Elicitation Induced α-Amyrin Synthesis in Tylophora indica In Vitro Cultures and Comparative Phytochemical Analyses of In Vivo and Micropropagated Plants
Abstract
:1. Introduction
2. Results
2.1. Callus Induction and Organogenesis
2.2. Rooting and Acclimatization
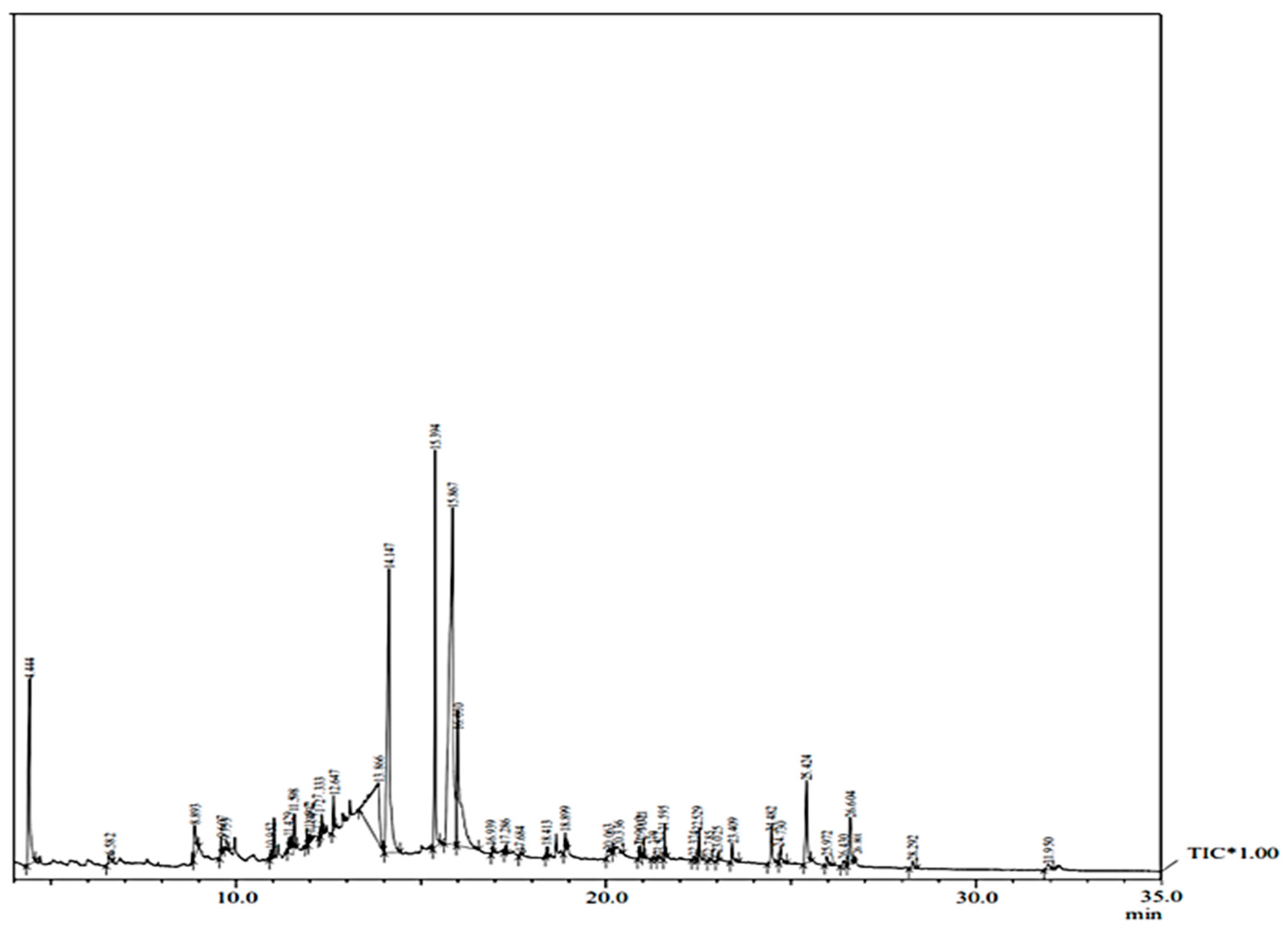
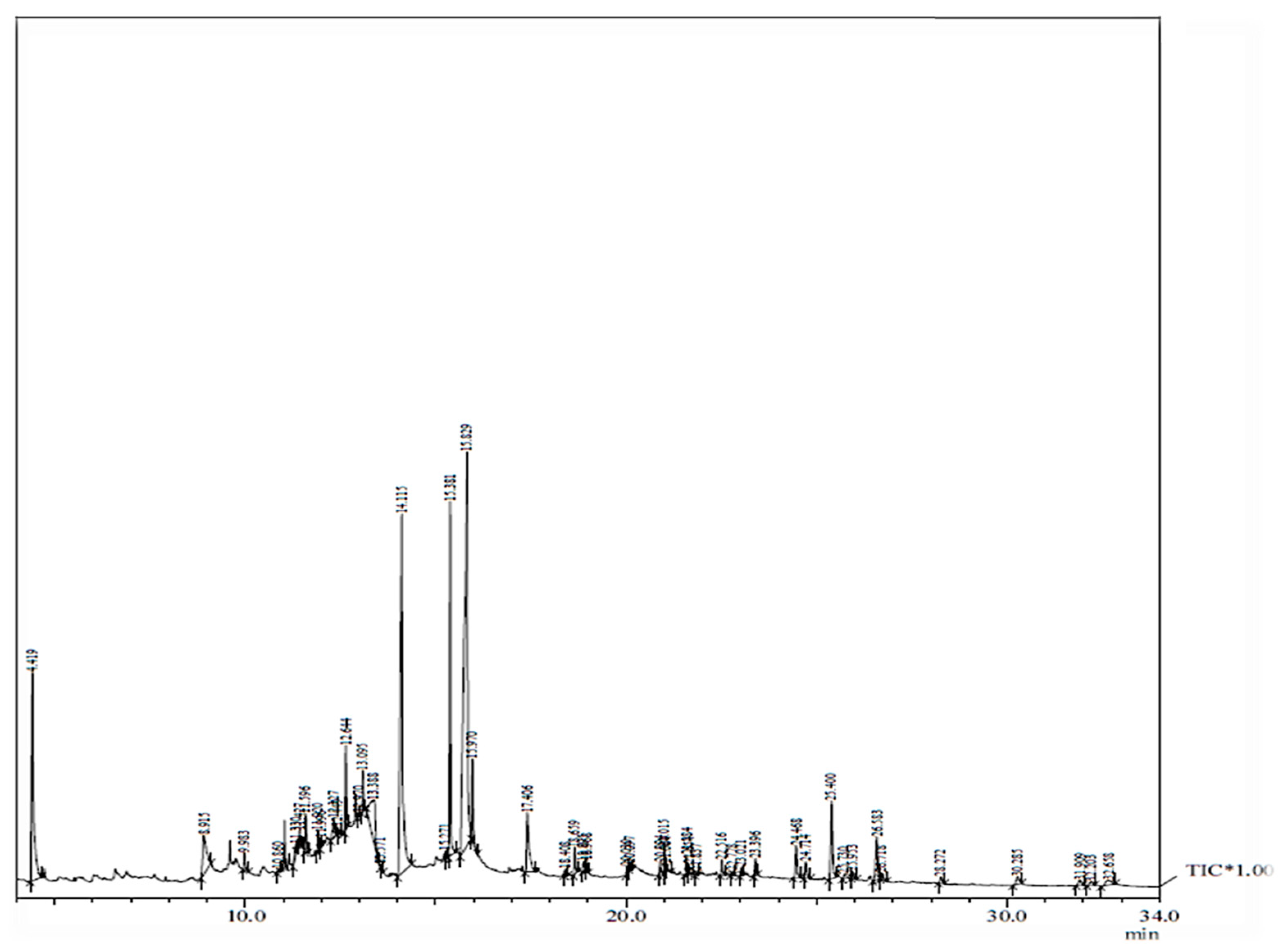
2.3. GC–MS Analysis
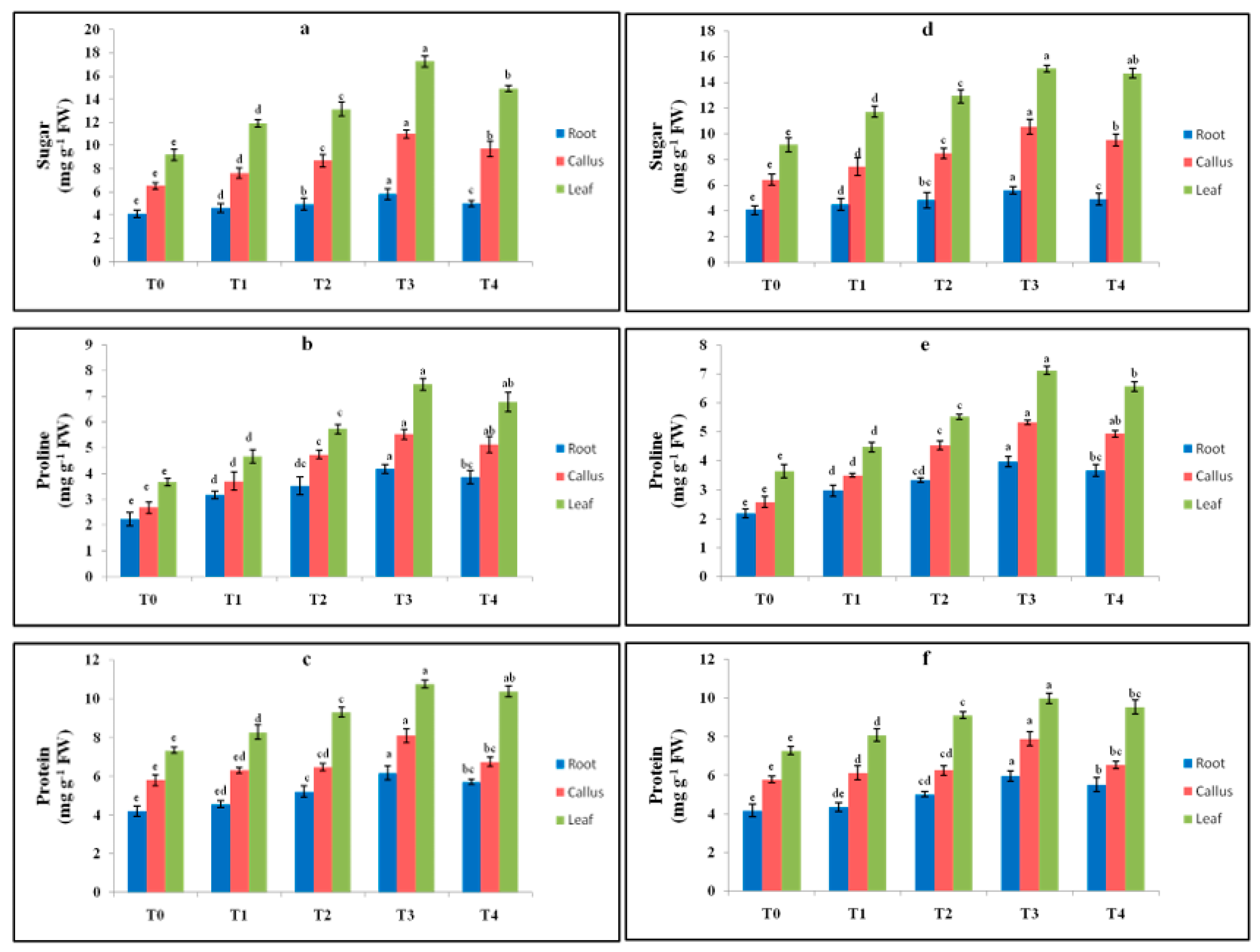
2.4. Effect of Elicitor Treatments on Sugar, Proline and Protein Contents in In Vitro Cultures
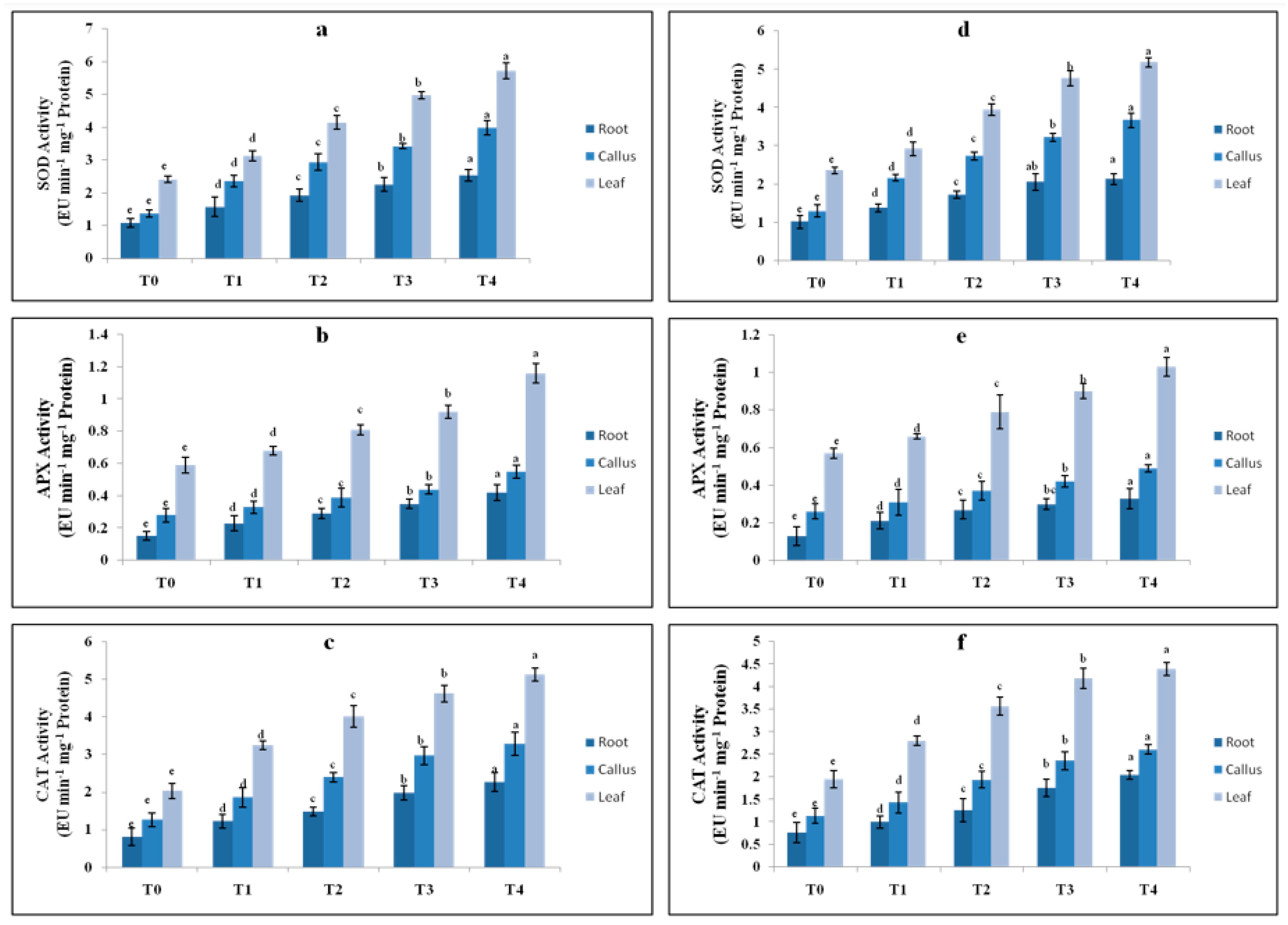
2.5. Effect of Elicitor Treatments on Antioxidant Enzyme Activity
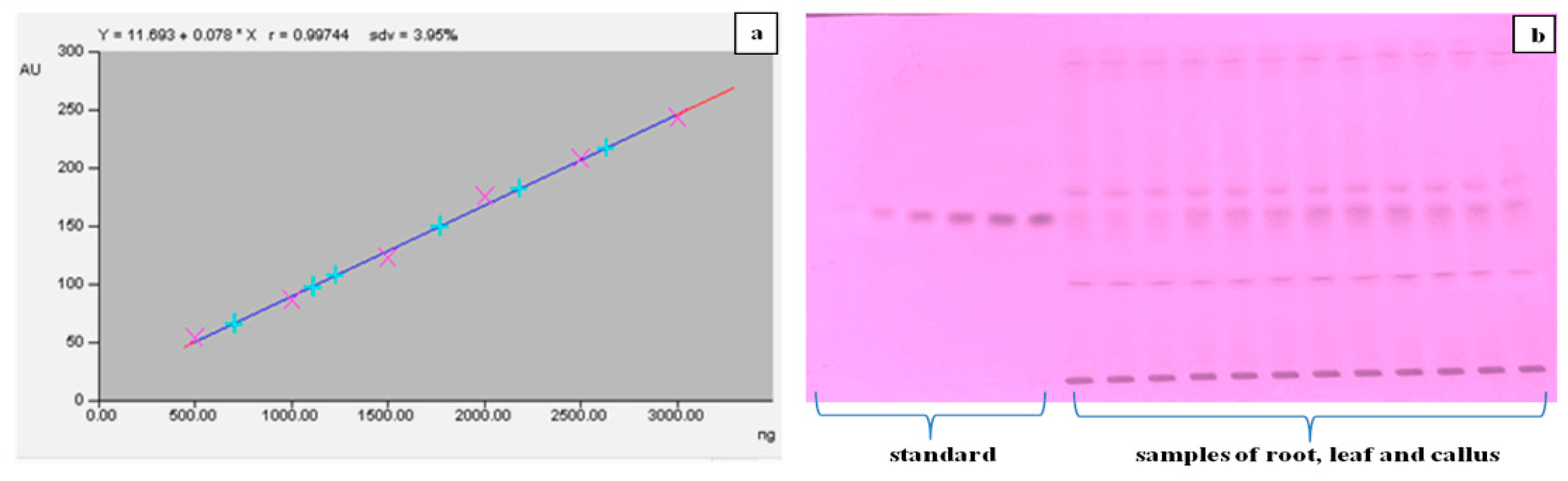
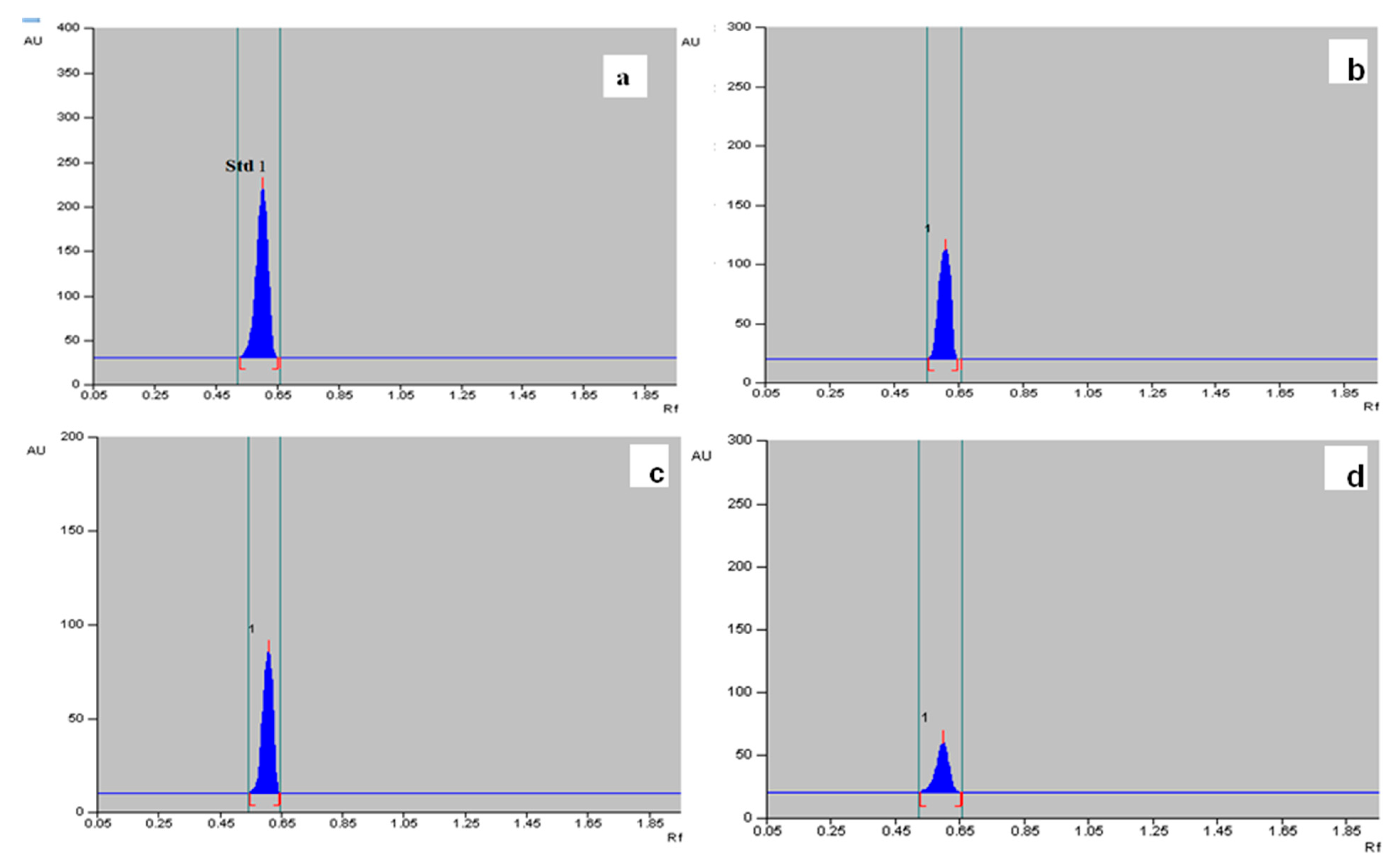
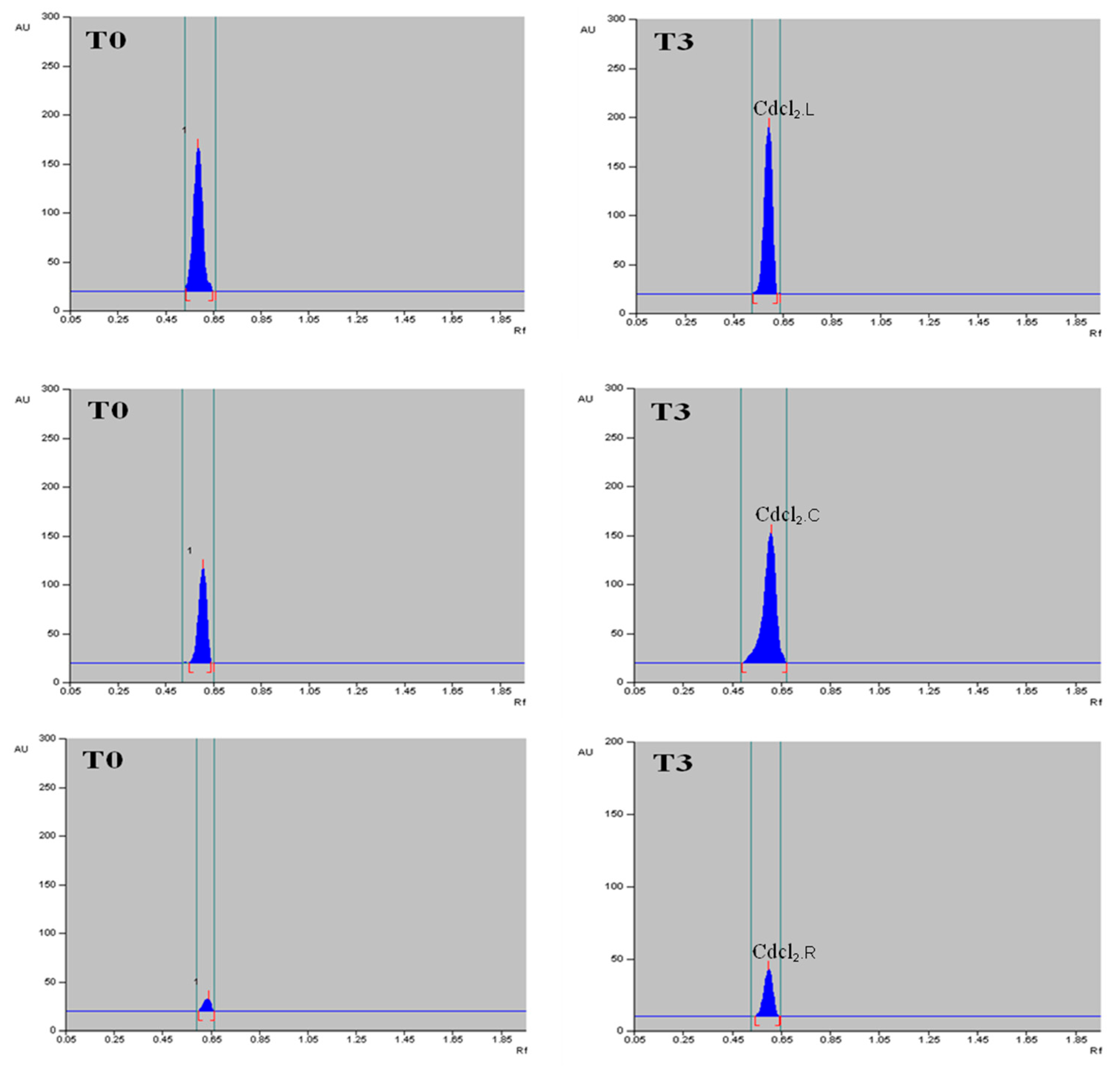
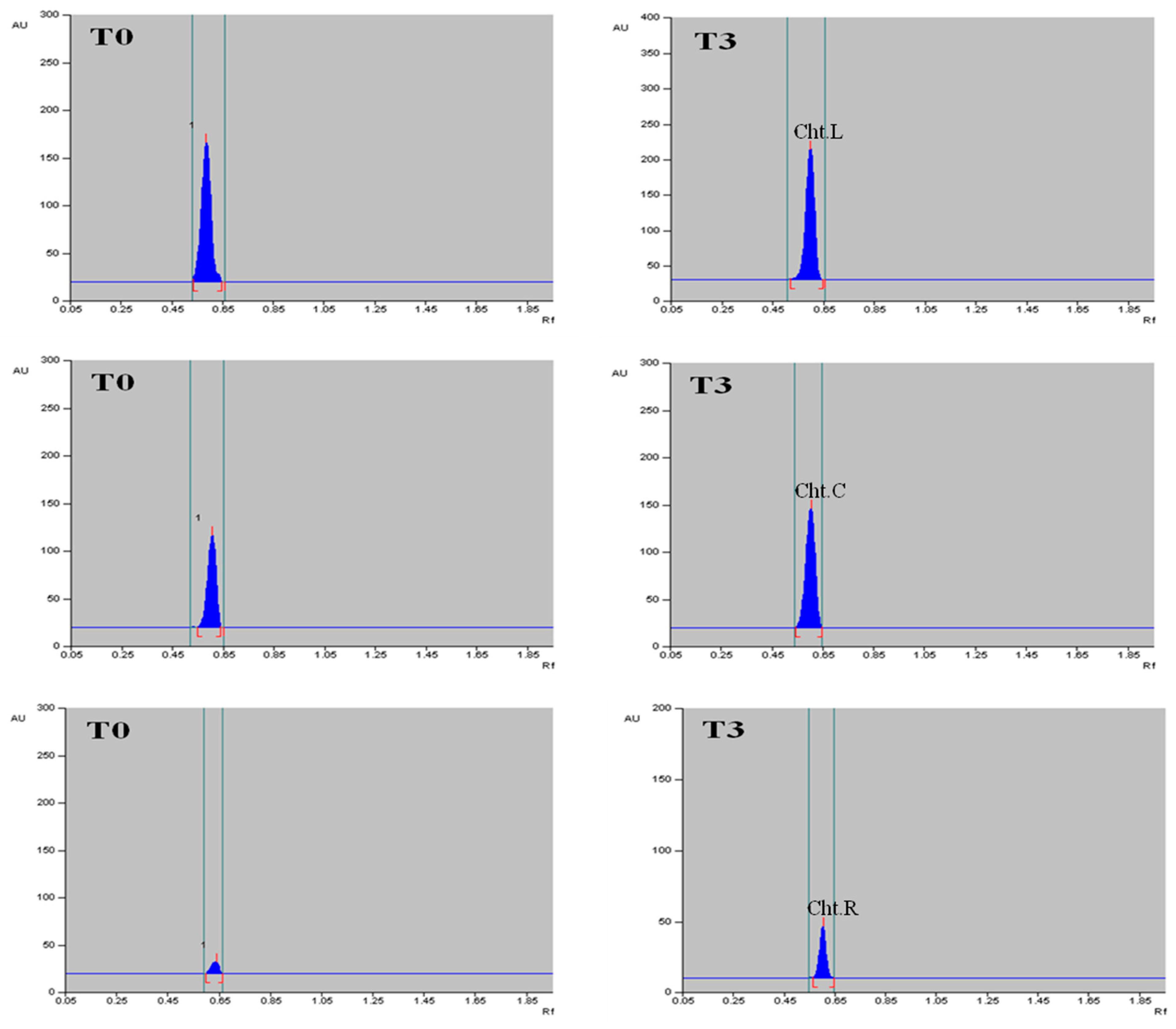
2.6. Quantification of α-Amyrin in Different Tissues of T. indica with HPTLC
3. Discussion
4. Materials and Methods
4.1. Explant Preparation and Culture Conditions
4.2. Callus Induction and Indirect Organogenesis
4.3. Root Induction and Acclimatization
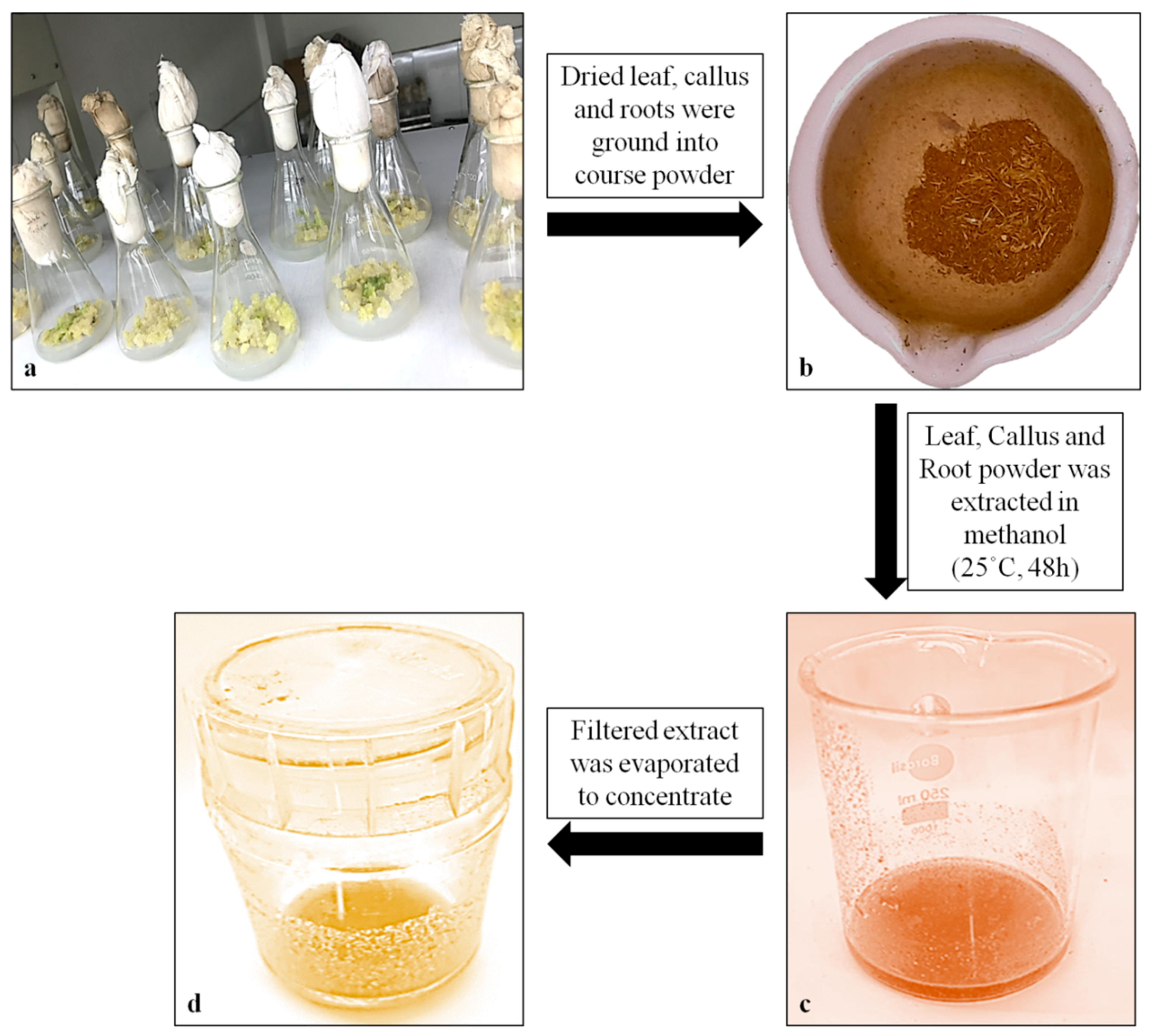
4.4. Extract Preparation and GC–MS Analysis
4.5. Elicitation Treatments
4.6. Biochemical Analysis
4.6.1. Sugar Content
4.6.2. Protein Content
4.6.3. Proline Content
4.6.4. Antioxidant Enzyme Assay
4.6.5. Quantification of α-Amyrin Using High-Performance Thin Layer Chromatography (HPTLC)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akram, W.; Saeed, T.; Ahmad, A.; Yasin, N.A.; Akbar, M.; Khan, W.U.; Ahmed, S.; Guo, J.; Luo, W.; Wu, T.; et al. Liquiritin Elicitation Can Increase the Content of Medicinally Important Glucosinolates and Phenolic Compounds in Chinese Kale Plants. J. Sci. Food Agric. 2020, 100, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Golkar, P.; Taghizadeh, M.; Yousefian, Z. The Effects of Chitosan and Salicylic Acid on Elicitation of Secondary Metabolites and Antioxidant Activity of Safflower under in Vitro Salinity Stress. Plant Cell Tissue Organ. Cult. 2019, 137, 575–585. [Google Scholar] [CrossRef]
- Taj, F.; Khan, M.A.; Ali, H.; Khan, R.S. Improved Production of Industrially Important Essential Oils through Elicitation in the Adventitious Roots of Artemisia Amygdalina. Plants 2019, 8, 430. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Nandy, S.; Nongdam, P.; Tikendra, L.; Mukherjee, A.; Mukherjee, S.; Pandey, D.K. Methyl Jasmonate and Salicylic Acid Elicit Indole Alkaloid Production and Modulate Antioxidant Defence and Biocidal Properties in Rauvolfia serpentina Benth. ex Kurz. in Vitro Cultures. S. Afr. J. Bot. 2020, 135, 1–17. [Google Scholar] [CrossRef]
- Ayoola-Oresanya, I.O.; Sonibare, M.A.; Gueye, B.; Abberton, M.T.; Morlock, G.E. Elicitation of Antioxidant Metabolites in Musa Species in Vitro Shoot Culture Using Sucrose, Temperature and Jasmonic Acid. Plant Cell Tissue Organ. Cult. 2021, 146, 225–236. [Google Scholar] [CrossRef]
- Golkar, P.; Moradi, M.; Garousi, G.A. Elicitation of Stevia Glycosides Using Salicylic Acid and Silver Nanoparticles under Callus Culture. Sugar Tech. 2019, 21, 569–577. [Google Scholar] [CrossRef]
