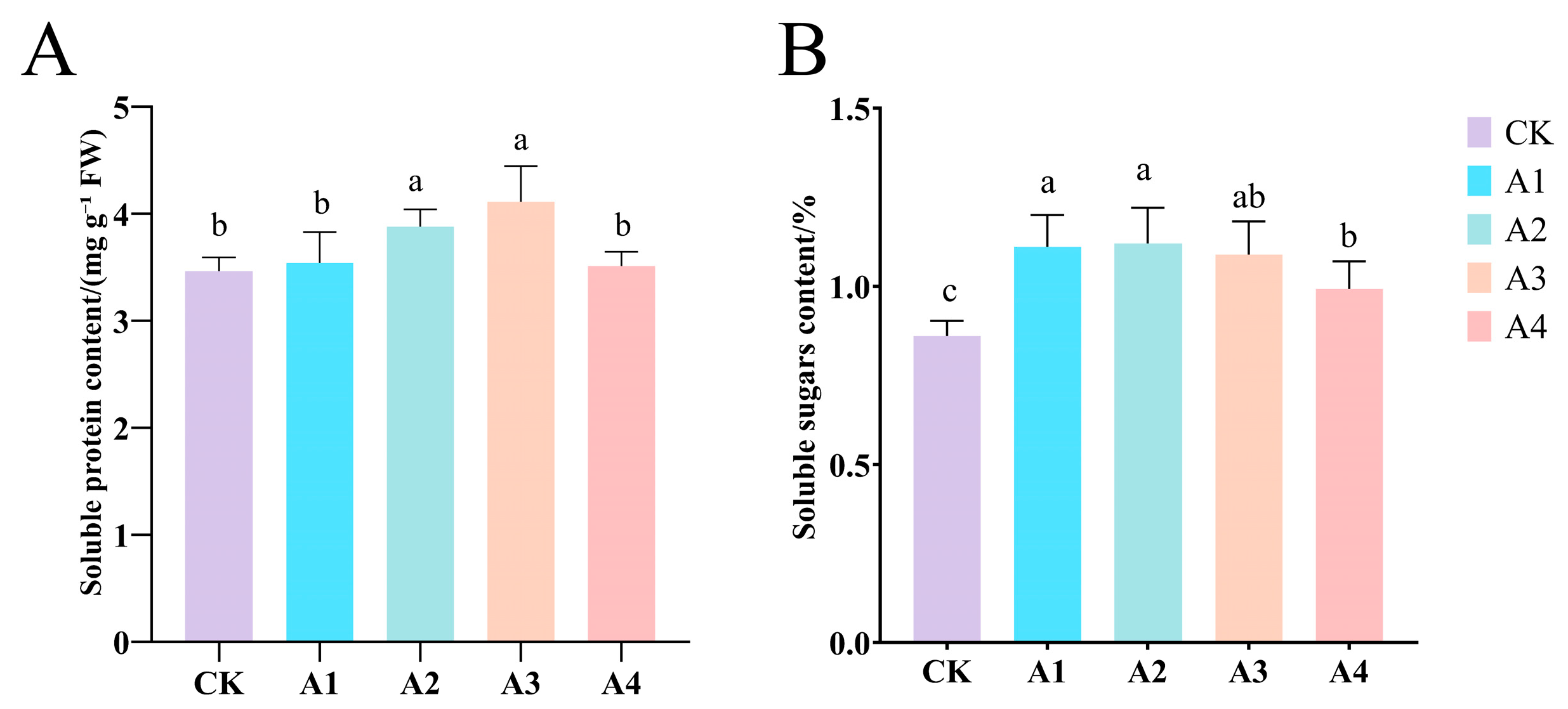
2.3. Impact of Vc on Permeating Substance Content of Grafted Cucumber Seedlings Under Low-Temperature Stress
2.4. Impact of Vc on the Content of Osmotic Regulatory Substances in Grafted Cucumber Seedlings Under Low-Temperature Stress
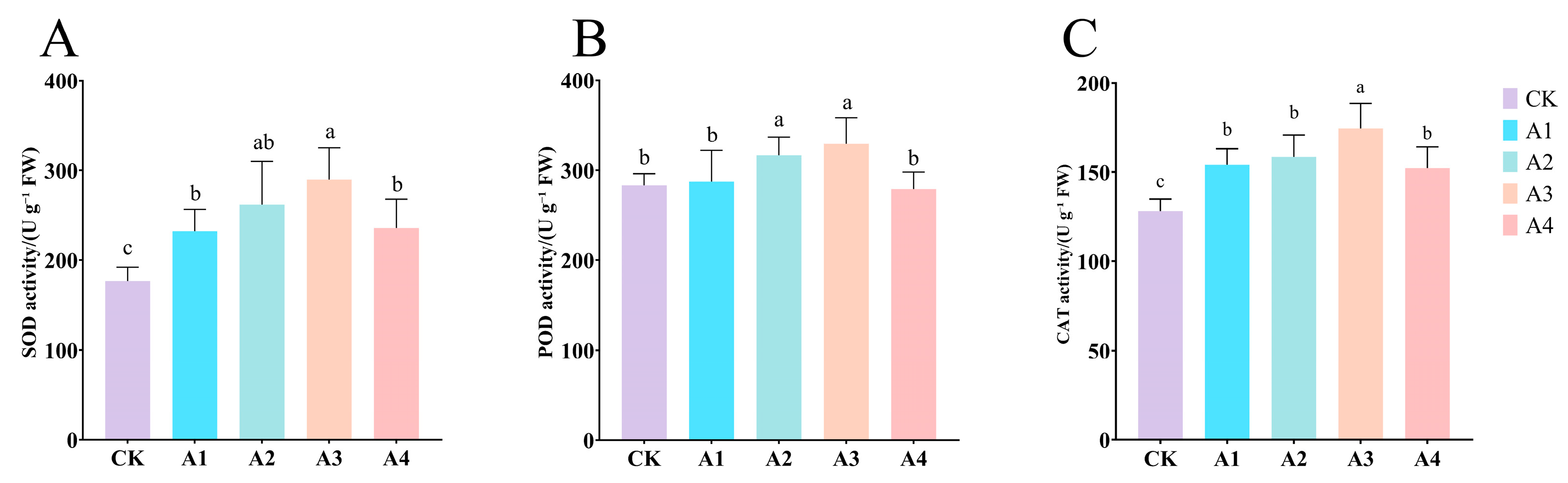
2.5. Impact of Vc on Antioxidant Enzyme Activities in Grafted Cucumber Seedlings Under Low-Temperature Stress
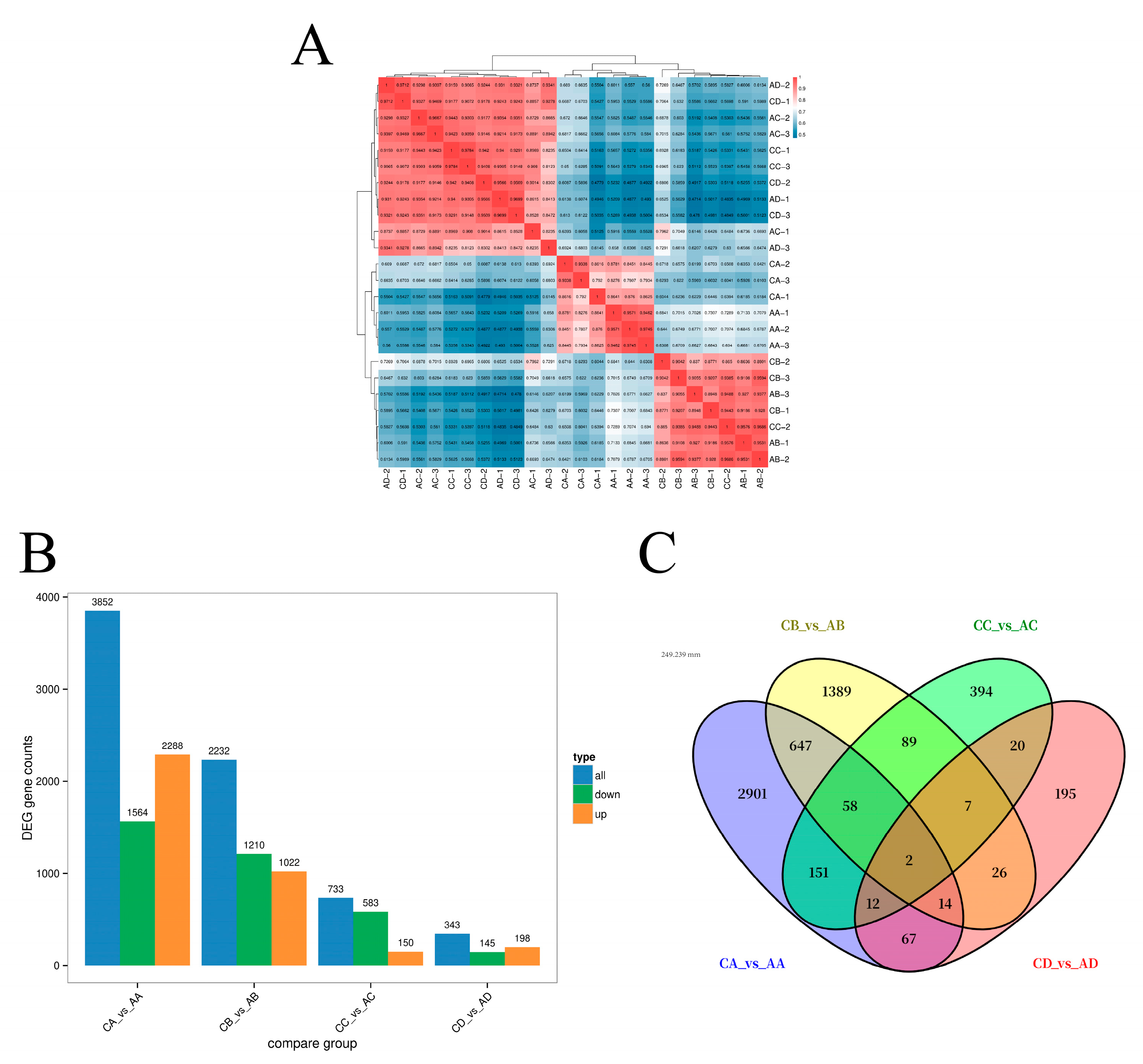
2.6. RNA Sequencing, Assembly, and Analysis of Differential Genes
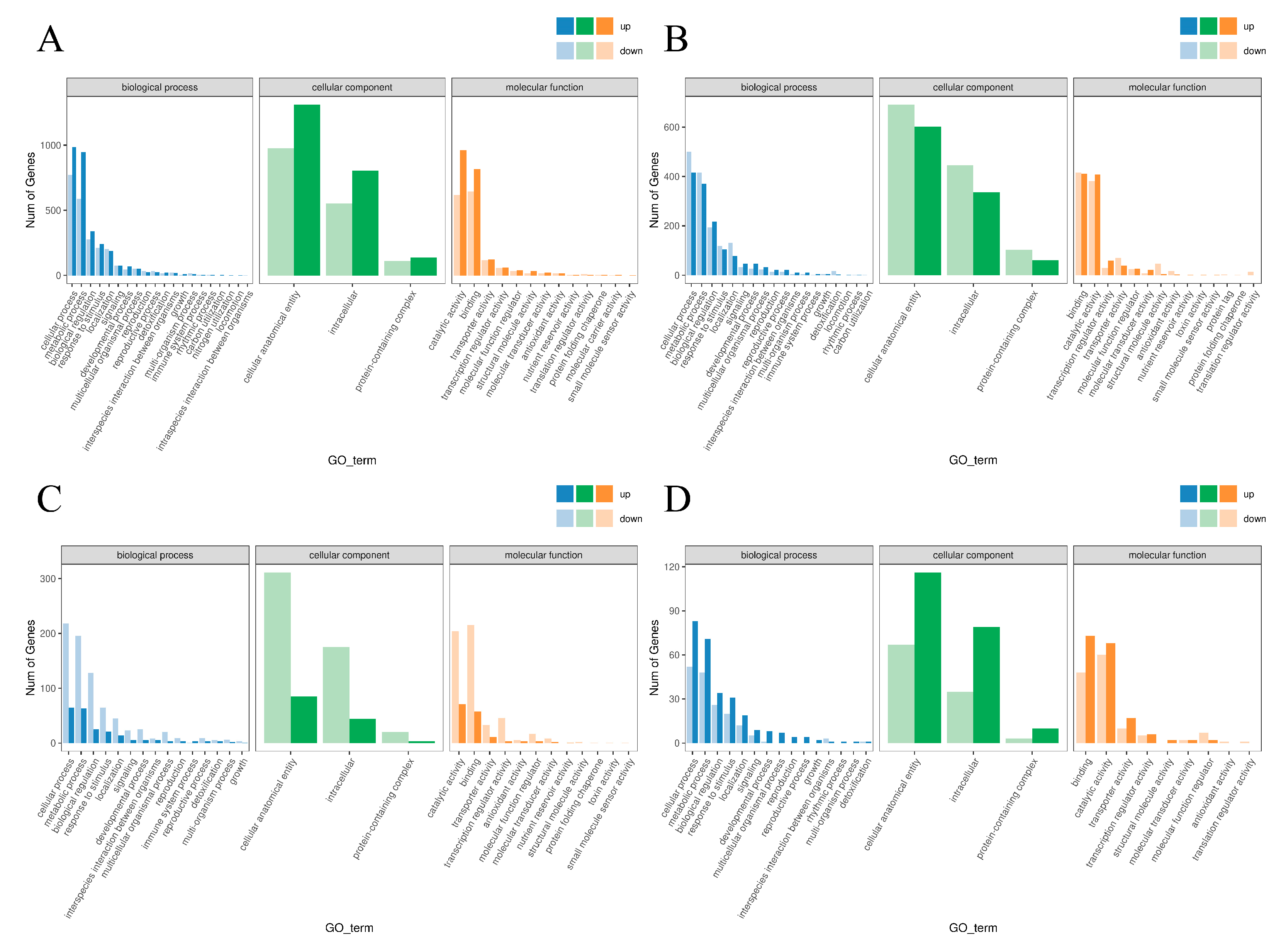
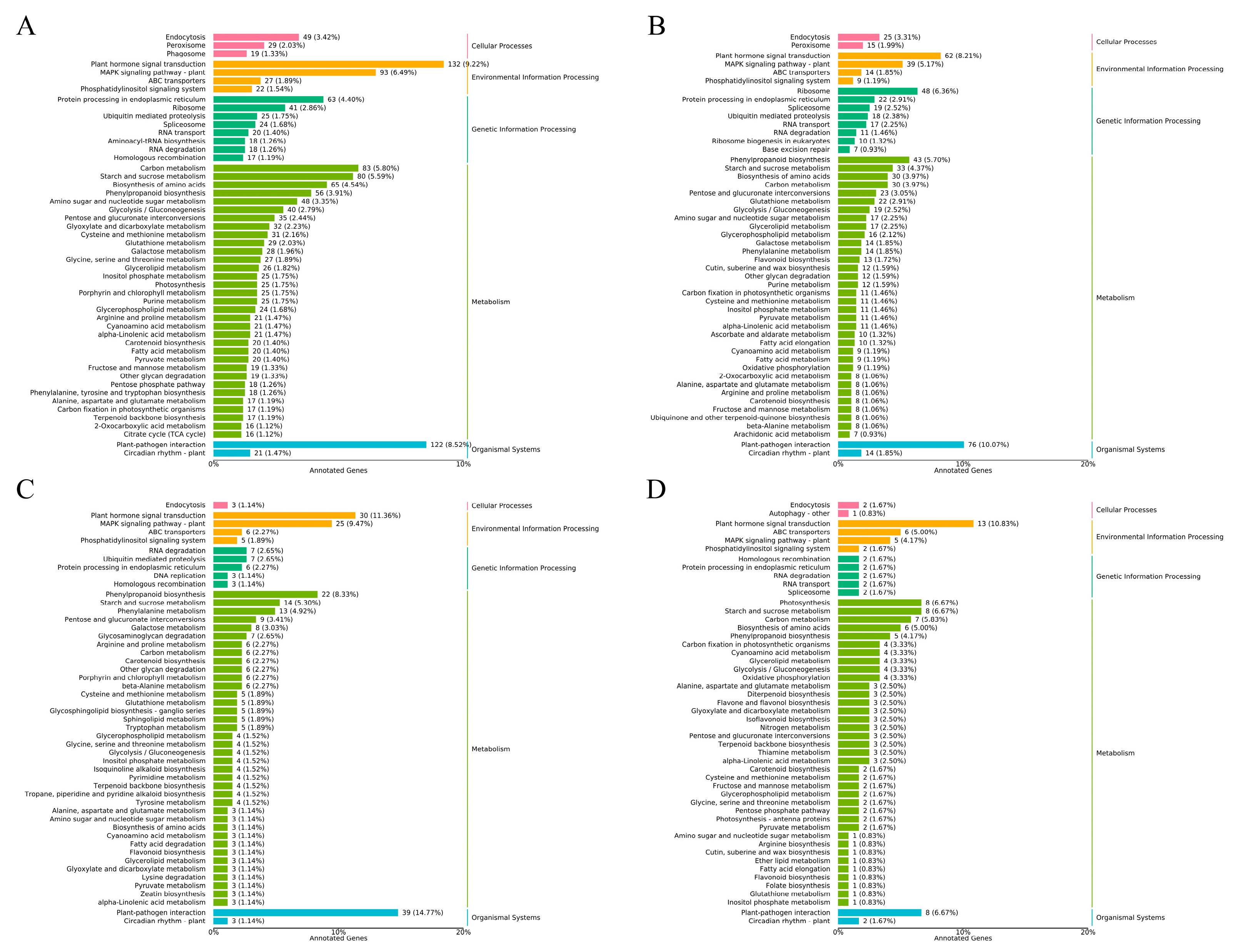
2.7. GO Functional Enrichment and KEGG Pathway Annotation Analysis of DEGs
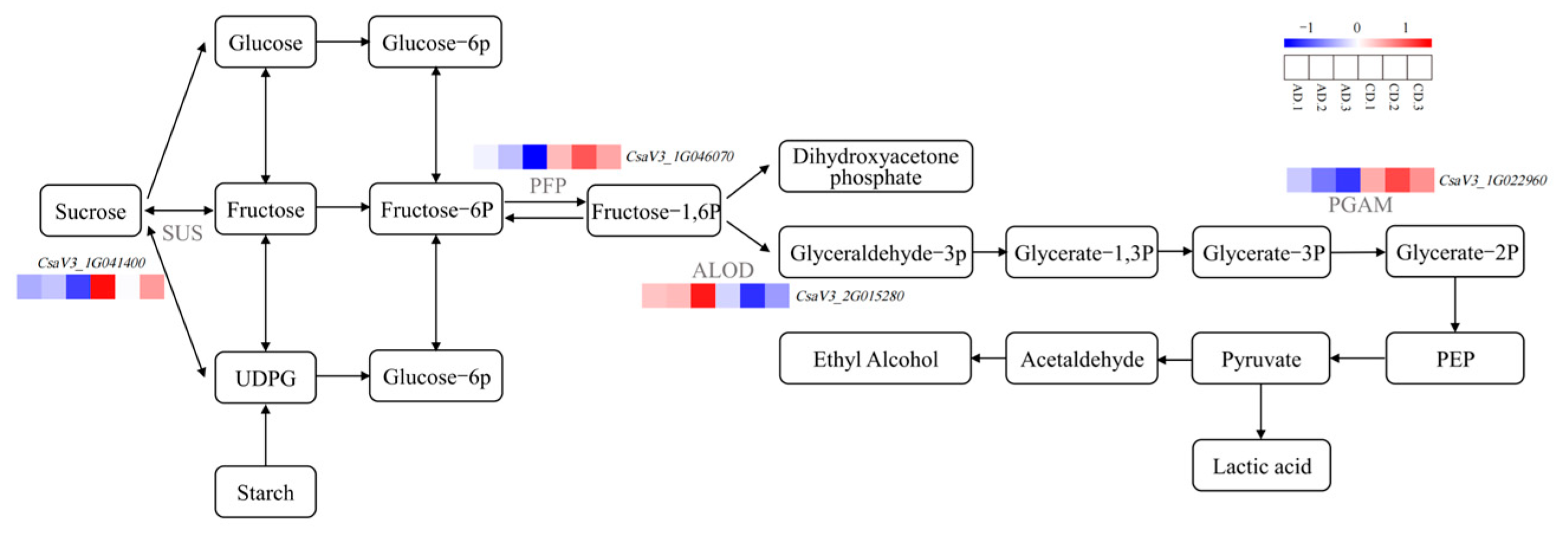
2.8. Key Genes in Signal Transduction Pathways Under Low-Temperature Stress
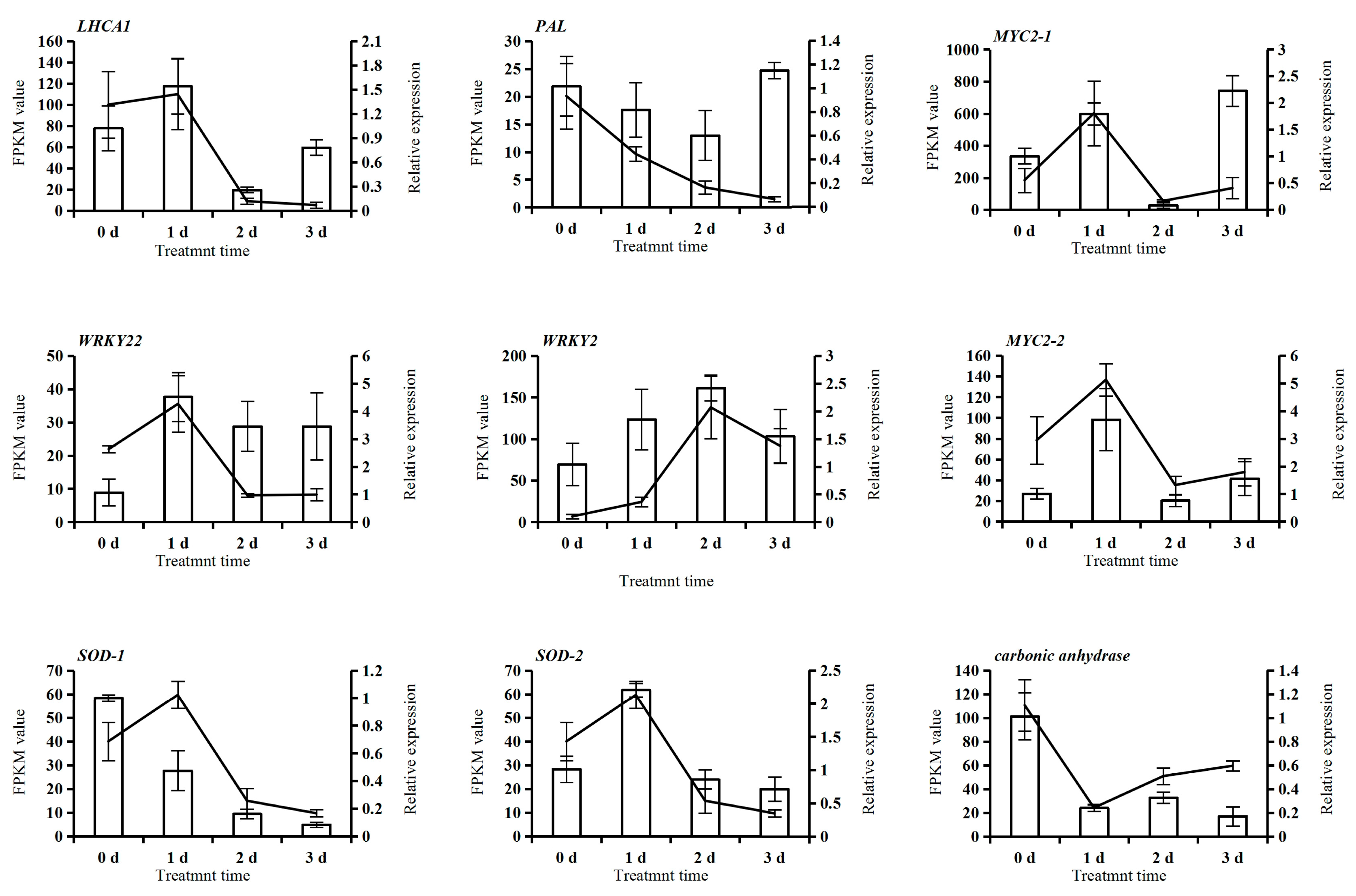
2.9. qRT–PCR Verification
3. Discussion
4. Materials and Methods
4.1. Experimental Site and Materials
4.2. Grafting and Treatment Application
4.3. Measurement of Morphological and Physiological Indicators
4.4. Transcriptome Sequencing and Differential Expression Gene Screening
4.5. qRT-PCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, X.; Lv, C.-Y.; Zhang, Y.-Y.; Ai, X.-Z.; Bi, H.-G. Comparative transcriptome analysis of grafting to improve chilling tolerance of cucumber. Protoplasma 2023, 260, 1349–1364. [Google Scholar] [CrossRef]
