Anti-Cholinergic Effects of the Phenolic Extract from the Astragalus crenatus Plant: A Computational and Network Pharmacology Study
Abstract
1. Introduction
2. Results
2.1. Identification and Quantification of Phenolic Compounds of A. crenatus
2.2. Anticholinesterase Activity
2.3. Binding Mode Analysis by the Molecular Docking Approach
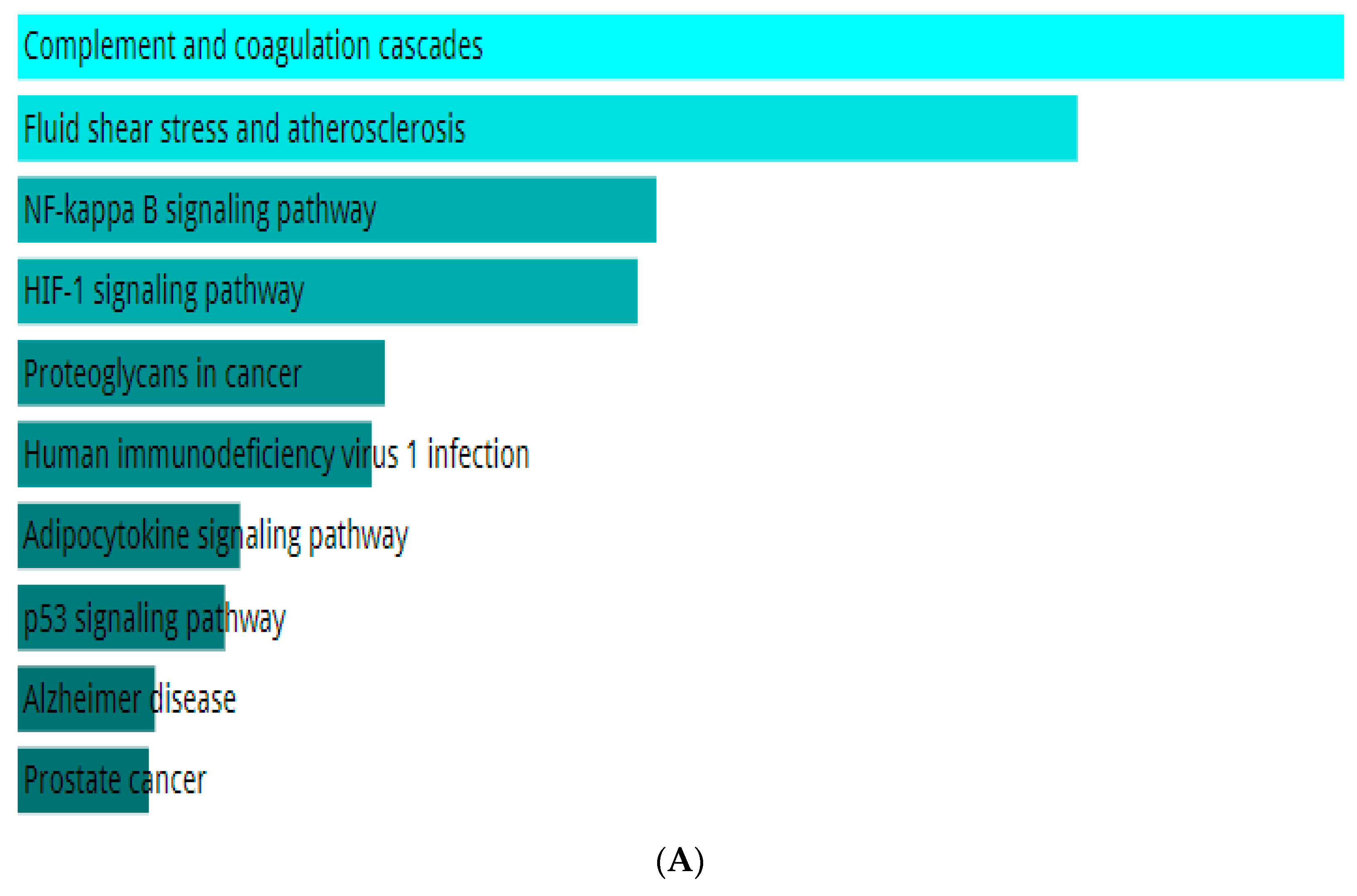
2.4. Network Pharmacology of the Top Ligand
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Plant Extract
4.3. Equipment and Chromatographic Parameters
4.4. Cholinesterases Inhibition
4.5. Statistical Analysis
4.6. Molecular Docking Protocol
4.7. Network Pharmacology of the Top Ligand
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Touliabah, H.E.; Refaay, D.A. Enhancement of Anticancer, Antibacterial, and Acetylcholinesterase Inhibition Activities from Oscillatoria sancta under Starvation Conditions. Water 2023, 15, 664. [Google Scholar] [CrossRef]
- Cuong, N.M.; Khanh, P.N.; Nhung, L.T.H.; Ha, N.X.; Huong, T.T.; Bauerova, K.; Kim, Y.H.; Tung, D.D.; Thuy, T.T.; Anh, N.T.H. Acetylcholinesterase Inhibitory Activities of Some Flavonoids from the Root Bark of Pinus krempfii Lecomte: In Vitro and in Silico Study. J. Biomol. Struct. Dyn. 2023, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, J.; Wang, C.; Wu, L.; Liu, Y. Screening Bifunctional Flavonoids of Anti-Cholinesterase and Anti-Glucosidase by In Vitro and In Silico Studies: Quercetin, Kaempferol and Myricetin. Food Biosci. 2023, 51, 102312. [Google Scholar] [CrossRef]
- Colavitta, M.F.; Barrantes, F.J. Therapeutic Strategies Aimed at Improving Neuroplasticity in Alzheimer Disease. Pharmaceutics 2023, 15, 2052. [Google Scholar] [CrossRef] [PubMed]
- Tanvir Kabir, M.; Sahab Uddin, M.; Al Mamun, A.; Jeandet, P.; Aleya, L.; Mansouri, R.A.; Md Ashraf, G.; Mathew, B.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Combination Drug Therapy for the Management of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3272. [Google Scholar] [CrossRef] [PubMed]
- Lekmine, S.; Bendjedid, S.; Benslama, O.; Martín-García, A.I.; Boussekine, S.; Kadi, K.; Akkal, S.; Nieto, G.; Sami, R.; Al-Mushhin, A.A.M.; et al. Ultrasound-Assisted Extraction, LC–MS/MS Analysis, Anticholinesterase, and Antioxidant Activities of Valuable Natural Metabolites from Astragalus armatus Willd.: In Silico Molecular Docking and In Vitro Enzymatic Studies. Antioxidants 2022, 11, 2000. [Google Scholar] [CrossRef]