- Bansal, Y.; Mujib, A.; Siddiqui, Z.H.; Mamgain, J.; Syeed, R.; Ejaz, B. Ploidy Status, Nuclear DNA Content and Start Codon Targeted (SCoT) Genetic Homogeneity Assessment in Digitalis purpurea L., Regenerated In Vitro. Genes 2022, 13, 2335. [Google Scholar] [CrossRef]
- Dulara, B.K.; Godara, P.; Barwer, N. In-Vivo and In-Vitro Phytochemical GC-MS Analysis of Volatile Constituents of Andrographis Paniculata (Burm.f.) Nees. Pharma Innov. J. 2019, 8, 255–261. [Google Scholar]
- Mamgain, J.; Mujib, A.; Syeed, R.; Ejaz, B.; Malik, M.Q.; Bansal, Y. Genome Size and Gas Chromatography-Mass Spectrometry (GC–MS) Analysis of Field-Grown and in Vitro Regenerated Pluchea lanceolata Plants. J. Appl. Genet. 2023, 64, 1–21. [Google Scholar] [CrossRef]
- Ferdausi, A.; Chang, X.; Jones, M. Enhancement of Galanthamine Production through Elicitation and NMR-Based Metabolite Profiling in Narcissus pseudonarcissus Cv. Carlton in Vitro Callus Cultures. In Vitro Cell. Dev. Biol.-Plant 2021, 57, 435–446. [Google Scholar] [CrossRef]
- Hussain, S.A.; Ahmad, N.; Anis, M.; Alatar, A.A. Influence of Meta-Topolin on in Vitro Organogenesis in Tecoma stans L., Assessment of Genetic Fidelity and Phytochemical Profiling of Wild and Regenerated Plants. Plant Cell Tissue Organ. Cult. 2019, 138, 339–351. [Google Scholar] [CrossRef]
- Jena, S.; Ray, A.; Sahoo, A.; Sahoo, S.; Dash, B.; Kar, B.; Nayak, S. Rapid Plant Regeneration in Industrially Important Curcuma zedoaria Revealing Genetic and Biochemical Fidelity of the Regenerants. 3 Biotech 2020, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Trettel, J.R.; Gazim, Z.C.; Gonçalves, J.E.; Stracieri, J.; Magalhães, H.M. Effects of Copper Sulphate (CuSO4) Elicitation on the Chemical Constitution of Volatile Compounds and the in Vitro Development of Basil. Sci. Hortic. 2018, 234, 19–26. [Google Scholar] [CrossRef]
- Narayani, M.; Srivastava, S. Elicitation: A Stimulation of Stress in in Vitro Plant Cell/Tissue Cultures for Enhancement of Secondary Metabolite Production. Phytochem. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Gorni, P.H.; Pacheco, A.C.; Silva, J.F.A.; Moreli, R.R.; Spera, K.D.; Silva, R.M.G. Plant Elicitation with Salicylic Acid Increases Bioactive Compounds Content and Antioxidant Activity in the Infusion of Achillea millefolium L. Biosci. J. 2019, 35, 289–295. [Google Scholar] [CrossRef]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A Biotechnological Tool for Enhanced Production of Secondary Metabolites in Hairy Root Cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, V.; Deshpande, J.; Wani, M.; Jeb, J.; Patil Biotechnology, D. Elicitation Based Enhancement of Solasodine Production in In-Vitro Cultures of Different Solanum Species Triveni Enterprises Triveni Enterprises (Educational Services Set-up) (Educational Services Set-up) Elicitation Based Enhancement of Solasodine Production in in-Vitro Cultures of Different Solanum Species. J. Environ. Biol. 2023, 44, 167–174. [Google Scholar] [CrossRef]
- Dasari, R.; Gopu, C.; Vankudoth, S.; Dharavath, S.; Taduri, S. Enhancement of Production of Pharmaceutically Important Anti-Cancerous Compound; Cucurbitacin E via Elicitation and Precursor Feeding of in Vitro Culture of Citrullus colocynthis (L.) Schard. Vegetos 2020, 33, 323–334. [Google Scholar] [CrossRef]