- Cheng, F.; Gao, M.; Lu, J.; Huang, Y.; Bie, Z. Spatial-Temporal Response of Reactive Oxygen Species and Salicylic Acid Suggest Their Interaction in Pumpkin Rootstock-Induced Chilling Tolerance in Watermelon Plants. Antioxidants 2021, 10, 2024. [Google Scholar] [CrossRef] [PubMed]
- Lo’aY, A.; El-Khateeb, A. Antioxidant enzyme activities and exogenous ascorbic acid treatment of ‘Williams’ banana during long-term cold storage stress. Sci. Hortic. 2018, 234, 210–219. [Google Scholar] [CrossRef]
- Ivanov, B.N. Role of ascorbic acid in photosynthesis. Biochem. Mosc. 2014, 79, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Elkelish, A.; Qari, S.H.; Mazrou, Y.S.A.; Abdelaal, K.A.A.; Hafez, Y.M.; Abu-Elsaoud, A.M.; Batiha, G.E.-S.; El-Esawi, M.A.; El Nahhas, N. Exogenous Ascorbic Acid Induced Chilling Tolerance in Tomato Plants Through Modulating Metabolism, Osmolytes, Antioxidants, and Transcriptional Regulation of Catalase and Heat Shock Proteins. Plants 2020, 9, 431. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Z.; Zhu, L.; Wang, J.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Wang, Y.; Zhang, Z. The Multiple Roles of Ascorbate in the Abiotic Stress Response of Plants: Antioxidant, Cofactor, and Regulator. Front. Plant Sci. 2021, 12, 598173. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, Y.; Lin, H.; Lin, M.; Fan, Z. Impacts of exogenous ROS scavenger ascorbic acid on the storability and quality attributes of fresh longan fruit. Food Chem. X 2021, 12, 100167. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, X.; Wang, W.; Pan, Y.; Huang, L.; Liu, X.; Zong, Y.; Zhu, L.; Yang, D.; Fu, B.; et al. Comparative Transcriptome Profiling of Chilling Stress Responsiveness in Two Contrasting Rice Genotypes. PLoS ONE 2018, 7, e43274. [Google Scholar] [CrossRef]
- Tang, K.; Zhao, L.; Ren, Y.; Yang, S.; Zhu, J.; Zhao, C. The Transcription Factor ICE1 Functions in Cold Stress Response by Binding to the Promoters of CBF and COR Genes. J. Integr. Plant Biol. 2020, 62, 258–263. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Sun, T.; Wang, X.; Zhu, C.; Ai, Y.; Gu, H. The Precise Regulation of Different COR Genes by Individual CBF Transcription Factors in Arabidopsis Thaliana. J. Integr. Plant Biol. 2017, 59, 118–133. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Yang, S. Molecular Regulation of CBF Signaling in Cold Acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef]
- Zhang, T.; Che, F.; Zhang, H.; Pan, Y.; Xu, M.; Ban, Q.; Han, Y.; Rao, J. Impact of Nitric Oxide Treatment on Chilling Injury, Antioxidant Enzymes and Expression of the CmCBF1 and CmCBF3 Genes in Cold-stored Hami melon (Cucumis melo L.) fruit. Postharvest Biol. Technol. 2017, 127, 88–98. [Google Scholar] [CrossRef]
- Yu, M.; Luobu, Z.; Zhuoga, D.; Wei, X.; Tang, Y. Advances in plant response to low-temperature stress. Plant Growth Regul. 2025, 105, 167–185. [Google Scholar] [CrossRef]
