1. Introduction
Camelina [
Camelina sativa (L.) Crantz] is an oilseed crop plant belonging to the family Brassicaceae. The first traces of its cultivation date back nearly 4000 years [
1]. Camelina remained an important oilseed crop until the 1950s, when it was largely replaced with other oil-producing plants, including rapeseed (
Brassica napus L.) [
2]. In recent decades, however, we have been witnessing a renaissance in the use of camelina. Not only can camelina be used as an intercrop thanks to its short growth period, but it also has low water and nutrient requirements and is comparatively resilient against various adverse abiotic stresses as well as pathogens and pests. The high oil content of its seeds, along with a unique composition of fatty acids, renders this crop particularly useful for food and industrial applications [
3]. The essential linoleic and α-linolenic acids make up more than 50% of the total fatty acid content. Especially the high proportion of the polyunsaturated omega-3 α-linolenic acid (30–43%) is only surpassed in linseed oil [
4]. The oil content of camelina seeds has been reported to vary between 30 and 49% depending on genotype, environmental factors, and agronomic practice [
5]. For similar reasons, the yield of oil per hectare can range by almost one order of magnitude [
6]. Nowadays, the quality characteristics of camelina are subject to both conventional breeding and biotechnological approaches, and camelina has become one of the very first crop plants for which genome-edited lines have been tested in the field [
7].
The in vitro regeneration of plants is a basic prerequisite for the development and application of various biotechnological methods. Camelina plants have been regenerated via adventitious shoot formation from seedling-derived explants such as hypocotyls, cotyledon petioles, and true leaves [
8,
9]. Immature zygotic embryos dissected from developing seeds proved to be particularly useful for in vitro plant regeneration via adventitious shoot formation or somatic embryogenesis in a variety of plant species. In cereals, plant regeneration from immature zygotic embryos has been broadly used [
10]. In addition, this principle was also established in dicotyledons and, more specifically, in species where less satisfactory results had been obtained by other approaches. Examples are sunflower [
11], pepper [
12], soybean [
13], grapevine [
14], woody species [
15,
16], and some brassicas [
17,
18,
19].
As a facile alternative for genetic engineering, the tissue culture-independent principle of dipping the flowers into
Agrobacterium suspension has been applied in camelina [
20,
21]. However, as in some other crop species, genetic engineering of camelina has remained limited owing to low or inconsistent efficiency and high genotype dependence, irrespective of the employed methods of DNA transfer and plant regeneration.
The aim of this study was to establish a novel method of in vitro regeneration based on the formation of adventitious shoots on immature zygotic embryo explants, which has the potential to be applicable to a wide range of camelina accessions. The rationale behind this approach is that immature embryos are more likely to contain totipotent cells as compared with explants taken from mature embryos or plants. As a result, a robust method was established that rests on neoplastic development at the hypocotyl of cultivated immature zygotic embryos. The resulting protuberances further develop via the emergence of leafy structures into adventitious shoots and, after rooting, give rise to plants. This method was largely established in the experimental line Cam139 and then adopted in the current cultivar, Ligena. In addition, to gain a deeper understanding of the underlying developmental process, the adventitious formation of regenerative structures was histologically traced back to the initiation of locally enhanced cell proliferation in the outermost layers of the hypocotyl. Our results constitute a promising precondition for the establishment of robust methods of genetic engineering, including genome editing in camelina.
3. Discussion
The aim of this study was to tap the expectedly high totipotency of cells in zygotic immature camelina embryos for the establishment of a new, robust regeneration system. The method to be established should not only be applicable to particularly suitable experimental lines but also to elite breeding material. The in vitro cultivation of such embryos under the conditions established in this study led to local protuberances at the surface of the embryonic hypocotyl. From these tissue areas with intensified cell division, adventitious shoots were formed, some of which converted into normally developing plants.
In grasses, as compared with many dicotyledonous species, it is, if not impossible, at least more challenging to redirect the development of vegetative tissue to the formation of regenerative structures such as adventitious shoots or somatic embryos [
22]. Therefore, immature zygotic embryos constitute the standard explant for in vitro regeneration in these species. However, this principle has not yet been widely applied to dicotyledonous plant species, with one of the exceptions being Chinese cabbage, where the efficiency of regenerative structure formation using immature embryos was indeed higher than with other explants [
23]. The present investigation was the first to utilize this principle in the genus
Camelina.