- Lekmine, S.; Boussekine, S.; Akkal, S.; Martín-García, A.I.; Boumegoura, A.; Kadi, K.; Djeghim, H.; Mekersi, N.; Bendjedid, S.; Bensouici, C.; et al. Investigation of Photoprotective, Anti-Inflammatory, Antioxidant Capacities and LC–ESI–MS Phenolic Profile of Astragalus gombiformis Pomel. Foods 2021, 10, 1937. [Google Scholar] [CrossRef]
- Benslama, O.; Lekmine, S.; Mansouri, N. Phytochemical Constituents of Astragalus monspessulanus and Integrative Analysis for Its Antioxidant, Photoprotective, and Antityrosinase Activities: Experimental and Computational Investigation. Eur. J. Integr. Med. 2023, 60, 102247. [Google Scholar] [CrossRef]
- Lekmine, S.; Boussekine, S.; Kadi, K.; Martín-García, A.I.; Kheddouma, A.; Nagaz, K.; Bensouici, C. A Comparative Study on Chemical Profile and Biological Activities of Aerial Parts (Stems, Flowers, Leaves, Pods and Seeds) of Astragalus gombiformis. Biocatal. Agric. Biotechnol. 2020, 27, 101668. [Google Scholar] [CrossRef]
- Jin, L.; Jin, W.; Zeng, Q.; Yu, L.; Yang, J.; Wan, H.; He, Y. Optimization of Green Extraction Process with Natural Deep Eutectic Solvent and Comparative in Vivo Pharmacokinetics of Bioactive Compounds from Astragalus-Safflower Pair. Phytomedicine 2023, 114, 154814. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Fayez, S.; Zengin, G.; Selvi, S.; Uba, A.I.; Mollica, A.; Bouyahya, A.; Ponniya, S.K.M.; Nilofar; Lekmine, S. Chemical Exploration of Different Extracts from Phytolacca americana Leaves and Their Potential Utilization for Global Health Problems: In Silico and Network Pharmacology Validation. J. Biomol. Struct. Dyn. [CrossRef]
- Bendjedid, S.; Lekmine, S.; Tadjine, A.; Djelloul, R.; Bensouici, C. Analysis of Phytochemical Constituents, Antibacterial, Antioxidant, Photoprotective Activities and Cytotoxic Effect of Leaves Extracts and Fractions of Aloe Vera. Biocatal. Agric. Biotechnol. 2021, 33, 101991. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Kadi, K.; Ignacio Martín-García, A.; Shamsul Ola, M.; Abdullah Yilmaz, M.; Ali, A. Therapeutic Potential of Hyoscyamus niger-Derived Compounds: Targeting Ovarian Cancer through Antioxidant Activity and EGFR Tyrosine Kinase Inhibition. J. King Saud. Univ. Sci. 2024, 36, 103103. [Google Scholar] [CrossRef]