- Fu, S.; Chen, J.; Wu, X.; Gao, H.; Lü, G. Comprehensive Evaluation of Low Temperature and Salt Tolerance in Grafted and Rootstock Seedlings Combined with Yield and Quality of Grafted Tomato. Horticulturae 2022, 8, 595. [Google Scholar] [CrossRef]
- Long, H.; Li, Z.; Suo, H.; Ou, L.; Miao, W.; Deng, W. Study on the Mechanism of Grafting to Improve the Tolerance of Pepper to Low Temperature. Agronomy 2023, 13, 1347. [Google Scholar] [CrossRef]
- Charrier, G.; Willick, I.R.; Takahashi, D. Cross-disciplinary insights into the mechanisms of plant cold hardiness: From molecules to ecosystems. Physiol. Plant. 2023, 175, 2. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef]
- Niu, J.; Chen, Z.; Yu, S.; Wang, Q. Ascorbic acid regulates nitrogen, energy, and gas exchange metabolisms of alfalfa in response to high-nitrate stress. Environ. Sci. Pollut. Res. 2022, 29, 24085–24097. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qin, N.; Sun, L.; Yu, M.; Hu, W.; Qi, Z. Selenium Improves Physiological Parameters and Alleviates Oxidative Stress in Strawberry Seedlings under Low-Temperature Stress. Int. J. Mol. Sci. 2018, 19, 1913. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, F.; Huang, Y.; Bie, Z. Grafting watermelon onto pumpkin increases chilling tolerance by up regulating arginine decarboxylase to increase putrescine biosynthesis. Front. Plant Sci. 2022, 12, 812396. [Google Scholar] [CrossRef]