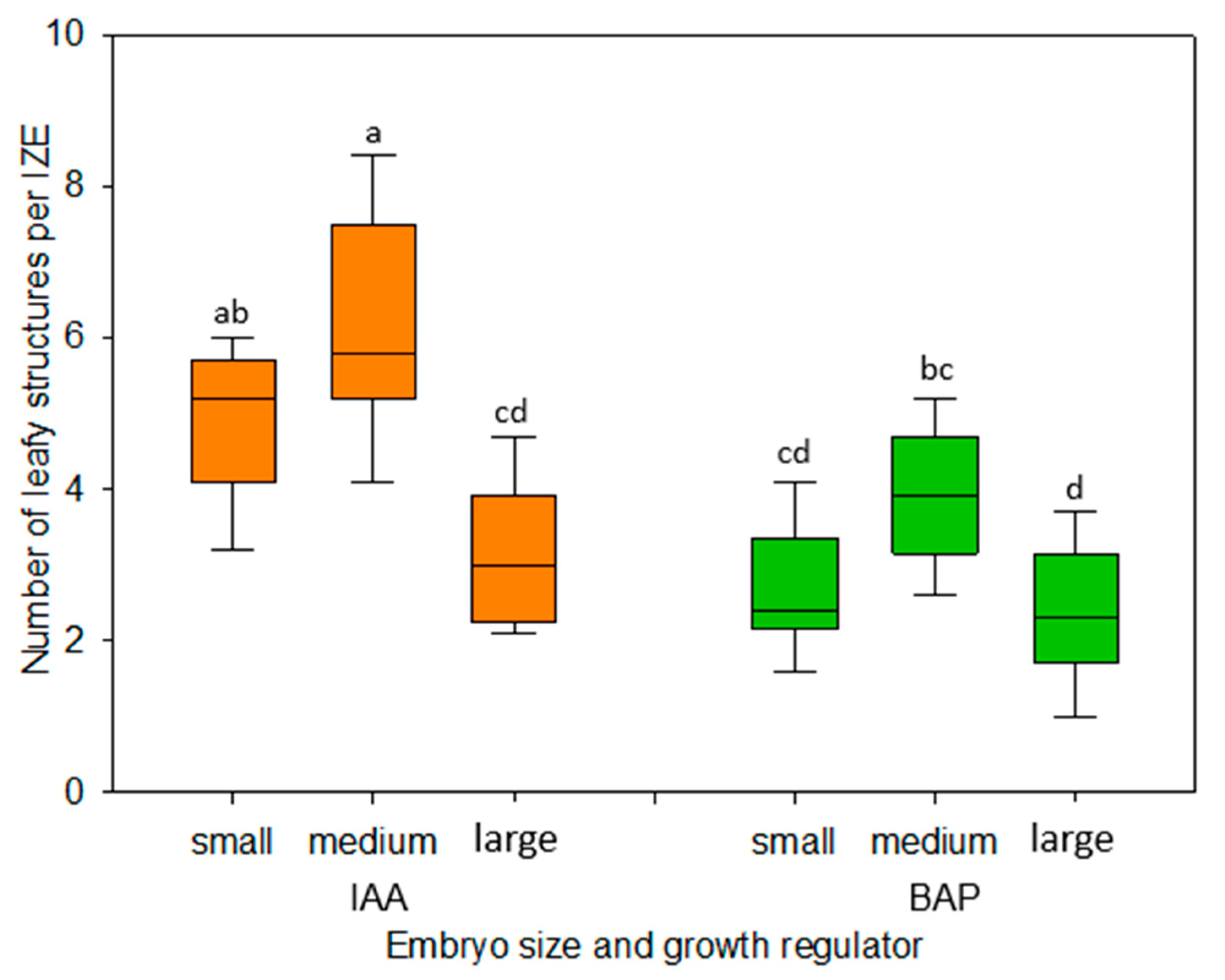
The dependence of the in vitro development of explants on their developmental stage is a well-known phenomenon. In the present study, immature embryos of the medium walking stick stage proved to be best suited for the formation of adventitious leafy structures (
Figure 4). While younger-stage embryos appeared to be only marginally more sensitive to the dissection procedure and their changing environment as compared with medium-size ones, embryos of the late walking stick stage exhibited a statistically significantly reduced formation of regenerative structures, especially when cultivated in the presence of IAA (
Figure 4). In this respect, our results are on par with earlier observations for cabbage, cauliflower, and kohlrabi [
18,
19] and support the hypothesis that, due to advanced cellular differentiation, embryos of the later developmental stage feature a decreasing totipotency of their cells.
Based upon histological preparations, the adventitious structures originated from the outer cell layers of the hypocotyl, i.e., epidermal cells, as well as subepidermal cells of the outer cortex. During the formation of the protuberances, the original order of the cell layers within the hypocotyl, largely based on anticlinal cell divisions, was abolished due to a strong increase in periclinal cell divisions. The simultaneous division of several juxtaposed cells and the broad base of the protuberances speak for a multicellular origin of regeneration, which may be relevant for the later employment of the established culture system for genetic engineering approaches. Similar cell division patterns were also observed at the beginning of the formation of regenerative structures in sunflower [
24] and Chinese tallow trees [
25], where an increasing proportion of periclinal cell divisions either contributed to internally unstructured protuberances or led to the formation of somatic embryos. By contrast, somatic embryos developed too rarely under the conditions described here to enable us to clarify their cellular origin with feasible effort using histological preparations.
