Pyrazolo[4,3-c]pyridine Sulfonamides as Carbonic Anhydrase Inhibitors: Synthesis, Biological and In Silico Studies
Abstract
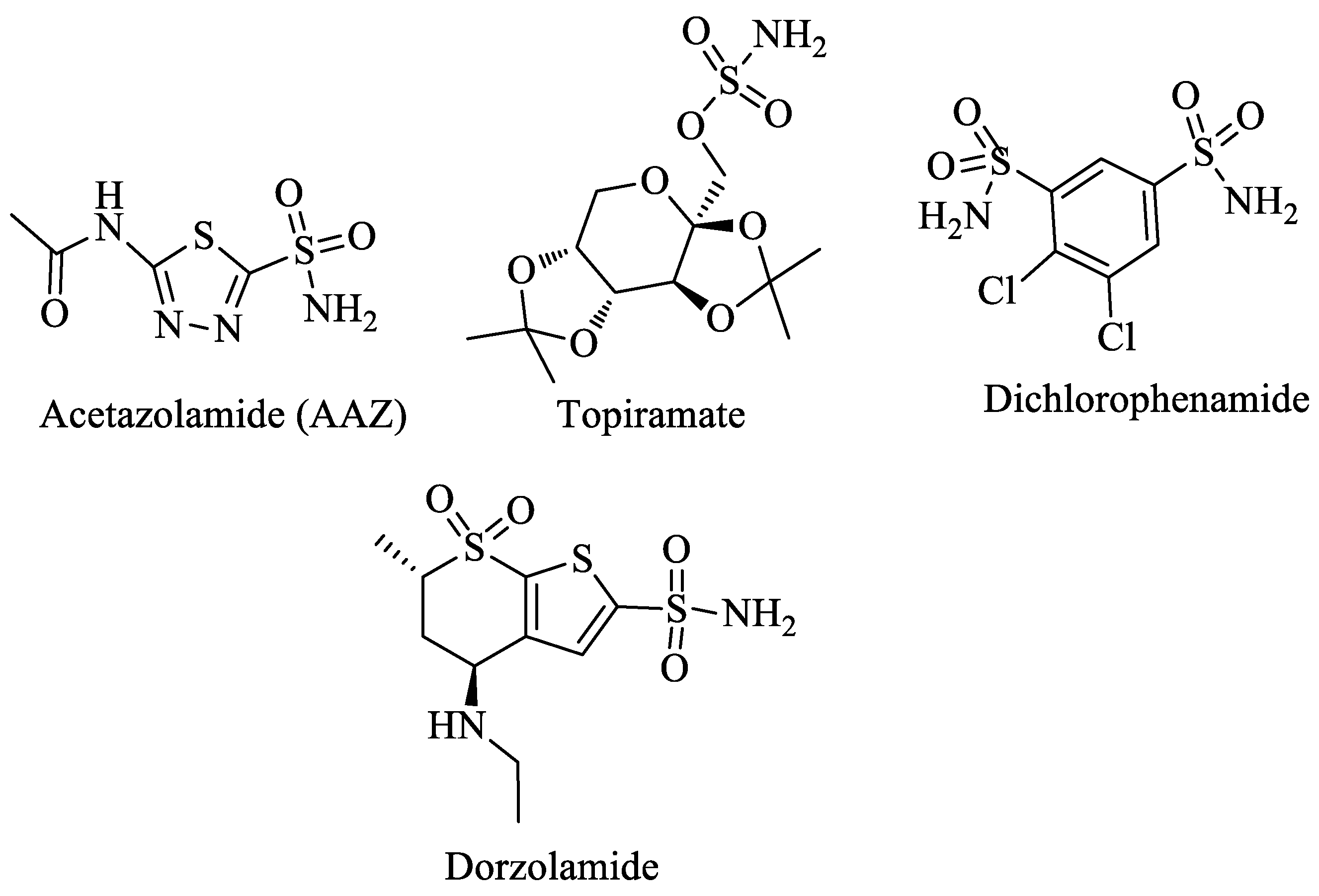
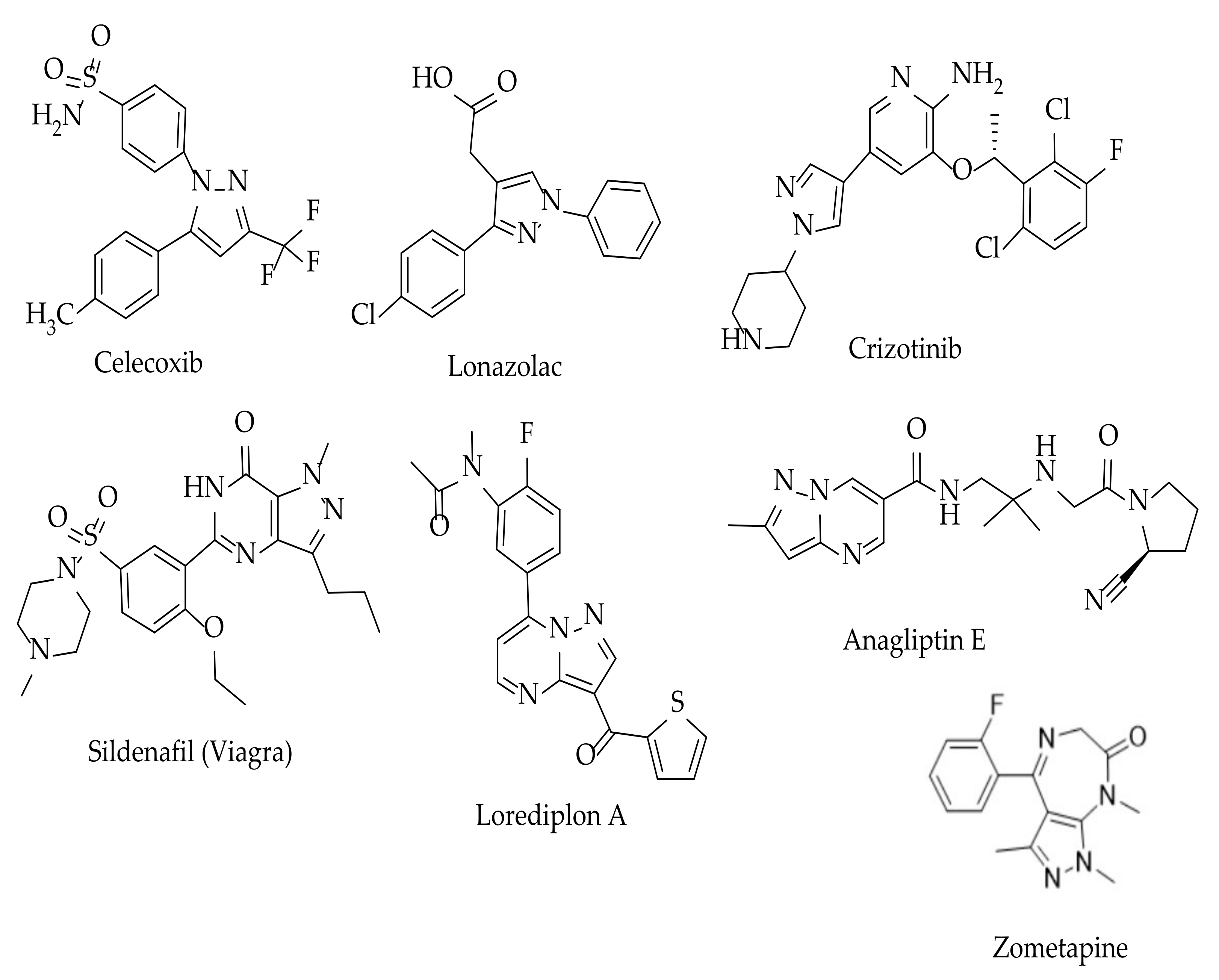
