Quality by Design: Development of the Quality Target Product Profile (QTPP) for Semisolid Topical Products
Abstract
1. Introduction
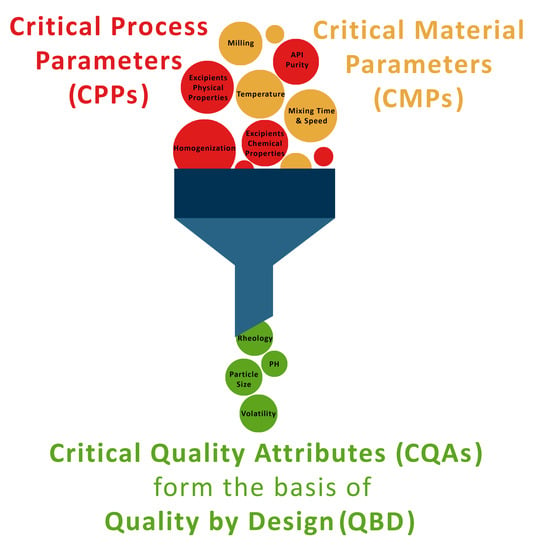
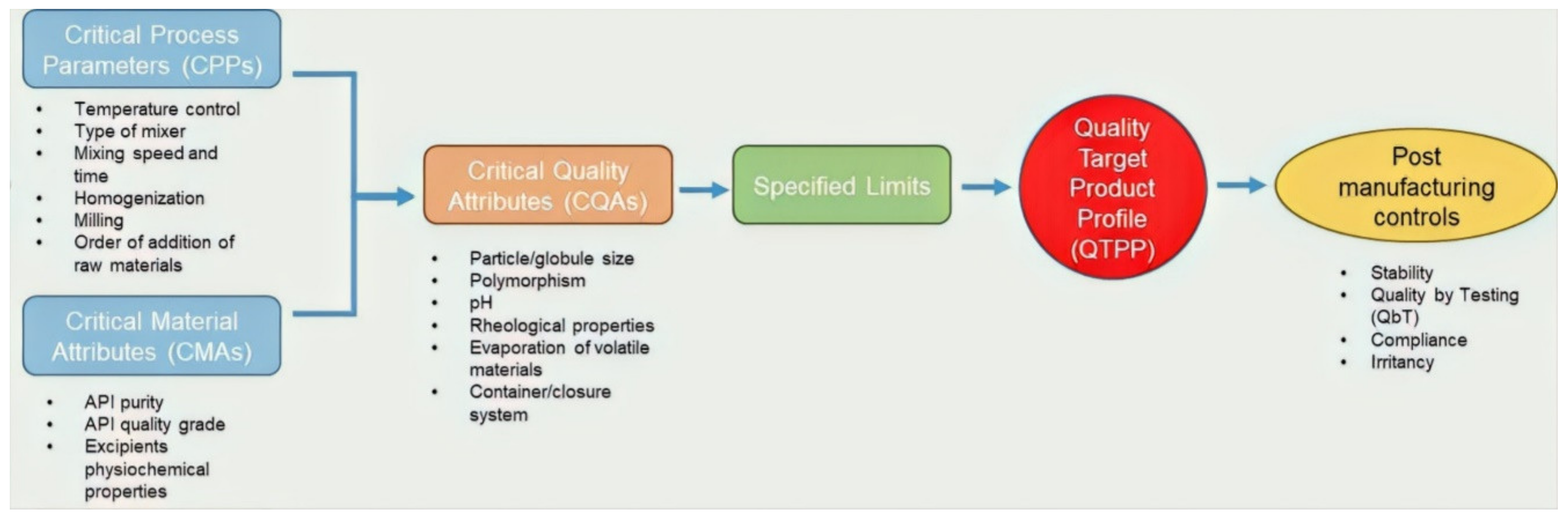
2. QbD and QTPP
| ⮚ Defining a QTPP; |  |
| ⮚ Specifying CMAs; | |
| ⮚ Identifying and developing CPPs; | |
| ⮚ Identifying CQAs; | |
| ⮚ Controlling product and manufacturing procedures to produce final products with consistent required quality over time [11,12]. | |
3. QAs of Topical Dosage Forms
4. Product Design and Development
4.1. CMAs
4.2. CPPs
5. Risk Assessment and Risk Control
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| QAs | quality attributes |
| API | active pharmaceutical ingredient |
| CQAs | critical quality attributes |
| QbD | quality by design |
| QTPP | quality target product profile |
| ICH Q8 | international conference on harmonization of technical requirements for registration of pharmaceuticals for human use. ICH harmonized tripartite guideline. Pharmaceutical development |
| CMAs | critical material attributes |
| CPPs | critical process parameters |
| FDA | the US food and drug administration |
| QC | quality control |
| DoE | design of experiment |
| QbT | quality by testing |
References
- Chang, R.K.; Raw, A.; Lionberger, R.; Yu, L. Generic Development of Topical Dermatologic Products, Part II: Quality by Design for Topical Semisolid Products. AAPS J. 2013, 15, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Nishino, K.; Nayar, S.K. The Apperance of Human Skin: A Survey. Found. Trends Comput. Graph. Vis. 2007, 3, 1–95. [Google Scholar] [CrossRef]
- Kimball, M. Manufacturing topical formulations: Scale-up from Lab to Pilot Production. In Handbook of Formulating Dermal Applications: A Definitive Practical Guide; Wiley: Hoboken, NJ, USA, 2016; pp. 167–232. [Google Scholar]
- Sivaraman, A.; Banga, A.K. Quality by design approaches for topical dermatological dosage forms. Res. Rep. Transdermal Drug Deliv. 2015, 4, 9–21. [Google Scholar] [CrossRef]
- Chang, R.K.; Raw, A.; Lionberger, R.; Yu, L. Generic Development of Topical Dermatologic Products: Formulation Development, Process Development, and Testing of Topical Dermatologic Products. AAPS J. 2013, 15, 41–52. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Considerations (ICH) Guideline Q8 (R2) on Pharmaceutical Development. 2009. Available online: https://www.ema.europa.eu/en/ich-q8-r2-pharmaceutical-development (accessed on 17 February 2020).
- Osborne, D.W. Impact of Quality by Design on Topical Product Excipient Suppliers, Part I: A Drug Manufacturer’s Perspective. Pharm. Technol. 2016, 40, 38–43. [Google Scholar]