- Zheng, S.; Hao, Y.; Fan, S.; Cai, J.; Chen, W.; Li, X.; Zhu, X. Metabolomic and Transcriptomic Profiling Provide Novel Insights into Fruit Ripening and Ripening Disorder Caused by 1-MCP Treatments in Papaya. Int. J. Mol. Sci. 2021, 22, 916. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Wu, H.; Xie, X.; Zhao, B.; Chen, P.; Ao, D.; Pan, H.; Lin, B. Comparative Transcriptomic Analyses of Anthocyanin Biosynthesis Genes in Eggplant Under Low Temperature and Weak Light. Plants 2025, 14, 478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, X.; Feng, Y.; Zhang, X.; Bi, H.; Ai, X. Abscisic Acid Mediates Salicylic Acid Induced Chilling Tolerance of Grafted Cucumber by Activating H2O2 Biosynthesis and Accumulation. Int. J. Mol. Sci. 2022, 23, 16057. [Google Scholar] [CrossRef] [PubMed]
- Crepin, A.; Kučerová, Z.; Kosta, A.; Durand, E.; Caffarri, S. Isolation and characterization of a large photosystem I–light-harvesting complex II supercomplex with an additional Lhca1–a4 dimer in Arabidopsis. Plant J. 2020, 102, 398–409. [Google Scholar] [CrossRef]
- Wang, R.; Yu, M.; Xia, J.; Xing, J.; Fan, X.; Xu, Q.; Cang, J.; Zhang, D. Overexpression of TaMYC2 Confers Freeze Tolerance by ICE-CBF-COR Module in Arabidopsis Thaliana. Front. Plant Sci. 2022, 13, 1042889. [Google Scholar] [CrossRef]
- Han, C.; Li, J.; Ma, Y.; Guo, J.; Guo, X.; Xu, J.; Beres, B.L. PlWRKY70: A Paeonia Lactiflora Transcription Factor that Sensitively Responds to Low-temperature, Salt, and Waterlogging Stresses. Can. J. Plant Sci. 2019, 100, 146–155. [Google Scholar] [CrossRef]