- Hashim, M.; Ahmad, B.; Drouet, S.; Hano, C.; Abbasi, B.H.; Anjum, S. Comparative Effects of Different Light Sources on the Production of Key Secondary Metabolites in Plants in Vitro Cultures. Plants 2021, 10, 1521. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Aldaej, M.I.; Al-Khayri, J.M.; Rezk, A.A.; Al-Mssallem, M.Q.; Sattar, M.N.; Ramadan, K.M.A. Production and Antioxidant Activity of Secondary Metabolites in Hassawi Rice (Oryza sativa L.) Cell Suspension under Salicylic Acid, Yeast Extract, and Pectin Elicitation. In Vitro Cell. Dev. Biol.-Plant 2022, 58, 615–629. [Google Scholar] [CrossRef]
- Nazar, S.; Hussain, M.A.; Khan, A.; Muhammad, G.; Bukhari, S.N.A. Alkaloid-Rich Plant Tylophora Indica; Current Trends in Isolation Strategies, Chemical Profiling and Medicinal Applications. Arab. J. Chem. 2020, 13, 6348–6365. [Google Scholar] [CrossRef]
- Gururani, R.; Patel, S.; Yaduvanshi, N.; Dwivedi, J.; Paliwal, S.; Sharma, S. Tylophora Indica (Burm. f.) Merr: An Insight into Phytochemistry and Pharmacology. J. Ethnopharmacol. 2020, 262, 113122. [Google Scholar] [CrossRef] [PubMed]
- Nagegowda, D.A.; Gupta, P. Advances in Biosynthesis, Regulation, and Metabolic Engineering of Plant Specialized Terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Viet, T.D.; Xuan, T.D.; Anh, L.H. α-Amyrin and β-Amyrin Isolated from Celastrus hindsii Leaves and Their Antioxidant, Anti-Xanthine Oxidase, and Anti-Tyrosinase Potentials. Molecules 2021, 26, 7248. [Google Scholar] [CrossRef] [PubMed]
- Mamgain, J.; Mujib, A.; Ejaz, B.; Gulzar, B.; Malik, M.Q.; Syeed, R. Flow Cytometry and Start Codon Targeted (SCoT) Genetic Fidelity Assessment of Regenerated Plantlets in Tylophora indica (Burm.f.) Merrill. Plant Cell Tissue Organ. Cult. 2022, 150, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Bhusare, B.P.; John, C.K.; Bhatt, V.P.; Nikam, T.D. Induction of Somatic Embryogenesis in Leaf and Root Explants of Digitalis Lanata Ehrh.: Direct and Indirect Method. S. Afr. J. Bot. 2020, 130, 356–365. [Google Scholar] [CrossRef]
- Rad, M.M.; Abdossi, V.; Moradi, P.; Rakhshandehroo, F.; Mehrafarin, A. Phytochemical Changes of Digitalis purpurea L. in Response to Polyamines and Methyl Jasmonate Application in Callus Culture. J. Plant Biochem. Biotechnol. 2022, 31, 310–319. [Google Scholar] [CrossRef]
- Zayova, E.; Nedev, T.; Petrova, D.; Zhiponova, M.; Kapchina, V.; Chaneva, G. Tissue Culture Applications of Artemisia annua L. Callus for Indirect Organogenesis and Production Phytochemical. Plant Tissue Cult. Biotechnol. 2020, 30, 97–106. [Google Scholar] [CrossRef]
- Kim, Y.G.; Okello, D.; Yang, S.; Komakech, R.; Rahmat, E.; Kang, Y. Histological Assessment of Regenerating Plants at Callus, Shoot Organogenesis and Plantlet Stages during the in Vitro Micropropagation of Asparagus cochinchinensis. Plant Cell Tissue Organ. Cult. 2021, 144, 421–433. [Google Scholar] [CrossRef]