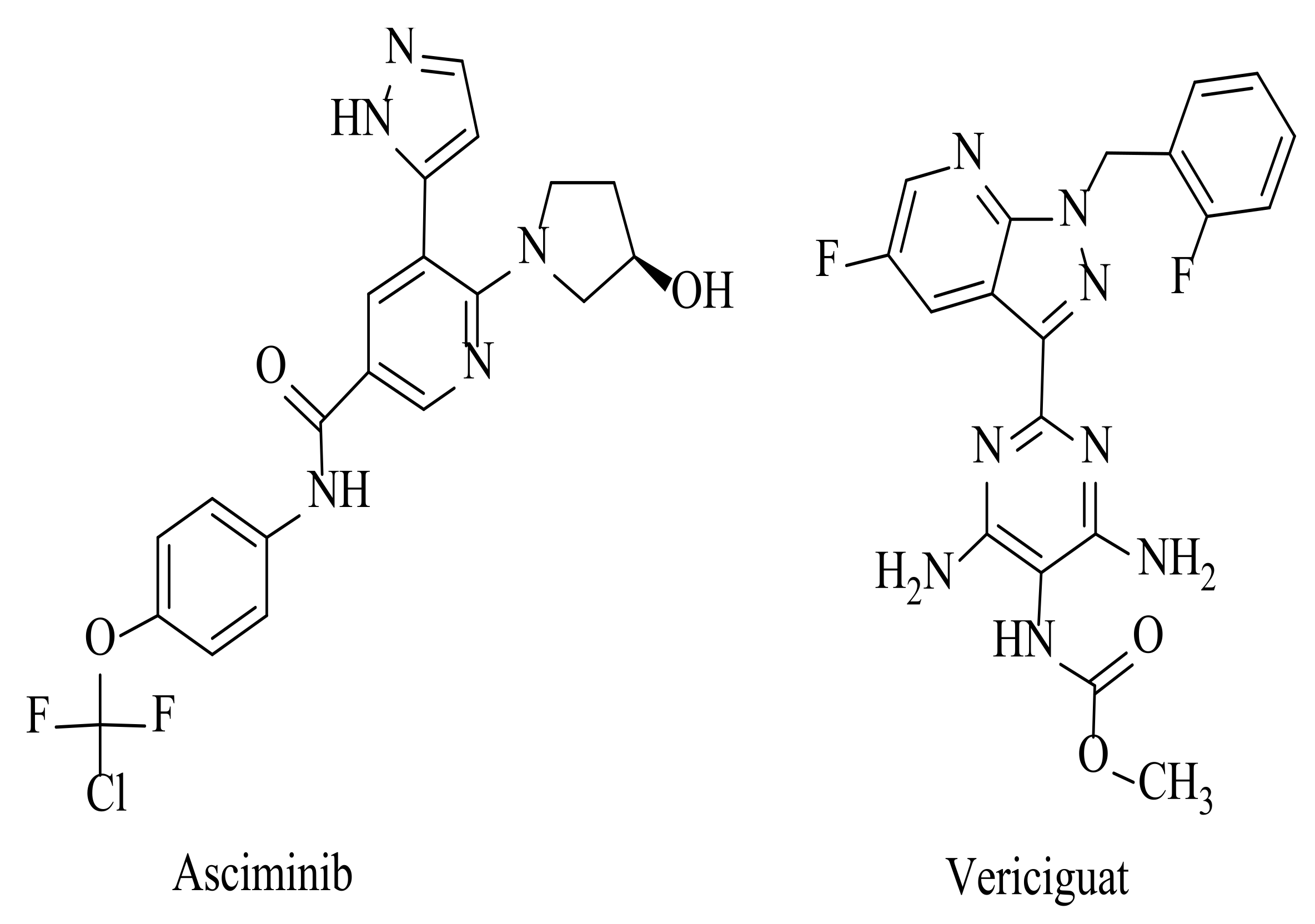
1. Introduction
2. Results and Discussion
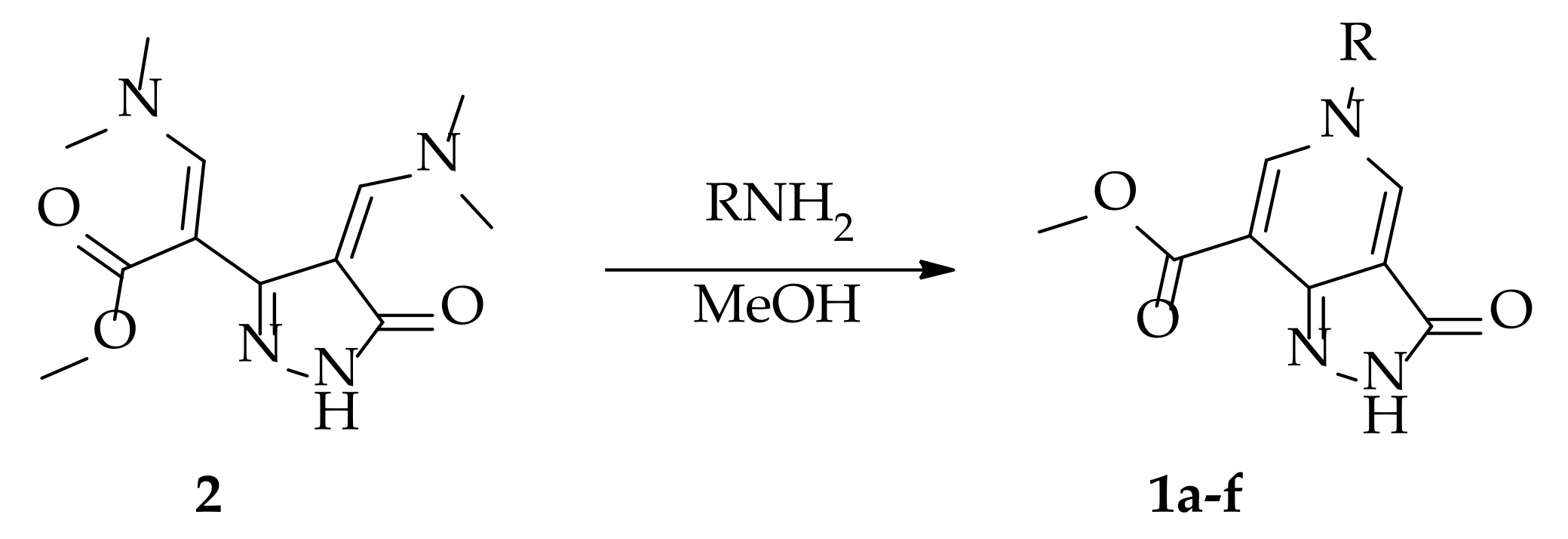
2.1. Chemistry