- Babich, O.; Larina, V.; Krol, O.; Ulrikh, E.; Sukhikh, S.; Gureev, M.A.; Prosekov, A.; Ivanova, S. In Vitro Study of Biological Activity of Tanacetum vulgare Extracts. Pharmaceutics 2023, 15, 616. [Google Scholar] [CrossRef]
- Rosheen; Sharma, S.; Utreja, D. Salicylic Acid: Synthetic Strategies and Their Biological Activities. ChemistrySelect 2023, 8, e202204614. [Google Scholar] [CrossRef]
- Kadi, K.; Mrah, R.; Hamli, S.; Lekmine, S.; Dib, D.; Addad, D.; Boukeria, S.; Gueboudji, Z.; Hafsaoui, I. Evaluation of the Anticoagulant Activity of Margins from Olives Extraction in the Khenchela Region. J. Fundam. Appl. Sci. 2020, 12, 634–649. [Google Scholar] [CrossRef]
- Boussekine, S.; Lekmine, S.; Gasmi, S.; Benkhedir, A.; Saker, H.; Lidoughi, A. The protective effect of selenium on diabetic nephropathy in Wistar rats. J. Microbiol. Biotechnol. Food Sci. 2022, 12, e5960. [Google Scholar] [CrossRef]
- Mekersi, N.; Kadi, K.; Casini, S.; Addad, D.; Amari, A.; Lekmine, S. Evaluation of the Effects of Short-Term Amendment with Olive Mill Pomace on Some Soil Properties. Soil Sci. Annu. 2022, 73, 150493. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Eweys, A.S.; Zhao, Y.-S.; Matter, I.A. Application of Environmental-Safe Fermentation with Saccharomyces Cerevisiae for Increasing the Cinnamon Biological Activities. Bioresour. Bioprocess. 2023, 10, 12. [Google Scholar] [CrossRef]
- Liu, F.; Cao, X.; Zhang, T.; Xing, L.; Sun, Z.; Zeng, W.; Xin, H.; Xue, W. Synthesis and Biological Activity of Myricetin Derivatives Containing Pyrazole Piperazine Amide. Int. J. Mol. Sci. 2023, 24, 10442. [Google Scholar] [CrossRef]
- Tobar-Delgado, E.; Mejía-España, D.; Osorio-Mora, O.; Serna-Cock, L. Rutin: Family Farming Products’ Extraction Sources, Industrial Applications and Current Trends in Biological Activity Protection. Molecules 2023, 28, 5864. [Google Scholar] [CrossRef] [PubMed]
- Kalaycı, B.; Özek, N.Ş.; Aysin, F.; Özbek, H.; Kazaz, C.; Önal, M.; Güvenalp, Z. Evaluation of Cytotoxic and Apoptotic Effects of the Extracts and Phenolic Compounds of Astragalus globosus Vahl and Astragalus Breviflorus DC. Saudi Pharm. J. 2023, 31, 101682. [Google Scholar] [CrossRef] [PubMed]