- Ejaz, B.; Mujib, A.; Mamgain, J.; Malik, M.Q.; Syeed, R.; Gulzar, B.; Bansal, Y. Comprehensive in Vitro Regeneration Study with SCoT Marker Assisted Clonal Stability Assessment and Flow Cytometric Genome Size Analysis of Carthamus tinctorius L.: An Important Medicinal Plant. Plant Cell Tissue Organ. Cult. 2022, 148, 403–418. [Google Scholar] [CrossRef]
- Mujib, A.; Malik, M.Q.; Bansal, Y.; Syeed, R.; Ejaz, B.; Mamgain, J. Somatic Embryogenesis in Catharanthus Roseus: Proteomics of Embryogenic and Non-Embryogenic Tissues; and Genome Size Analysis of Regenerated Plant. In The Catharanthus Genome; Springer International Publishing: Cham, Switzerland, 2022; pp. 85–100. [Google Scholar]
- Erişen, S.; Kurt-Gür, G.; Servi, H. In Vitro Propagation of Salvia sclarea L. by Meta-Topolin, and Assessment of Genetic Stability and Secondary Metabolite Profiling of Micropropagated Plants. Ind. Crops Prod. 2020, 157, 112892. [Google Scholar] [CrossRef]
- Shalini, K.; Ilango, K. Preliminary Phytochemical Studies, Gc-Ms Analysis and in Vitro Antioxidant Activity of Selected Medicinal Plants and Its Polyherbal Formulation. Pharmacogn. J. 2021, 13, 648–659. [Google Scholar] [CrossRef]
- Bansal, Y.; Mujib, A.; Mamgain, J.; Dewir, Y.H.; Rihan, H.Z. Phytochemical Composition and Detection of Novel Bioactives in Anther Callus of Catharanthus roseus L. Plants 2023, 12, 2186. [Google Scholar] [CrossRef] [PubMed]
- Ekalu, A.; Gbekele-Oluwa Ayo, R.; Habila, J.D.; Hamisu, I. Bioactivities of Phaeophytin a, α-Amyrin, and Lupeol from Brachystelma togoense Schltr. J. Turk. Chem. Soc. Sect. A Chem. 2019, 6, 411–419. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, X.; Xie, L.; Deng, M.; Chen, H.; Song, J.; Long, J.; Li, X.; Luo, J. Lupeol and Its Derivatives as Anticancer and Anti-Inflammatory Agents: Molecular Mechanisms and Therapeutic Efficacy. Pharmacol. Res. 2021, 164, 105373. [Google Scholar] [CrossRef] [PubMed]
- Zareei, E.; Zaare-Nahandi, F.; Hajilou, J.; Oustan, S. Eliciting Effects of Magnetized Solution on Physiological and Biochemical Characteristics and Elemental Uptake in Hydroponically Grown Grape (Vitis vinifera L. Cv. Thompson seedless). Plant Physiol. Biochem. 2021, 167, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.Q.; Mujib, A.; Gulzar, B.; Zafar, N.; Syeed, R.; Mamgain, J.; Ejaz, B. Kanchan Enrichment of Alliin in Different in Vitro Grown Tissues of Allium Sativum through CdCl2 Elicitation as Revealed by High Performance Thin Layer Chromatography (HPTLC). Ind. Crops Prod. 2020, 158, 113007. [Google Scholar] [CrossRef]
- Siddiqui, Z.H.; Mujib, A.; Abbas, Z.K.; Noorani, M.S.; Khan, S. Vinblastine Synthesis under the Influence of CaCl2 Elicitation in Embryogenic Cell Suspension Culture of Catharanthus Roseus. S. Afr. J. Bot. 2023, 154, 319–329. [Google Scholar] [CrossRef]
- Gulzar, B.; Mujib, A.; Mushtaq, Z.; Malik, M.Q. Old Catharanthus Roseus Culture (14 Years) Produced Somatic Embryos and Plants and Showed Normal Genome Size; Demonstrated an Increased Antioxidant Defense Mechanism; and Synthesized Stress Proteins as Biochemical, Proteomics, and Flow-Cytometry Studies Reveal. J. Appl. Genet. 2021, 62, 43–57. [Google Scholar] [CrossRef]