- Rehman, S.; Rashid, A.; Manzoor, M.A.; Li, L.; Sun, W.; Riaz, M.W.; Li, D.; Zhuge, Q. Genome-Wide Evolution and Comparative Analysis of Superoxide Dismutase Gene Family in Cucurbitaceae and Expression Analysis of Lagenaria siceraria Under Multiple Abiotic Stresses. Front. Genet. 2022, 12, 784878. [Google Scholar] [CrossRef]
- Misle, E.; Kahlaoui, B.; Hachicha, M.; Alvarado, P. Leaf area estimation in muskmelon by allometry. Photosynthetica 2013, 51, 613–620. [Google Scholar] [CrossRef]
- Semeniuk, P.; Moline, H.; Abbott, J. A Comparison of the Impacts of ABA and an Antitranspirant on Chilling Injury of Coleus, Cucumbers, and Dieffenbachia. J. Am. Soc. Hortic. Sci. 2025, 111, 866–868. [Google Scholar] [CrossRef]
- Wei, C.; Ma, L.; Cheng, Y.; Guan, Y.; Guan, J. Exogenous ethylene alleviates chilling injury of ‘Huangguan’pear by enhancing the proline content and antioxidant activity. Sci. Hortic. 2019, 257, 108671. [Google Scholar] [CrossRef]
- Zhou, W.; Qi, Z.; Chen, J.; Tan, Z.; Wang, H.; Wang, C.; Yi, Z. Rooting ability of rice seedlings increases with higher soluble sugar content from exposure to light. PLoS ONE 2020, 15, e0241060. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, J.; Qin, Y.; Hu, G. Study on The Graft Compatibility Between ‘Jingganghongnuo’ and other Litchi Cultivars. Sci. Hortic. 2016, 199, 56–62. [Google Scholar] [CrossRef]
- Stewart, R.R.; Bewley, J.D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef]
- Maehly, A.C. Plant peroxidase. Methods Enzymol. 1955, 2, 801–813. [Google Scholar] [CrossRef]