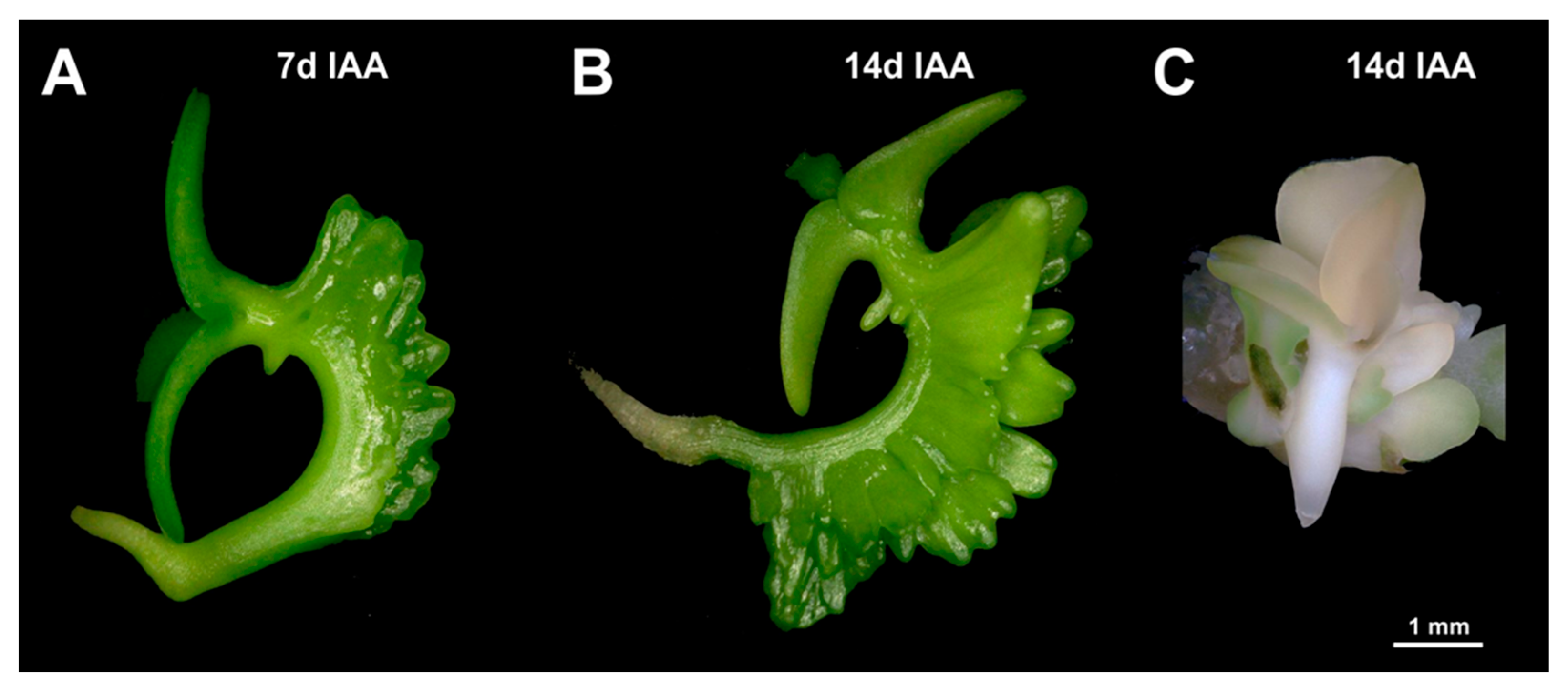
An interesting observation in the present study was that the preferential site of adventitious emergence of leafy structures was dependent on the exogenously supplied growth regulator. When the cytokinin analog BAP was administered as the sole growth regulator, the leafy structures emerged preferentially at the basal end of the hypocotyl, a phenomenon that was also observed in sunflower and Chinese tallow trees [
24,
25]. In the presence of the auxin IAA, however, the leafy structures were formed either at the upper end of the hypocotyl (in Cam139) or along its entire length (in cv. Ligena).
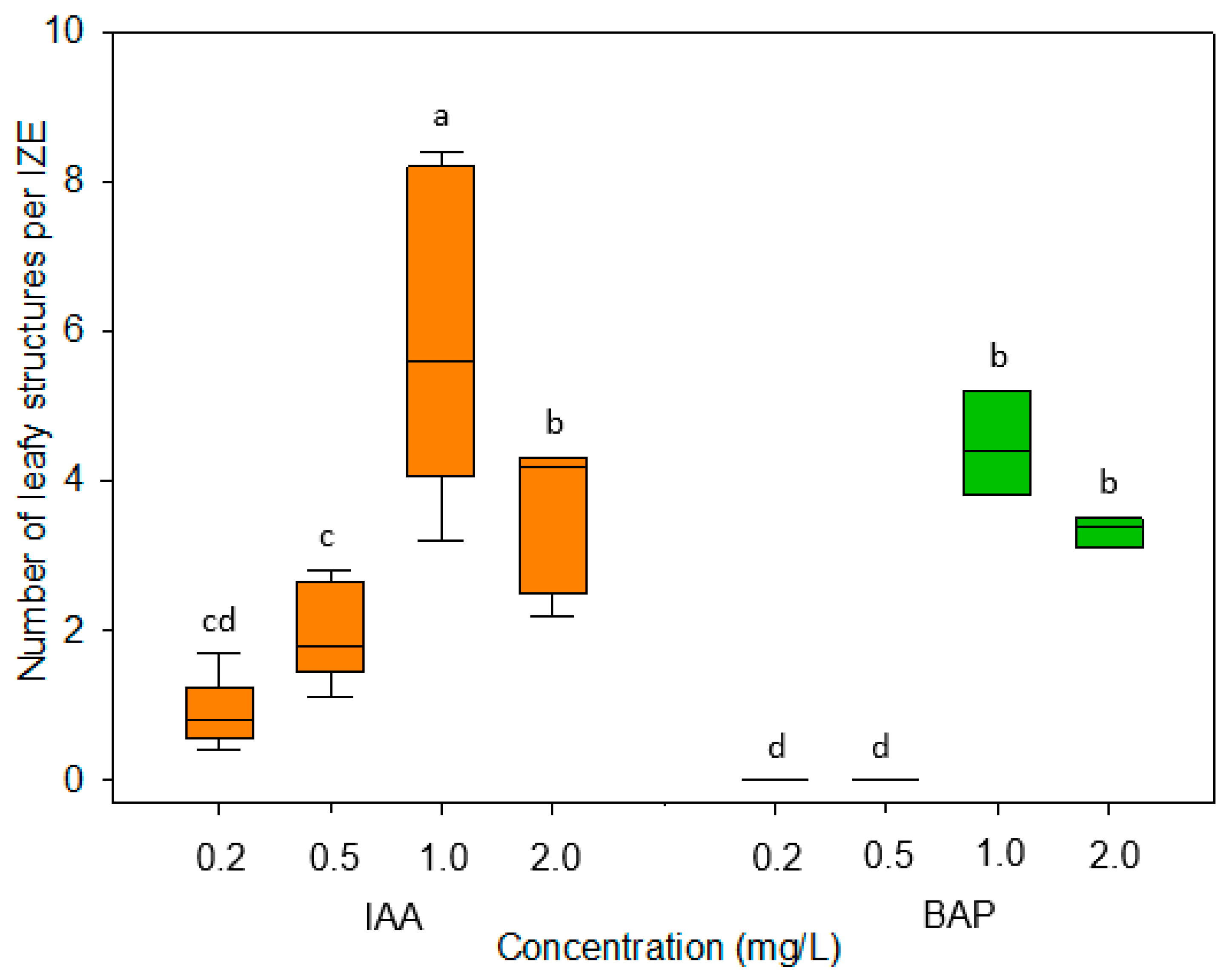
Cultivation of immature embryos on induction medium without growth regulators overall led to a strong cell expansion driven by vacuole enlargement in cortex and epidermis of the hypocotyl but had no obvious effect on cell division. The formation of adventitious structures was observed exclusively when the culture medium was supplemented with auxin- or cytokinin-type growth regulators. It was somewhat surprising that the auxin analogs NAA, 2,4-D, and Dicamba did not lead to a more intensive formation of adventitious structures compared to IAA (
Figure 3) that is known to be comparatively unstable in aqueous solutions. Further investigations showed that the formation of regenerative structures occurred most efficiently at a concentration of 1 mg/L of IAA or BAP (