- Yasinov, R.K.; Khaitov, I.K. Flavonoids of Astragalus kabadianus. Chem. Nat. Compd. 1988, 24, 386. [Google Scholar] [CrossRef]
- Yasinov, R.K.; Syrovezhko, N.V.; Yakovelev, G.P. Flavonoids of Astragalus Quisqualis. Chem. Nat. Compd. 1983, 19, 368. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Kadi, K.; Martín-García, A.I.; Yilmaz, M.A.; Akkal, S.; Boumegoura, A.; Alhomida, A.S.; Ola, M.S.; Ali, A. LC/MS-MS Analysis of Phenolic Compounds in Hyoscyamus albus L. Extract: In Vitro Antidiabetic Activity, In Silico Molecular Docking, and In Vivo Investigation against STZ-Induced Diabetic Mice. Pharmaceuticals 2023, 16, 1015. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.S.; Morsy, N.M.; Aboulthana, W.M.; Ragab, A. In Vitro Enzymatic Evaluation of Some Pyrazolo [1,5-a] Pyrimidine Derivatives: Design, Synthesis, Antioxidant, Anti-diabetic, Anti-Alzheimer, and Anti-arthritic Activities with Molecular Modeling Simulation. Drug Dev. Res. 2023, 84, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Karagecili, H.; İzol, E.; Kirecci, E.; Gulcin, İ. Determination of Antioxidant, Anti-Alzheimer, Antidiabetic, Antiglaucoma and Antimicrobial Effects of Zivzik pomegranate (Punica granatum)—A Chemical Profiling by LC-MS/MS. Life 2023, 13, 735. [Google Scholar] [CrossRef] [PubMed]
- Lakhera, S.; Devlal, K.; Rana, M.; Celik, I. Study of Nonlinear Optical Responses of Phytochemicals of Clitoria ternatea by Quantum Mechanical Approach and Investigation of Their Anti-Alzheimer Activity with in Silico Approach. Struct. Chem. 2023, 34, 439–454. [Google Scholar] [CrossRef]
- Güven, L.; Erturk, A.; Miloğlu, F.D.; Alwasel, S.; Gulcin, İ. Screening of Antiglaucoma, Antidiabetic, Anti-Alzheimer, and Antioxidant Activities of Astragalus alopecurus Pall—Analysis of Phenolics Profiles by LC-MS/MS. Pharmaceuticals 2023, 16, 659. [Google Scholar] [CrossRef]
- Koçyiğit, Ü.M.; Eruygur, N.; Mehmet, A.; Tekin, M.; Taslimi, P.; Gökalp, F.; Gülçin, İ. Evaluation of Anticholinergic, Antidiabetic and Antioxidant Activity of Astragalus dumanii, an Endemic Plant. Kahramanmaraş Sütçü İmam Üniver. Tarım Doğa Derg. 2022, 25, 1–10. [Google Scholar] [CrossRef]