- Li, H.X.; Xiao, Y.; Cao, L.L.; Yan, X.; Li, C.; Shi, H.Y.; Wang, J.W.; Ye, Y.H. Cerebroside C increases tolerance to chilling injury and alters lipid composition in wheat roots. PLoS ONE 2013, 8, e73380. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Diameter of Scion Stem (mm) | Height of Scion Plant (cm) | Leaf Area (cm2) | Fresh Weight (g) | Chilling Injury Index |
|---|---|---|---|---|---|
| CK | 3.51 ± 0.11 b | 3.42 ± 0.30 b | 43.22 ± 3.93 b | 3.168 ± 0.371 b | 0.580 ± 0.014 a |
| A1 | 3.54 ± 0.23 b | 3.77 ± 0.23 a | 48.86 ± 4.30 b | 3.414 ± 0.314 ab | 0.552 ± 0.022 ab |
| A2 | 3.49 ± 0.25 b | 3.70 ± 0.25 ab | 64.04 ± 6.31 a | 3.538 ± 0.553 ab | 0.532 ± 0.020 b |
| A3 | 3.84 ± 0.12 a | 3.78 ± 0.19 a | 58.09 ± 5.76 a | 3.787 ± 0.364 a | 0.518 ± 0.015 b |
| A4 | 3.44 ± 0.20 b | 3.52 ± 0.27 ab | 48.57 ± 6.28 b | 3.068 ± 0.483 b | 0.536 ± 0.020 b |
| Gene Name | Gene ID | Forward Primers (5′–3′) | Reverse Primers (5′–3′) |
|---|---|---|---|
| EF-1α | EF-1α | ACTGGTGGTTTTGAGGCTGGT | CTTGGAGTATTTGGGTGTGGT |
| PAL | CsaV3_6G014060 | TCGTCCTAATGCCAAGGCTG | ACCTCGGACAACAAAGCCAA |
| MYC2-1 | CsaV3_3G001710 | GCGATTTTCTGGCAGTCGTC | CGTAACCTCCTCATCCACCG |
| MYC2-2 | CsaV3_3G007980 | CAGAGTGAGGTCGTCAGCAG | TGGCACATGCTTCCCATGAT |
| WRKY22 | CsaV3_4G034570 | GGATATCAAGCCGTCGCAGA | CGGAGGTACCGAAATCGGAG |
| WRKY2 | CsaV3_3G026920 | GGAGGCGGTAAGTGGGTTTT | AGCTCCTCGGGAACTTGTTG |
| SOD-1 | CsaV3_4G013220 | TGCTCTTGGCGATACCACAA | ACGACTGCCCGACCAATTAC |