Figure 2). In some previous studies, however, adventitious shoot formation on cultured immature embryos in the
Brassicaceae cabbage, kohlrabi, and cauliflower was most efficient without supplemented growth regulators [
18,
19]. Apparently, in these species, the dissection of the immature embryo from the seed and the altered conditions associated with the cultivation in vitro were sufficient to trigger the developmental changes.
In the literature, the use of phytohormones or their synthetic analogs to induce regenerative structures on immature embryos of dicotyledonous species ranges from IAA (
Datura innoxia [
26]), NAA (rapeseed [
27]), 2,4-D (Arabidopsis [
28], pepper [
12],
Brassica rapa [
23], sunflower [
11,
13]), BAP (sunflower [
24], neem [
15]) to various combinations of auxins and cytokinins. Adventitious shoots and somatic embryos have been obtained from the cotyledons of immature embryos of, e.g., rapeseed [
27] and Arabidopsis [
29] in addition to the hypocotyl of
Datura innoxia [
26], sunflower [
13], kohlrabi [
18], cabbage, and cauliflower [
19].
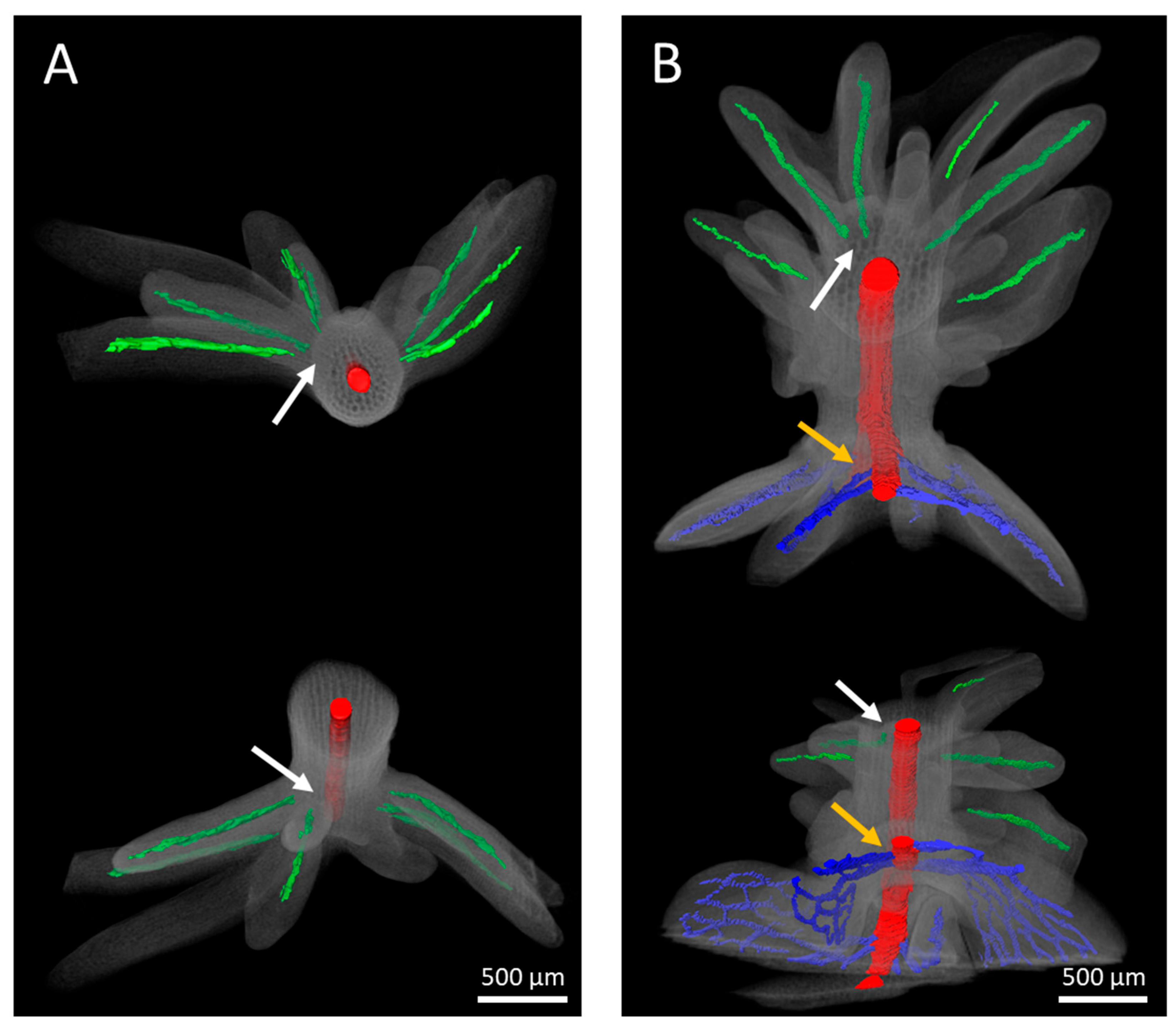
Serial section analysis of developing explants revealed that the vascular bundles of the adventitious leafy structures are largely straight, singular, and without branches. Irrespective of the use of either IAA or BAP for the induction of adventitious structures, the vasculature appears to originate within the protuberances, and definitively does not extend towards the main vasculature of the hypocotyl (