- Bhaskara, G.B.; Yang, T.-H.; Verslues, P.E. Dynamic Proline Metabolism: Importance and Regulation in Water Limited Environments. Front. Plant Sci. 2015, 6, 484. [Google Scholar] [CrossRef]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline Accumulation in Plants: Roles in Stress Tolerance and Plant Development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Springer: New Delhi, India, 2016; pp. 155–166. [Google Scholar]
- Ali, M.; Mujib, A.; Gulzar, B.; Zafar, N. Essential Oil Yield Estimation by Gas Chromatography–Mass Spectrometry (GC–MS) after Methyl Jasmonate (MeJA) Elicitation in in Vitro Cultivated Tissues of Coriandrum sativum L. 3 Biotech 2019, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Maurya, B.; Pandey, N.; Sharma, L.; Goswami, N. Influence of salicylic acid elicitation on secondary metabolites and biomass production in in-vitro cultured Withaniacoagulans (l.) Dunal. Plant Arch. 2019, 19, 1308–1316. [Google Scholar]
- Malar, S.; Shivendra Vikram, S.; Favas, P.J.C.; Perumal, V. Lead Heavy Metal Toxicity Induced Changes on Growth and Antioxidative Enzymes Level in Water Hyacinths [Eichhornia crassipes (Mart.)]. Bot. Stud. 2016, 55, 54. [Google Scholar] [CrossRef] [PubMed]
- Farrokhzad, Y.; Rezaei, A. Aluminum Elicitation Improves Antioxidant Potential and Taxol Production in Hazelnut (Corylus avellana L.) Cell Suspension Culture. Agric. Conspec. Sci. 2020, 85, 229–236. [Google Scholar]
- ShenavaieZare, A.; Ganjeali, A.; VaeziKakhki, M.R.; Cheniany, M.; Mashreghi, M. Plant Elicitation and TiO2 Nanoparticles Application as an Effective Strategy for Improving the Growth, Biochemical Properties, and Essential Oil of Peppermint. Physiol. Mol. Biol. Plants 2022, 28, 1391–1406. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of Chitosan on Plant Responses with Special Reference to Abiotic Stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef]
- Nandy, S.; Hazra, A.K.; Pandey, D.K.; Ray, P.; Dey, A. Elicitation of Industrially Promising Vanillin Type Aromatic Compound 2-Hydroxy 4-Methoxy Benzaldehyde (MBAlD) Yield in the in-Vitro Raised Medicinal Crop Hemidesmus indicus (L) R. Br. by Methyl Jasmonate and Salicylic Acid. Ind. Crops Prod. 2021, 164, 113375. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Soundar, S.; Parekh, K.; Lokhande, S.; Chowdhary, A. Media Optimization in Immobilized Culture to Enhance the Content of Kaempferol in Tylophora indica (Asclepeadaceae) and Curcumin in Curcuma longa (Zingiberaceae). IOSR J. Pharm. Biol. Sci. 2014, 9, 86–90. [Google Scholar] [CrossRef]
- Pathak, A.R.; Patel, S.R.; Joshi, A.G.; Shrivastava, N.; Sindhav, G.; Sharma, S.; Ansari, H. Elicitor Mediated Enhancement of Shoot Biomass and Lupeol Production in Hemidesmus indicus (L.) R. Br. Ex. Schult. and Tylophora indica (Burm. F.) Merrill Using Yeast Extract and Salicylic Acid. Nat. Prod. Res. 2023, 37, 1767–1773. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Dey, P.M. Oligosaccharides. In Methods in Plant Biochemistry; Academic Press: Cambridge, MA, USA, 1990; Volume 2, pp. 189–218. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]