| SOD-2 | CsaV3_6G014400 | CGCTGTCCTCAAGGGAACTT | ATGTCGGATTTCGTCCTCGG |
| carbonic anhydrase | CsaV3_2G030320 | AGCAAACATTGTTCCGCCAT | AGCTGCTTTTGCATTCACCA |
| LHCA1 | CsaV3_5G025740 | CCATGTCCGCTGAGTGGATG | GGAACTGCTGCCCATTCTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Wang, J.; Zhang, X.; Weng, Z.; Huo, K.; Yi, Q.; Wu, C.; Kumar, S.; Gao, H.; Fu, L.; et al. Physiological and Transcriptomic Mechanisms Underlying Vitamin C-Mediated Cold Stress Tolerance in Grafted Cucumber. Plants 2025, 14, 2398. https://doi.org/10.3390/plants14152398
Yu P, Wang J, Zhang X, Weng Z, Huo K, Yi Q, Wu C, Kumar S, Gao H, Fu L, et al. Physiological and Transcriptomic Mechanisms Underlying Vitamin C-Mediated Cold Stress Tolerance in Grafted Cucumber. Plants. 2025; 14(15):2398. https://doi.org/10.3390/plants14152398
Chicago/Turabian StyleYu, Panpan, Junkai Wang, Xuyang Zhang, Zhenglong Weng, Kaisen Huo, Qiuxia Yi, Chenxi Wu, Sunjeet Kumar, Hao Gao, Lin Fu, and et al. 2025. "Physiological and Transcriptomic Mechanisms Underlying Vitamin C-Mediated Cold Stress Tolerance in Grafted Cucumber" Plants 14, no. 15: 2398. https://doi.org/10.3390/plants14152398
APA StyleYu, P., Wang, J., Zhang, X., Weng, Z., Huo, K., Yi, Q., Wu, C., Kumar, S., Gao, H., Fu, L., Chen, Y., & Zhu, G. (2025). Physiological and Transcriptomic Mechanisms Underlying Vitamin C-Mediated Cold Stress Tolerance in Grafted Cucumber. Plants, 14(15), 2398. https://doi.org/10.3390/plants14152398