Figure 9). By contrast, the connection between the vasculatures of regenerative structures and explants was previously reported to be associated with adventitious shoot formation, for instance in
Hypericum perforatum and Arabidopsis [
29,
30]. Based on such observations, the prevailing view in the field has emerged that the vascular connection with the explant is a suitable distinguishing feature between adventitious shoot formation and somatic embryogenesis [
31]. However, the microscopic investigations presented in our study suggest that such a generalization is no longer justifiable. Irrespective of this new insight, the unipolar development of adventitious shoots and the bipolar development of the apical shoot meristem and apical root meristem of somatic embryos remain unambiguous differentiation criteria.
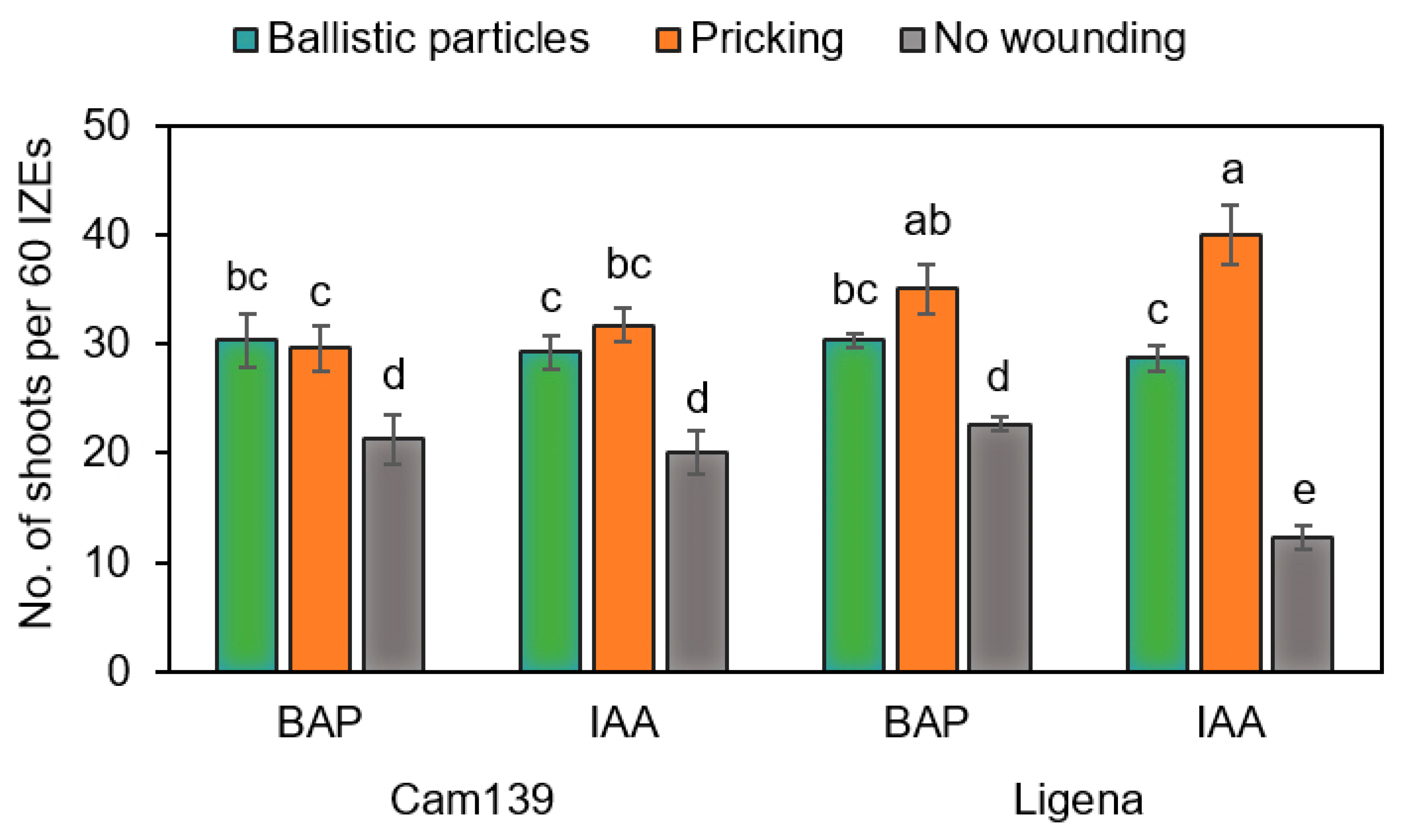
In this study, we tested the effect of mechanical wounding by ballistic particles or pricking with an injection needle on the formation of regenerative structures. Both approaches promoted the emergence of adventitious structures as compared with non-wounded hypocotyls grown under otherwise identical culture conditions (
Figure 6). The current hypothesis is that wounding causes the transfer of endogenous auxin to the wounded sites and hence an accumulation there [
32]. In this respect, results in line with ours were previously reported in, e.g., soybean and Chinese tallow trees [
13,
25].