- Triki, Z.; Fergani, Z.; Lekmine, S.; Tahraoui, H.; Amrane, A.; Zamouche, M.; Kebir, M.; Assadi, A.A.; Khezami, L.; Zhang, J. Numerical Modelling and Performance Evaluation of Vacuum Membrane Distillation for Energy-Efficient Seawater Desalination: Towards Energy-Efficient Solutions. Water 2023, 15, 3612. [Google Scholar] [CrossRef]
- Anwar, S.; Rehman, W.; Hussain, R.; Khan, S.; Alanazi, M.M.; Alsaif, N.A.; Khan, Y.; Iqbal, S.; Naz, A.; Hashmi, M.A. Investigation of Novel Benzoxazole-Oxadiazole Derivatives as Effective Anti-Alzheimer’s Agents: In Vitro and In Silico Approaches. Pharmaceuticals 2023, 16, 909. [Google Scholar] [CrossRef]
- Li, S.; Li, A.J.; Travers, J.; Xu, T.; Sakamuru, S.; Klumpp-Thomas, C.; Huang, R.; Xia, M. Identification of Compounds for Butyrylcholinesterase Inhibition. SLAS Discov. 2021, 26, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Ertas, A.; Yener, I. A Comprehensive Study on Chemical and Biological Profiles of Three Herbal Teas in Anatolia; Rosmarinic and Chlorogenic Acids. S. Afr. J. Bot. 2020, 130, 274–281. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Lagunin, A.; Ivanov, S.; Rudik, A.; Filimonov, D.; Poroikov, V. DIGEP-Pred: Web Service for in Silico Prediction of Drug-Induced Gene Expression Profiles Based on Structural Formula. Bioinformatics 2013, 29, 2062–2063. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P. STRING V11: Protein–Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
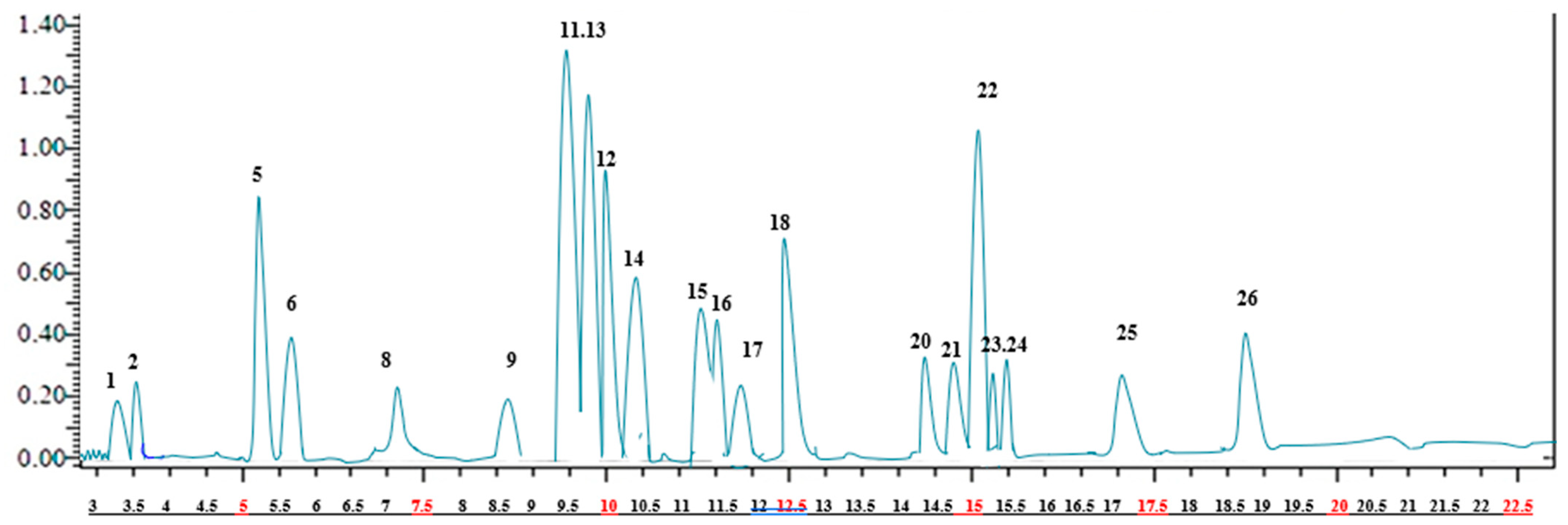





| Compound | (m/z) | MS2 | Quantification (mg Analyte/g Extract) | |
|---|---|---|---|---|
| 1 | Quinic acid | 191.0 | 85 (22), 93 (22) | 0.221 ± 2.3 g |
| 2 | Malic acid | 133.1 | 115 (14), 71 (17) | 0.334 ± 2.3 i |
| 3 | tr-Aconitic acid | 172.9 | 85 (12). 129 (9) | N.D. |
| 4 | Gallic acid | 169.1 | 125 (14), 79 (25) | N.D. |
| 5 | Chlorogenic acid | 353.0 | 191 (17) | 67.645 ± 1.5 a |
| 6 | Protocatechuic acid | 153.0 | 109 (16), 108 (26) | 39.986 ± 2.2 b |
| 7 | Tannic acid | 183.0 | 124 (22), 78 (34) | N.D. |
| 8 | trans-Caffeic acid | 179.0 | 135 (15), 134 (24), 89 (31) | 0.256 ± 2.3 i |
| 9 | Vanillin | 151.1 | 136 (17), 92 (21) | 0.558 ± 1.4 h |
| 10 | p-Coumaric acid | 163.0 | 119 (15), 93 (31) | N.D. |
| 11 | Rosmarinic acid | 358.9 | 161 (17), 133 (42) | 96.675 ± 1.3 a |
| 12 | Rutin | 609.1 | 300 (37), 271 (51), 301 (38) | 68.156 ± 1.6 g |