| PGRs (mg L−1) | Callus Induction (%) | Organogenesis (%) | |||
|---|---|---|---|---|---|
| 2,4-D | BAP | NAA | Leaf Explant | Shoot Formation (%) | Mean No. of Shoot/ Callus Mass |
| 0.5 | 68.31 ± 0.88 c | 0 | 0 | ||
| 1.0 | 76.43 ± 0.91 b | 0 | 0 | ||
| 1.5 | 82.25 ± 0.41 a | 0 | 0 | ||
| 2.0 | 68.81 ± 0.98 c | 0 | 0 | ||
| 1 | 0.1 | 56.22 ± 1.24 d | 44.13 ± 3.88 d | 2.60 ± 0.12 c | |
| 1 | 0.5 | 51.32 ± 1.15 e | 51.40 ± 3.20 d | 2.79 ± 0.14 c | |
| 1 | 1.0 | 47.19 ± 0.99 f | 39.33 ± 3.21 e | 2.32 ± 0.07 c | |
| 2 | 0.1 | 27.68 ± 0.61 g | 63.50 ± 2.90 b | 3.34 ± 0.03 b | |
| 2 | 0.5 | 20.11 ± 0.91 h | 68.15 ± 4.55 a | 4.19 ± 0.15 a | |
| 2 | 1.0 | 0 | 56.10 ± 4.51 c | 3.21 ± 0.10 b | |
| 3 | 0.1 | 0 | 37.50 ± 3.79 e | 1.90 ± 0.08 d | |
| 3 | 0.5 | 0 | 34.70 ± 4.40 e | 1.72 ± 0.09 d | |
| 3 | 1.0 | 0 | 30.36 ± 2.70 f | 1.48 ± 0.01 e | |
| PGR (mg L−1) | % Root Induction | Mean No. Roots/ Shoots | |
|---|---|---|---|
| IBA | NAA | ||
| 0.5 | 68.31 ± 1.18 c | 7.01 ± 0.01 c | |
| 1.0 | 83.14 ± 1.57 a | 8.12 ± 0.02 a | |
| 1.5 | 78.03 ± 0.56 b | 7.92 ± 0.01 b | |
| 0.25 | 31.52 ± 1.24 h | 2.47 ± 0.01 i | |
| 0.5 | 47.57 ± 1.29 f | 4.77 ± 0.03 g | |
| 1.0 | 34.37 ± 1.11 g | 3.16 ± 0.02 h | |
| 1.0 | 0.25 | 58.41 ± 1.18 e | 6.02 ± 0.01 e |
| 1.0 | 0.5 | 63.35 ± 1.06 d | 6.89 ± 0.02 d |
| 0.5 | 1.0 | 55.64 ± 1.23 e | 5.32 ± 0.01 f |
| Peak | R. Time | Area | Area% | Name of Compound |
|---|---|---|---|---|
| 1 | 4.444 | 58413741 | 7.20 | 4h-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methane |
| 2 | 6.582 | 2895162 | 0.36 | 2-methoxy-4-vinylphenol |
| 3 | 8.893 | 12285838 | 1.52 | ethanone, 1-(4-hydroxy-3-methoxyphenyl) |
| 4 | 9.607 | 2496143 | 0.31 | 1,6,10-dodecatrien-3-ol |
| 5 | 9.753 | 2661528 | 0.33 | benzamide |
| 6 | 10.952 | 1948509 | 0.24 | 3-cyclohexen-1-ol |
| 7 | 11.429 | 2135516 | 0.26 | 2,6,10-dodecatrien-1-ol |
| 8 | 11.598 | 6546426 | 0.81 | 6-(p-tolyl)-2-methyl-2-heptenol |
| 9 | 11.922 | 2922553 | 0.36 | (r,z)-2-methyl-6-(4-methylcyclohexa-1,4-dien-1-yl) |
| 10 | 11.999 | 1632825 | 0.20 | spiro[androst-5-ene-17,1′-cyclobutan]-2′-one |
| 11 | 12.277 | 1760491 | 0.22 | phenol, 2,4,5-trimethoxy-3-methane |
| 12 | 12.333 | 4624633 | 0.57 | 2(4h)-benzofuranone |
| 13 | 12.647 | 6157467 | 0.76 | neophytadiene |
| 14 | 13.866 | 96537127 | 11.91 | inositol |
| 15 | 14.147 | 128600912 | 15.86 | n-hexadecanoic acid |
| 16 | 15.394 | 68133736 | 8.40 | phytol |
| 17 | 15.867 | 224738398 | 27.72 | 9,12,15-octadecatrienoic acid |
| 18 | 16.010 | 74847773 | 9.23 | [dodecanoyl(methyl)amino]acetic acid |
| 19 | 16.939 | 965421 | 0.12 | carbonic acid, 2-dimethylaminoethyl isobutyl ester |
| 20 | 17.286 | 1059839 | 0.13 | 9-octadecenal, (z) |
| 21 | 17.684 | 879834 | 0.11 | 9-octadecenoic acid (z) |
| 22 | 18.413 | 1611301 | 0.20 | 3-cyclopentylpropionic acid, 2-dimethylaminoethyl ester |
| 23 | 18.899 | 4168486 | 0.51 | hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester |
| 24 | 20.063 | 3893883 | 0.48 | 1,13-tetradecadiene |
| 25 | 20.336 | 4435408 | 0.55 | methyl (z)-5,11,14,17-eicosatetraenoate |
| 26 | 20.900 | 1621582 | 0.20 | 9-octadecenamide, (z) |