The achievements of the present investigation are difficult to compare with previous studies in which immature embryos were used, since the overall efficiency of the established methods was rarely provided. In one of the exceptions to this, Kraut et al. [
17], working on Arabidopsis, reported an average of about one regenerated plant per immature embryo explant, which is comparable to our results. A comparison with previous studies on the in vitro culture of other sorts of camelina explants also proves to be difficult. Pollard et al. [
33] cultivated immature embryos of camelina cv. Sunesson to investigate the contents and fluxes of metabolites. However, the development of the cultured embryos was beyond the scope of their study. In contrast, Tattersall and Millam [
8] and Yemets et al. [
9], aiming to achieve multiple shoot formation from seedling explants, used segments from leaves of a Danish camelina accession and from hypocotyls or petioles of the Ukrainian camelina cultivars Peremozhets and Mirazh, respectively. In both studies, adventitious shoots developed from calluses that had initially formed. Unfortunately, only anecdotal information was provided in the two studies [
8,
9] on shoot formation and the conversion of the shoots into plants. In contrast to the previous reports on camelina, the regeneration of shoots and plants in the method developed by us usually takes place without an intermediate formation of callus, which can be of great advantage with regard to the genetic and epigenetic stability of the resulting plants. Also worth mentioning in this context is that comparatively high numbers of leafy structures can be obtained by the method established here, so that the conversion of these structures into meristem-bearing shoots and plants may be further improved.
While the dipping method is exclusively suitable for
Agrobacterium-mediated DNA transfer, the peripheral localization of the founder cells of plant regeneration in the method established here suggests that they may be well accessible for the ballistic transfer of transgene-coding plasmids or other reagents such as pre-produced ribonucleoprotein complexes (RNPs) of Cas-endonuclease and guide RNA. This opens additional opportunities, especially for genome editing, where the delivery of RNPs as well as DNA repair templates at high dosages is of great interest for homologous recombination-mediated precise editing [
34].
Taken together, a robust method of rapid plant regeneration was established using immature zygotic embryos of camelina, including the current cultivar Ligena, for which no regeneration or transformation method had previously been available. Under further consideration, the fact that the regenerated plants have their cellular origin in the outermost cell layers of the explants represents an extraordinarily favorable starting point for the intended application of this regeneration principle towards the development of methods of transgenesis and genome editing in this crop.
4. Materials and Methods
Experiments were performed using camelina accession Cam139 (provided by the Genebank of the Leibniz Institute of Plant Genetics and Crop Plant Research (IPK) Gatersleben, Gatersleben, Germany) and camelina cultivar Ligena (provided by Dr. Dieter Stelling from Deutsche Saatveredelung, Lippstadt-Bremen, Germany). Plants were grown in a greenhouse under natural daylight supplemented with 16 h illumination by sodium high-pressure lamps, providing an additional photon flux density of 300 to 500 μmol m
−2 s