| 13 | Hesperidin | 611.1 | 303, 465 | 79.613 ± 1.2 c |
| 14 | Hyperoside | 463.1 | 300, 301 | 63.173 ± 1.5 c |
| 15 | 4-OH Benzoic acid | 137.0 | 93, 65 | 58.184 ± 1.3 b |
| 16 | Salicylic acid | 137.0 | 93, 65, 75 | 55.637 ± 1.3 h |
| 17 | Myricetin | 317.0 | 179, 151, 137 | 4.158 ± 1.5 |
| 18 | Fisetin | 285.0 | 135, 121 | 66.647 ± 2.3 h |
| 19 | Coumarin | 147.0 | 103, 91, 77 | N.D. |
| 20 | Quercetin | 300.9 | 179, 151, 121 | 7.853 ± 1.1 d |
| 21 | Naringenin | 271.0 | 151, 119, 107 | 6.253 ± 1.1 de |
| 22 | Hesperetin | 301.0 | 164, 136, 108 | 75.102 ± 1.4 h |
| 23 | Luteolin | 285.0 | 175, 151, 133 | 4.291 ± 1.3 e |
| 24 | Kaempferol | 285.0 | 217, 133, 151 | 5.461 ± 1.4 ef |
| 25 | Apigenin | 269.0 | 151, 117 | 25.147 ± 1.6 |
| 26 | Rhamnetin | 315.0 | 165, 121, 300 | 35.658 ± 2.3 h |
| 27 | Chrysin | 253.0 | 143, 119, 107 | N.D. |
| Samples | AChE IC50 (μg/mL) | BChE IC50 (μg/mL) |
|---|---|---|
| Galantamine | 12.37 ± 1.37 | 32.16 ± 0.74 |
| A. crenatus extract | 7.48 ± 0.23 | 37.14 ± 0.26 |
| Compounds | Binding Energy (kcal/mol) | Hydrogen Interactions (Distance in Å) | Hydrophobic Interactions | van der Waals Interactions |
|---|---|---|---|---|
| Galantamine | −9.8 | Ser203 (2.91 Å), Glu202 (2.65 Å), His447 (3.58 Å), Asp74 (3.72 Å), Tyr124 (3.46 Å) | Tyr337, Trp86, Gly121, Phe338, Phe295, Phe297, His447 | Gly122, Ser125, Tyr341, Gly120, Tyr133, Gly448 |
| Hesperidin | −10.5 | Tyr124 (3.65 Å), Arg296 (3.27 Å), Phe295 (2.84 Å), Tyr72 (2.91 Å), Tyr72 (1.98 Å), Trp286 (3.02 Å), Trp286 (1.93 Å), Ser293 (2.08 Å) | Tyr341, Tyr337, Trp286 | Glu292, Gln291, Leu289, His287, Thr75, Leu76, Phe297, His447, Gly122, Gly121, Phe338, Val294 |
| Luteolin | −10.4 | Phe295 (2.84 Å), Arg296 (2.93 Å), Tyr124 (3.43 Å), Tyr124 (4.19 Å), Gly122 (2.88 Å) | Trp286, Tyr341 | Tyr337, His447, Ser203, Gly121, Ala204, Phe338, Phe297, Val294, Ser293 |
| Rosmarinic acid | −10.3 | Ser203 (2.92 Å), Glu202 (2.79 Å), His447 (2.23 Å), Asp74 (2.58 Å), Tyr337 (2.93 Å), Tyr124 (3.04 Å), Tyr341 (2.88 Å), Arg296 (2.40 Å), Ser293 (2.9 Å 3) | Trp86, Tyr341 | Trp439, Phe295, Trp286, Phe297, Val294, Gly121, Phe338, Gly122, Ala204, Gly448 |
| Apigenin | −10.3 | Ser203 (2.96 Å), Tyr124 (3.51 Å) | Tyr341, Trp286 | Ala204, Gly121, His447, Ser293, Val294, Phe297, Phe338, Tyr337, Phe295, Arg296 |