| 27 | 21.021 | 2253178 | 0.28 | squalene |
| 28 | 21.270 | 648175 | 0.08 | α tocospiro a |
| 29 | 21.422 | 842781 | 0.10 | α tocospiro b |
| 30 | 21.595 | 5338594 | 0.66 | indolizine, 7-(3,4-dimethoxyphenyl)-1,2,3,5,8,8 |
| 31 | 22.374 | 727963 | 0.09 | ergosta-5,22-dien-3-ol, (3.beta.,22e,24s) |
| 32 | 22.529 | 5792185 | 0.71 | (+)-septicine |
| 33 | 22.785 | 607484 | 0.07 | Gamma tocopherol |
| 34 | 23.025 | 1455700 | 0.18 | stigmasta-5,22-dien-3-ol, acetate, (3.beta.)- |
| 35 | 23.409 | 4931963 | 0.61 | vitamin E |
| 36 | 24.482 | 11552025 | 1.42 | ergost-5-en-3-ol |
| 37 | 24.730 | 5072544 | 0.63 | stigmasta-5,22-dien-3-ol |
| 38 | 25.424 | 26109537 | 3.22 | Gamma sitosterol |
| 39 | 25.972 | 3018664 | 0.37 | 4,4,6a,6b,8a,11,11,14b-octamethyl-1,4,4a,5,6,6a,6 |
| 40 | 26.430 | 2263070 | 0.28 | lanosterol |
| 41 | 26.604 | 16351452 | 2.02 | α amyrin |
| 42 | 27.823 | 1929876 | 0.24 | lupeol |
| 43 | 28.292 | 2844723 | 0.35 | phytyl decanoate |
| 44 | 31.950 | 3036051 | 0.37 | isopropyl linoleate |
| Peak | R. Time | Area | Area% | Name of Compound |
|---|---|---|---|---|
| 1 | 4.419 | 44896275 | 9.14 | 1,5-anhydro-6-deoxyhexo-2,3-diulose |
| 2 | 8.915 | 16295910 | 3.32 | ethanone, 1-(4-hydroxy-3-methoxyphenyl) |
| 3 | 9.983 | 2665097 | 0.54 | 1,2-benzenedicarboxylic acid |
| 4 | 10.860 | 449741 | 0.09 | 1-(4-isopropylphenyl)-2-methylpropyl acetate |
| 5 | 11.330 | 2313565 | 0.47 | 2-(2-iodo-ethyl)-1,3,3-trimethyl-cyclohexene |
| 6 | 11.427 | 1709951 | 0.35 | 2,6,10-dodecatrien-1-ol, 3,7,11-trimethane |
| 7 | 11.596 | 5409117 | 1.10 | 6-(p-tolyl)-2-methyl-2-heptenol |
| 8 | 11.920 | 2398279 | 0.49 | (r,z)-2-methyl-6-(4-methylcyclohexa-1,4-dien-1-yl) |
| 9 | 11.996 | 1228296 | 0.25 | spiro[androst-5-ene-17,1′-cyclobutan]-2′-one |
| 10 | 12.327 | 4067868 | 0.83 | 2(4h)-benzofuranone |
| 11 | 12.449 | 1022344 | 0.21 | 2-cyclohexen-1-one |
| 12 | 12.644 | 8962082 | 1.82 | neophytadiene |
| 13 | 12.970 | 369300 | 0.08 | 1,2-benzenedicarboxylic acid, |
| 14 | 13.095 | 4317055 | 0.88 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol |
| 15 | 13.388 | 22914178 | 4.66 | inositol |
| 16 | 13.571 | 346778 | 0.07 | eicosanoic acid, methyl ester |
| 17 | 14.115 | 92093974 | 18.75 | n-hexadecanoic acid |
| 18 | 15.271 | 722974 | 0.15 | 11,14,17-eicosatrienoic acid, methyl ester |
| 19 | 15.381 | 39588281 | 8.06 | phytol |
| 20 | 15.829 | 141327110 | 28.77 | 9,12,15-octadecatrienoic acid, (z,z,z) |
| 21 | 15.970 | 9835497 | 2.00 | 9-octadecenoic acid (z) |
| 22 | 17.406 | 16012414 | 3.26 | n-benzyl-p-toluene sulfonamide |
| 23 | 18.408 | 703718 | 0.14 | 3-cyclopentylpropionic acid, 2-dimethylaminoethyl ester |
| 24 | 18.659 | 3753517 | 0.76 | 2-methyltetracosane |
| 25 | 18.895 | 1471172 | 0.30 | hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) |
| 26 | 18.968 | 820931 | 0.17 | di-n-octyl phthalate |
| 27 | 20.030 | 921001 | 0.19 | ethyl (9z,12z)-9,12-octadecadienoate |
| 28 | 20.097 | 664029 | 0.14 | 9,12,15-octadecatrienoic acid |
| 29 | 20.894 | 1868283 | 0.38 | 9-octadecenamide |
| 30 | 21.270 | 538175 | 0.19 | α tocospiro a |
| 31 | 21.422 | 782781 | 0.21 | α tocospiro b |