- Fowler, M. Quality by Design (QbD) Approach to Generic Transdermal or Topical Product Development. American Pharmaceutical Review 2015 [cited 2020 20/01/2020]. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/172883-Quality-by-Design-QbD-Approach-to-Generic-Transdermal-or-Topical-Product-Development/ (accessed on 20 January 2020).
- NPolitis, S.; Colombo, P.; Colombo, G.; MRekkas, D. Design of experiments (DoE) in pharmaceutical development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, S. Application of quality by design in the current drug development. Asian J. Pharm. Sci. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Yu, L.X. Pharmaceutical Quality by Design: Product and Process Development, Understanding, and Control. Pharm. Res. 2008, 25, 781–791. [Google Scholar] [CrossRef]
- Rosas, J.G.; Blanco, M.; González, J.M.; Alcalá, M. Quality by design approach of a pharmaceutical gel manufacturing process, part 1: Determination of the design space. J. Pharm. Sci. 2011, 100, 4432–4441. [Google Scholar] [CrossRef]
- Jain, S. Quality by design (QBD): A comprehensive understanding of implementation and challenges in pharmaceuticals development. Int. J. Pharm. Pharm. Sci. 2014, 6, 29–35. [Google Scholar]
- Chavda, H. Qbd in developing topical dosage forms. Ely. J. Pharm. Res. 2016, 2, 1–2. [Google Scholar]
- European Medicines Agency. Draft Guideline on Quality and Equivalence of Topical Products. 2018. Available online: https://www.ema.europa.eu/en/quality-equivalence-topical-products (accessed on 18 March 2020).
- Gonyon, T.; Patel, P.; Owen, H.; Dunham, A.J.; Carter, P.W. Physicochemical stability of lipid injectable emulsions: Correlating changes in large globule distributions with phase separation behavior. Int. J. Pharm. 2007, 343, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Nagaich, U.; Gulati, N.; Sharma, V.K.; Khosa, R.L.; Partapur, M.U. Enhancement of solubilization and bioavailability of poorly soluble drugs by physical and chemical modifications: A recent review. J. Adv. Pharm. Educ. Res. 2012, 2, 32–67. [Google Scholar]
- Bauer, J.F. Polymorphism—A critical consideration in pharmaceutical development, manufacturing, and stability. J. Valid. Technol. 2008, 14, 15–24. [Google Scholar]
- FDA Guidance for Industry: ANDAs: Pharmaceutical Solid Polymorphism: Chemistry, Manufacturing, and Controls Information. 2007. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/andaspharmaceutical-solid-polymorphism-chemistry-manufacturing-and-controls-information (accessed on 19 December 2019).