| Hesperetin | −10.2 | Ser203 (3.04 Å), Tyr124 (2.96 Å), Phe295 (2.82 Å), Arg296 (2.16 Å) | Phe338, Tyr124, Trp286 | Val294, Tyr341, Tyr337, Phe297, Ala204, Gly121, His447 |
| Myricetin | −10.0 | Trp86 (2.82 Å), Asn87 (2.67 Å), Tyr133 (3.03 Å), Ser125 (3.68 Å) | Tyr124, Trp86 | Tyr72, Tyr341, Tyr337, Gly448, His447, Ile451, Gly120, Ser203, Ser125, Pro88, Gly126, Val73, Gln71 |
| Quercetin | −9.8 | Tyr72 (3.06 Å), Tyr341 (2.91 Å), Tyr124 (3.53 Å), Val294 (3.22 Å) | Tyr341, Trp286 | Ser293, Arg296, Phe297, Phe338, Gly122, Gly121, His447 |
| Naringenin | −9.8 | Ser203 (2.53 Å), Tyr124 (3.13 Å) | Phe338, Trp286, Tyr341 | Tyr337, His447, Gly121, Gly120, Ala204, Phe297, Val294, Ser293, Arg296, Phe295 |
| Compounds | Binding Energy (kcal/mol) | Hydrogen Interactions (Distance Å) | Hydrophobic Interactions | van der Waals Interactions | Electrostatic Interactions |
|---|---|---|---|---|---|
| Ethopropazine (co-crystallized) | −8.9 | - | Trp82, Tyr332, Gly116, Phe329, Leu286, Trp231 | Phe398, Ser198, Trp430, Ser79, Thr120, Gln119, Ser287, Gly117, Pro285, Val288 | Asp70 |
| Galantamine | −9.3 | Trp82 (3.58 Å), Gly116 (3.62 Å), Thr120 (2.16 Å) | Trp82, Leu125 | Ser79, Met437, Tyr440, Ala328, Trp430, His438, Gly115, Gly121, Asp70 | Trp82 |
| Hesperidin | −9.8 | Glu276 (3.50 Å), Asn289 (3.08 Å), Leu286 (3.66 Å), Gly117 (3.03 Å), Gly117 (2.99 Å), Ser198 (3.27 Å), Glu197 (2.77 Å), Trp82 (2.33 Å), Ala328 (2.76 Å) | Phe329, Trp231 | Phe398, Ala199, Gly439, Tyr332, Thr120, Gln119, Pro285, Gly116, Ser287, Val288 | |
| Rhamnetin | −9.7 | Ser198 (2.77 Å) | Leu289, Trp231, Phe329, Trp430, Tyr440, Met437, Trp82, Ala328, His438 | Ser287, Val288, Phe398, Gly117, Ala199, Gly115, Glu197, Gly439 | |
| Rutin | −9.7 | Asn289 (3.68 Å), Tyr440 (3.85 Å), Gly78 (4.28 Å), His438 (3.65 Å) | Tyr332, Trp82, Ala328, Phe329 | Asp70, Pro285, Phe398, Leu286, Gly117, Ala277, Asn68, Val288, Gln119, Gly439, Met437, Trp430, Ser79 | - |
| Hyperoside | −9.5 | Ser198 (3.15 Å), Glu197 (2.15 Å), Glu197 (2.55 Å), Tyr128 (2.55 Å), His438 (1.63 Å), Tyr440 (2.94 Å), Gly78 (2.83 Å), Thr120 (3.13 Å), Gly116 (3.22 Å) | Ala328, Trp82 | Ala199, Gly115, Ile442, Tyr114, Gly439, Met437, Trp430, Ser79, Tyr332, Phe329, Gly117 | Asp70 |