−1 depending on the plants’ vertical distance and individual position. The day/night temperature regime was adjusted to 20/18 °C, and the relative humidity to 65%. To obtain zygotic embryos, silicles were harvested 12–14 days after pollination (
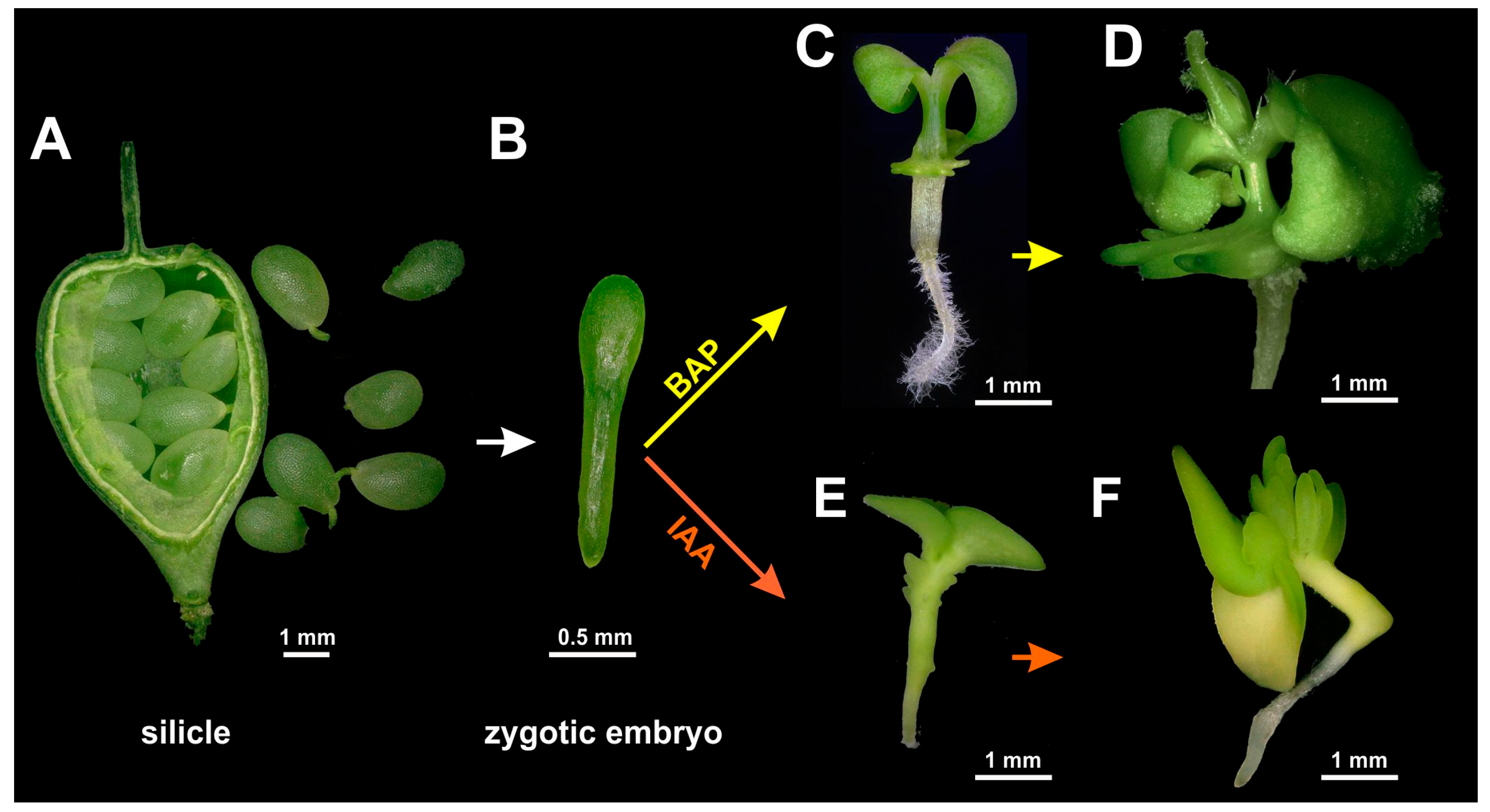
Figure 1) without distinguishing any different fruit size classes. After surface sterilization for 20 min with commercial bleach diluted to 4% NaOCl and supplemented with 0.1% Tween 20, silicles were rinsed three times with distilled water and stored at 4 °C overnight, which facilitates the dissection of the immature seeds. Isolation of immature zygotic embryos and all subsequent processing steps were performed under aseptic conditions in a laminar airflow workstation.
4.1. Culture of Immature Zygotic Embryo Explants
Zygotic embryos were dissected from immature seeds using a Zeiss Stemi 2000 binocular (Carl Zeiss Microscopy GmbH, Jena, Germany), with the embryos being sorted according to their individual length (for embryo size classes, see next paragraph) and cultivated on various culture media to establish conditions for the formation of adventitious regenerative structures. Per 90-mm plastic plate, 25 mL of induction medium was used, which contained, if not stated otherwise, ammonium-free MS basal minerals (Duchefa Biochemie, Haarlem, The Netherlands), 5 mM NH4NO3, B5 vitamins (Sigma-Aldrich, St. Louis, MO, USA), 3% (w/v) sucrose, and 0.4% Phytagel (w/v) (Sigma-Aldrich) for solidification, with the pH being adjusted to 5.7 with KOH. Growth regulators were added, as specified below. A 2× stock solution of induction medium without Phytagel was filter-sterilized (nonpyrogenic, sterile polystyrene filter, Corning, New York, NY, USA), while a 2× stock solution of Phytagel was autoclaved at 120 °C for 20 min. After warming the former and cooling the latter to about 50 °C, the two solutions were mixed 1:1 and distributed to the culture plates for solidification.
The plant growth regulators Dicamba, α-naphthaleneacetic acid (NAA), indole-3-acetic acid (IAA), 2,4-dichlorophenoxyacetic acid (2,4-D), or 6-benzylaminopurine (BAP) (all from Duchefa Biochemie Co., Haarlem, The Netherlands) were added to the medium as filter-sterilized, 1 mg/L stock solutions. After testing IAA and BAP at concentrations of 0.2, 0.5, 1.0, and 2.0 mg/L, final experiments were carried out using either IAA or BAP at a concentration of 1 mg/L. The developmental stages of regular embryonic development were specified according to Feeney et al. [
35]. To identify the most suitable developmental stage for adventitious shoot formation, early (>0.7–1.2 mm in length), medium (>1.2–1.7 mm), and late walking stick (>1.7–2.1 mm) zygotic embryos were tested. Ten dissected embryos were cultivated in a 9-cm culture dish. Cultures were kept overnight in the dark at 24 °C before being incubated at 25 °C with a 16 h-photoperiod using white fluorescent lamps with a 60 µmol m
−2 s
−1 photon flux density. After 2 weeks of culture, the number of leafy structures formed per immature zygotic embryo was counted.
4.2. Adventitious Shoot Formation and Plant Regeneration
After two weeks of cultivation, the cotyledon and root parts were removed, and the remaining hypocotyls carrying the emerging leafy structures were placed horizontally onto the shooting medium. This medium had the same composition as the induction medium in the case of explants cultivated in the presence of BAP, whereas for explants from the IAA-containing induction medium, sucrose was reduced to 2% (w/v) and IAA to 0.1 mg/L.