- Nakauma, M.; Funami, T.; Noda, S.; Ishihara, S.; Al-Assaf, S.; Nishinari, K.; Phillips, G.O. Comparison of sugar beet pectin, soybean soluble polysaccharide, and gum arabic as food emulsifiers. 1. Effect of concentration, pH, and salts on the emulsifying properties. Food Hydrocoll. 2008, 22, 1254–1267. [Google Scholar]
- Siewert, M.; Dressman, J.; Brown, C.K.; Shah, V.P.; Aiache, J.M.; Aoyagi, N.; Crison, J. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech 2003, 4, 43–52. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S.; Al-Dhubiab, B.; Attimarad, M.; Harsha, S. Basic considerations in the dermatokinetics of topical formulations. Braz. J. Pharm. Sci. 2013, 49, 423–434. [Google Scholar] [CrossRef]
- Yacobi, A.; Shah, V.P.; Bashaw, E.D.; Benfeldt, E.; Davit, B.; Ganes, D.; Lionberger, R. Current Challenges in Bioequivalence, Quality, and Novel Assessment Technologies for Topical Products. Pharm. Res. 2014, 31, 837–846. [Google Scholar] [CrossRef]
- Stokes, J.R.; Telford, J.H. Measuring the yield behaviour of structured fluids. J. Non Newton. Fluid Mech. 2004, 124, 137–146. [Google Scholar] [CrossRef]
- Nae, H. Rheological properties of topical formulations. In Handbook of Formulating Dermal Applications: A Definitive Practical Guide; Wiley: Hoboken, NJ, USA, 2013; pp. 287–348. [Google Scholar]
- Cross, S.E.; Roberts, M.S.; Jiang, R.; Benson, H.A. Can Increasing the Viscosity of Formulations be used to Reduce the Human Skin Penetration of the Sunscreen Oxybenzone? J. Investig. Dermatol. 2001, 117, 147–150. [Google Scholar] [CrossRef]
- Batheja, P.; Sheihet, L.; Kohn, J.; Singer, A.J.; Michniak-Kohn, B. Topical drug delivery by a polymeric nanosphere gel: Formulation optimization and in vitro and in vivo skin distribution studies. J. Control. Release 2011, 149, 159–167. [Google Scholar] [CrossRef]
- Shah, V.P.; Flynn, G.L.; Yacobi, A.; Maibach, H.I.; Bon, C.; Fleischer, N.M.; Marty, J.P. Bioequivalence of Topical Dermatological Dosage Forms-Methods of Evaluation of Bioequivalence. J. Pharm. Res. 1998, 15, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Ikeda, H.; Kondou, Y.; Kihira, K. Comparison of pharmaceutical properties of topical non-steroidal anti-inflammatory drug preparations on quality of life. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2005, 125, 397–404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Calixto, L.S.; Infante, V.H.P.; Campos, P.M.M. Design and Characterization of Topical Formulations: Correlations Between Instrumental and Sensorial Measurements. AAPS PharmSciTech 2018, 19, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Buhse, L.; Kolinski, R.; Westenberger, B.; Wokovich, A.; Spencer, J.; Chen, C.W.; Heintzelman, B. Topical drug classification. Int. J. Pharm. 2005, 295, 101–112. [Google Scholar] [CrossRef]
- Akala, E.O. Effect of packaging on stability of drugs and drug products. In Pharmaceutical Sciences Encyclopedia: Drug Discovery, Development, and Manufacturing; Wiley: Hoboken, NJ, USA, 2010; pp. 1–46. [Google Scholar]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Oxybutynin permeation in skin: The influence of drug and solvent activity. Int. J. Pharm. 2010, 384, 67–72. [Google Scholar] [CrossRef]
- Hadgraft, J.; Whitefield, M.; Rosher, P.H. Skin Penetration of Topical Formulations of Ibuprofen 5%: An in vitro Comparative Study. Skin Pharmacol. Physiol. 2003, 16, 137–142. [Google Scholar] [CrossRef]
- Dave, V.S.; Saoji, S.D.; Raut, N.A.; Haware, R.V. Excipient variability and its impact on dosage form functionality. J. Pharm. Sci. 2015, 104, 906–915. [Google Scholar] [CrossRef]
- Maqbool, A.; Mishra, M.K.; Pathak, S.; Kesharwani, A.; Kesharwani, A. Semisolid dosage forms manufacturing: Tools, critical process parameters, strategies, optimization, and recent advances. Indo. Am. J. Pharm. Res. 2017, 7, 882–893. [Google Scholar]
- Anju, G.; Pandey, P. Process Validation of Pharmaceutical Dosages Form: A Review. Biomed. J. 2017, 1, 1467–1475. [Google Scholar]
- Gramaglia, D.; Conway, B.R.; Kett, V.L.; Malcolm, R.K.; Batchelor, H.K. High speed DSC (hyper-DSC) as a tool to measure the solubility of a drug within a solid or semi-solid matrix. Int. J. Pharm. 2005, 301, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Agalloco, J.P.; Carleton, F.J. Validation of Pharmaceutical Processes, 3rd ed.; CRS Press: Boca Raton, FL, USA, 2013; pp. 122–127. [Google Scholar]