| Fisetin | −9.5 | Ser198 (1.98 Å), Gly117 (2.87 Å), Gly116 (2.98 Å) | Leu286, Trp231, Phe329, His438, Trp82 | Phe398, Ala328, Trp430, Glu197, Gly115, Ala199, Ser287, Pro285, Val288 | |
| Quercetin | −9.5 | Gly117 (2.89 Å), Ser287 (3.12 Å), Leu286 (3.08 Å) | Trp82, Trp231, Phe329, His438, Leu286 | Pro285, Ala199, Ser198, Gly115, Glu197, Gly439, Ala328, Phe398 | - |
| Hesperetin | −9.4 | His438 (2.96 Å), Glu197 (2.38 Å), Thr120 (3.78 Å) | Trp82 | Gly439, Ile442, Tyr128, Gly115, Gly116, Pro84, Ile69, Asn68 | Asp70 |
| Kaempferol | −9.4 | Ser198 (2.83 Å) | Phe329, Leu286, Trp231, Gly116, His438, Trp82 | Val288, Phe398, Ala199, Gly115, Glu197, Met437, Ala328 | |
| Luteolin | −9.4 | His438 (3.10 Å), Tyr128 (2.12) Å, Thr120 (4.85 Å) | Trp82 | Ile442, Gly115, Gly116, Pro84, Asn83, Ile69, Asn68, Gly439 | Asp70 |
| Myricetin | −9.3 | His438 (2.66 Å), Tyr128 (3.52 Å) | Trp82 | Ser79, Gly439, Ile442, Gly115, Gly116, Gly116, Tyr114, Pro84, Asn83, Ile69, Asn68 | Asp70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekmine, S.; Benslama, O.; Tahraoui, H.; Ola, M.S.; Laouani, A.; Kadi, K.; Martín-García, A.I.; Ali, A. Anti-Cholinergic Effects of the Phenolic Extract from the Astragalus crenatus Plant: A Computational and Network Pharmacology Study. Pharmaceuticals 2024, 17, 348. https://doi.org/10.3390/ph17030348
Lekmine S, Benslama O, Tahraoui H, Ola MS, Laouani A, Kadi K, Martín-García AI, Ali A. Anti-Cholinergic Effects of the Phenolic Extract from the Astragalus crenatus Plant: A Computational and Network Pharmacology Study. Pharmaceuticals. 2024; 17(3):348. https://doi.org/10.3390/ph17030348
Chicago/Turabian StyleLekmine, Sabrina, Ouided Benslama, Hichem Tahraoui, Mohammad Shamsul Ola, Aicha Laouani, Kenza Kadi, Antonio Ignacio Martín-García, and Ahmad Ali. 2024. "Anti-Cholinergic Effects of the Phenolic Extract from the Astragalus crenatus Plant: A Computational and Network Pharmacology Study" Pharmaceuticals 17, no. 3: 348. https://doi.org/10.3390/ph17030348
APA StyleLekmine, S., Benslama, O., Tahraoui, H., Ola, M. S., Laouani, A., Kadi, K., Martín-García, A. I., & Ali, A. (2024). Anti-Cholinergic Effects of the Phenolic Extract from the Astragalus crenatus Plant: A Computational and Network Pharmacology Study. Pharmaceuticals, 17(3), 348. https://doi.org/10.3390/ph17030348