| 32 | 21.584 | 2285700 | 0.47 | indolizine |
| 33 | 21.654 | 1131106 | 0.23 | octacosanol |
| 34 | 21.837 | 412305 | 0.08 | hexacosanoic acid, methyl ester |
| 35 | 22.516 | 2113879 | 0.43 | (+)-septicine |
| 36 | 22.778 | 563696 | 0.11 | Gamma tocopherol |
| 37 | 23.021 | 1013780 | 0.21 | stigmasta-5,22-dien-3-ol |
| 38 | 23.396 | 2252853 | 0.46 | vitamin E |
| 39 | 24.468 | 6169960 | 1.26 | ergost-5-en-3-ol |
| 40 | 24.714 | 3149052 | 0.64 | stigmasta-5,22-dien-3-ol |
| 41 | 25.400 | 15934409 | 3.24 | Gamma sitosterol |
| 42 | 25.730 | 1261223 | 0.26 | dl-2-phenyltryptophane |
| 43 | 25.953 | 1345166 | 0.27 | 4,4,6a,6b,8a,11,11,14b-octamethyl-1,4,4a,5,6,6a,6b,7,8,8a, |
| 44 | 26.583 | 9789411 | 4.13 | α amyrin |
| 45 | 27.618 | 1396959 | 0.58 | lupeol |
| 46 | 28.272 | 1633211 | 0.33 | phytyltetradecanoate |
| 47 | 30.285 | 2595029 | 0.53 | 3.beta.-myristoylolean-12-en-16.beta.-ol |
| 48 | 31.909 | 1795754 | 0.37 | isopropyl linoleate |
| 49 | 32.203 | 1231679 | 0.25 | 9,12-octadecadienal, dimethyl acetate |
| 50 | 32.658 | 2943911 | 0.60 | chromium |
| Plant Parts Used | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| Leaf | 1.78 ± 0.03 e | 1.99 ± 0.02 d | 2.21 ± 0.03 c | 2.72 ± 0.01 a | 2.56 ± 0.02 b |
| Callus | 0.98 ± 0.02 e | 1.11 ± 0.03 d | 1.26 ± 0.03 c | 1.51 ± 0.02 a | 1.38 ± 0.02 b |
| Root | 0.25 ± 0.02 e | 0.31 ± 0.03 d | 0.45 ± 0.02 c | 0.68 ± 0.02 a | 0.53 ± 0.03 b |
| Plant Parts Used | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| Leaf | 1.67 ± 0.02 e | 1.88 ± 0.03 d | 2.11 ± 0.03 c | 2.64 ± 0.02 a | 2.37 ± 0.01 b |
| Callus | 0.76 ± 0.03 e | 1.08 ± 0.02 d | 1.19 ± 0.01 c | 1.45 ± 0.02 a | 1.31 ± 0.01 b |
| Root | 0.18 ± 0.02 e | 0.29 ± 0.02 d | 0.40 ± 0.02 c | 0.61 ± 0.03 a | 0.51 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamgain, J.; Mujib, A.; Bansal, Y.; Gulzar, B.; Zafar, N.; Syeed, R.; Alsughayyir, A.; Dewir, Y.H. Elicitation Induced α-Amyrin Synthesis in Tylophora indica In Vitro Cultures and Comparative Phytochemical Analyses of In Vivo and Micropropagated Plants. Plants 2024, 13, 122. https://doi.org/10.3390/plants13010122
Mamgain J, Mujib A, Bansal Y, Gulzar B, Zafar N, Syeed R, Alsughayyir A, Dewir YH. Elicitation Induced α-Amyrin Synthesis in Tylophora indica In Vitro Cultures and Comparative Phytochemical Analyses of In Vivo and Micropropagated Plants. Plants. 2024; 13(1):122. https://doi.org/10.3390/plants13010122
Chicago/Turabian StyleMamgain, Jyoti, Abdul Mujib, Yashika Bansal, Basit Gulzar, Nadia Zafar, Rukaya Syeed, Ali Alsughayyir, and Yaser Hassan Dewir. 2024. "Elicitation Induced α-Amyrin Synthesis in Tylophora indica In Vitro Cultures and Comparative Phytochemical Analyses of In Vivo and Micropropagated Plants" Plants 13, no. 1: 122. https://doi.org/10.3390/plants13010122
APA StyleMamgain, J., Mujib, A., Bansal, Y., Gulzar, B., Zafar, N., Syeed, R., Alsughayyir, A., & Dewir, Y. H. (2024). Elicitation Induced α-Amyrin Synthesis in Tylophora indica In Vitro Cultures and Comparative Phytochemical Analyses of In Vivo and Micropropagated Plants. Plants, 13(1), 122. https://doi.org/10.3390/plants13010122