- Lachman, L.; Lieberman, H.A.; Kanig, J.L. The Theory and Practice of Industrial Pharmacy, 2nd ed.; Wiley: Hoboken, NJ, USA, 1986. [Google Scholar]
| QTPP Elements | Target | CQAs | Justification | |
|---|---|---|---|---|
| Dosage form | Cream | - | - | |
| Route of administration | Topical semisolid product | - | Skin targeted without systemic side impacts | |
| Dosage strength | % w/w | - | - | |
| Stability | At least 12 month shelf life at room temperature | Yes | Affect the product quality | |
| Particle/globule size | Yes | Affect the drug permeation | ||
| Molecular weight of Active Pharmaceutical Ingredient (API) | Yes | Affect the drug permeation | ||
| Polymorphism | Yes | Affect the formulation uniformity and rheological properties | ||
| pH | Yes | Affect the physiochemical stability | ||
| Solubility | Yes | Affect the drug permeation | ||
| Log P | Yes | Affect the drug release and skin retention | ||
| Rheological properties | Viscosity as a function of shear stress and shear rate | Yes | ||
| G′ (storage modulus) | Yes | |||
| G″ (loss modulus) | Yes | |||
| LVR region (linear viscoelastic region) | Yes | |||
| Yield stress | Yes | Affect the formulation performance | ||
| Volatile materials content | Yes | Affect the physiochemical stability | ||
| Container closure system | - | Affect the formulation performance | ||
| Content uniformity | Yes | |||
| Microbial limitation | Yes | Affect the formulation stability and safety |
| CQAs | Related to CMAs | Related to CPPs | Failure Mode |
|---|---|---|---|
| Particle/Globule size | • Change in raw material particle sizes | • Low- or high-speed mixing • Low or high duration of mixing time | • Changes in content uniformity, drug release and dermal distribution of the drug • Patient compliance due to perceptive attributes of the product |
| Rheology - Viscosity -Yield stress - Tan ɣ | • Variations in viscosity of liquid/semisolid raw materials | • The order of addition of rheology modifying materials • Low- or high-speed mixing • High duration of mixing | • Changes in skin retention of the formulation and drug penetration through the skin • Changing in patient acceptability/compliance • Impact on sensorial attributes of the product |
| Evaporation of volatiles | • Change in proportion of volatile and non-volatile substances in the formulation | • Process temperature • High duration of mixing | • Changes in formulation microstructure (crystallization or polymorphism) • Changes in skin retention and permeation of the active • Impact on sensorial attributes of the product |
| Homogeneity and uniformity | • Impurity in API or excipients | • Low- or high-speed mixing • Low duration of mixing • Low temperature • Use of improper mixer type | • Differences in distribution of active through the product affecting skin permeation and therapeutic performance |
| Precipitation/aggregation | • Dependent on the type of emulsifier, gelling agent or volatiles | • The order of addition • High duration of mixing | • Influence on API partitioning within the formulation • Amount of drug permeating through the skin |
| Microbial limitations | • Contaminated materials • Ineffective preservative system | • Contaminated manufacturing and packaging equipment • Lack of or un-validated cleaning protocols for the manufacturing plant and equipment | • Microbiological contamination and both physically and chemically unstable product |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namjoshi, S.; Dabbaghi, M.; Roberts, M.S.; Grice, J.E.; Mohammed, Y. Quality by Design: Development of the Quality Target Product Profile (QTPP) for Semisolid Topical Products. Pharmaceutics 2020, 12, 287. https://doi.org/10.3390/pharmaceutics12030287
Namjoshi S, Dabbaghi M, Roberts MS, Grice JE, Mohammed Y. Quality by Design: Development of the Quality Target Product Profile (QTPP) for Semisolid Topical Products. Pharmaceutics. 2020; 12(3):287. https://doi.org/10.3390/pharmaceutics12030287
Chicago/Turabian StyleNamjoshi, Sarika, Maryam Dabbaghi, Michael S. Roberts, Jeffrey E. Grice, and Yousuf Mohammed. 2020. "Quality by Design: Development of the Quality Target Product Profile (QTPP) for Semisolid Topical Products" Pharmaceutics 12, no. 3: 287. https://doi.org/10.3390/pharmaceutics12030287
APA StyleNamjoshi, S., Dabbaghi, M., Roberts, M. S., Grice, J. E., & Mohammed, Y. (2020). Quality by Design: Development of the Quality Target Product Profile (QTPP) for Semisolid Topical Products. Pharmaceutics, 12(3), 287. https://doi.org/10.3390/pharmaceutics12030287