After two weeks on shooting medium, resultant shoots were transferred onto rooting medium consisting of half-strength ammonium-free MS minerals (Duchefa Biochemie, Haarlem, The Netherlands), 2.5 mM NH4NO3, 1% (w/v) sucrose, and 0.1 mg/L IAA. After another four weeks of cultivation, rooted shoots were counted and transferred to pots with soil substrate to grow them under the conditions used for the donor plants. Images of explants and their development in vitro were documented using a KEYENCE VHX-5000 microscope (Keyence Germany GmbH, Neu-Isenburg, Germany).
4.3. Mechanical Wounding of Hypocotyls
To examine whether the formation of adventitious shoots can be enhanced by mechanical wounding, medium walking stick stage embryos were first cultivated for 72 h on induction medium containing either 1 mg/L BAP or IAA. In this experiment, 20 explants were used per culture dish. The hypocotyls were then carefully pricked at 3 to 5 positions with an injection needle (0.3 mm × 25 mm, BD PrecisionGlideTM, Fort Pierce, FL, USA), followed by cultivation under the same conditions for another two weeks. For comparison, a second series of immature zygotic embryos were wounded by accelerated particles using a PDS-1000/He device equipped with a hepta-adapter (Bio-Rad Laboratories, Hercules, CA, USA). For this, 30 mg of gold particles (ca. 0.6 μm in diameter; Bio-Rad, order no. 1652262) were washed twice with 200 µL of H2O in a 0.5-mL microcentrifuge tube by briefly vortexing. After the supernatant was removed, the particles were resuspended in 60 µL of 100% ethanol. Each macro-carrier (Bio-Rad, order no. 1652335) was loaded with 5 µL particle suspension. Prior to the experiment, immature zygotic embryos were transferred from the induction medium to the osmotic medium (ammonium-free MS minerals, B5 vitamins (Sigma-Aldrich), 0.35 M mannitol, and 0.4% Phytagel) and incubated for four to six hours at 24 °C in the dark before use. The particle gun was employed with a vacuum of 27 inches and 1100-psi rupture discs (Bio-Rad, order no. 1652329). Per shot, 20 immature zygotic embryos were used. After this ballistic treatment, the embryos remained on the osmotic medium overnight in the dark at 24 °C. The next day, the explants were transferred back to induction medium and incubated in the dark at 24 °C for another two weeks. Wounded embryos were compared with non-wounded, immature embryos serving as controls.
4.4. Statstical Analyses
Using the culture dishes as experimental repetitions, the experimental datasets were tested for normal (Gaussian) distribution and equal variance. Provided these tests were passed, an analysis of variance (ANOVA) was conducted, followed by all pairwise multiple comparisons of the treatments using the Tukey test. By contrast, datasets that did not pass both the normal distribution and equal variance tests were subjected to the non-parametric Kruskal-Wallis analysis of variance on ranks, followed by all pairwise multiple comparisons of the treatments using the Student-Newman-Keuls method. All statistical analyses were performed using the SigmaPlot 14.0 software package (Inpixon GmbH, Düsseldorf, Germany).
4.5. Microscopic Analyses
For histological analysis, medium-size walking stick zygotic embryos were used either freshly prepared or after 3 days of culture on BAP- or 4 days on IAA-containing media. The longer cultivation in the presence of IAA was used to compensate for the apparently faster development with BAP. For comparison, embryos were cultivated for four days on growth regulator-free medium.
After removal of the cotyledons’ node and the root, hypocotyl segments were fixed with 2.0% (v/v) glutaraldehyde and 2.0% (w/v) formaldehyde in 50 mM cacodylate buffer (pH 7.2) for 2 h at room temperature. After three rinses with buffer and one rinse with aqua dest., samples were post-fixed with 1% (w/v) aqueous OsO4 for 30 min. After another three washing steps with aqua dest., samples were dehydrated in an ascending ethanol series of 30% to 100%. After an additional incubation with 100% propylene oxide followed by a stepwise infiltration with Spurr’s resin (Plano GmbH, Marburg, Germany), fully infiltrated samples were transferred into embedding forms (BEEM capsules, Plano GmbH, Wetzlar, Germany) and polymerized overnight in a heating cabinet at 70 °C. Resin-embedded samples were trimmed with a Leica EM TRIM2 (Leica Microsystems, Wetzlar, Germany) device. Sections of 2-µm thickness were cut using a Leica Ultracut Ultramicrotome (Leica Microsystems, Wetzlar, Germany) and stained with 1% (w/v) methylene blue/1% (w/v) Azur II in 1% (w/v) aqueous borax. Stained sections were examined in a Zeiss Axio Imager 2 light microscope (Carl Zeiss GmbH, Jena, Germany).
For 3-D reconstruction of explants with adventitious leafy structures, samples were serially sliced to generate 2-µm-thick sections, of which those positioned at 10-µm intervals were collected. Sections were transferred onto 20-µL droplets of 1:400 diluted Crystal Violet (2% stock) on poly-l-lysine-coated slides and placed on a heating plate set at 90 °C to simultaneously stain and bake the sections. Recordings were made with a Zeiss Axioscan 7 slide reader (Carl Zeiss, Oberkochen, Germany) using a 10× NA 0.45 objective. Serial recordings were stacked and aligned using the open-source Fiji image processing software (version 1.54c, National Institutes of Health and Laboratory for Optical and Computational Instrumentation, University of Wisconsin, USA). For subsequent segmentation and 3-D reconstruction, Amira software (version 5.6, Thermo Fischer, Karlsruhe, Germany) was used.
Ultrastructure analyses by transmission electron microscopy of resin-embedded samples were performed as described previously [
36].