2.2. Carbonic Anhydrase Inhibition
2.3. Molecular Docking Studies
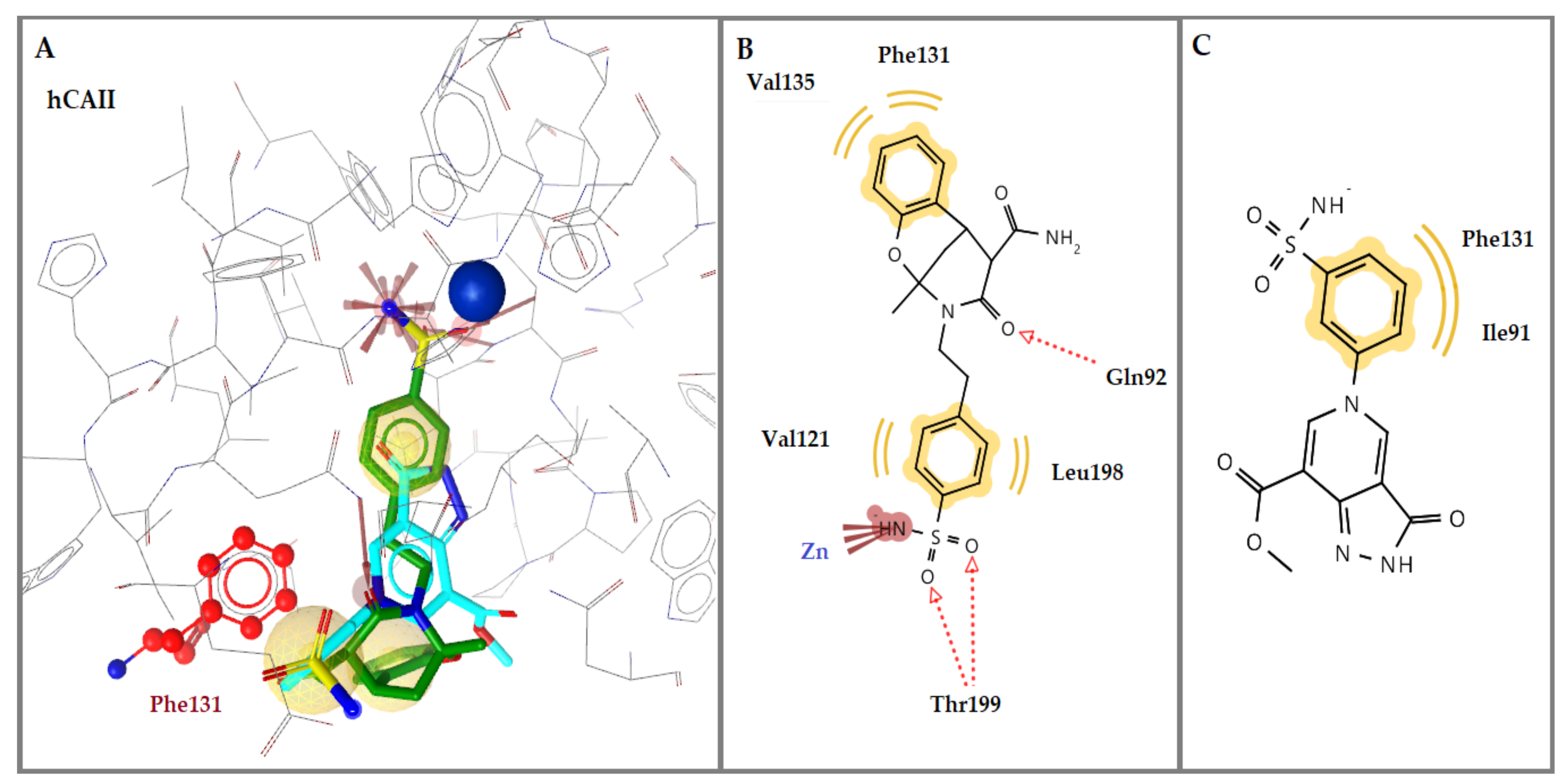
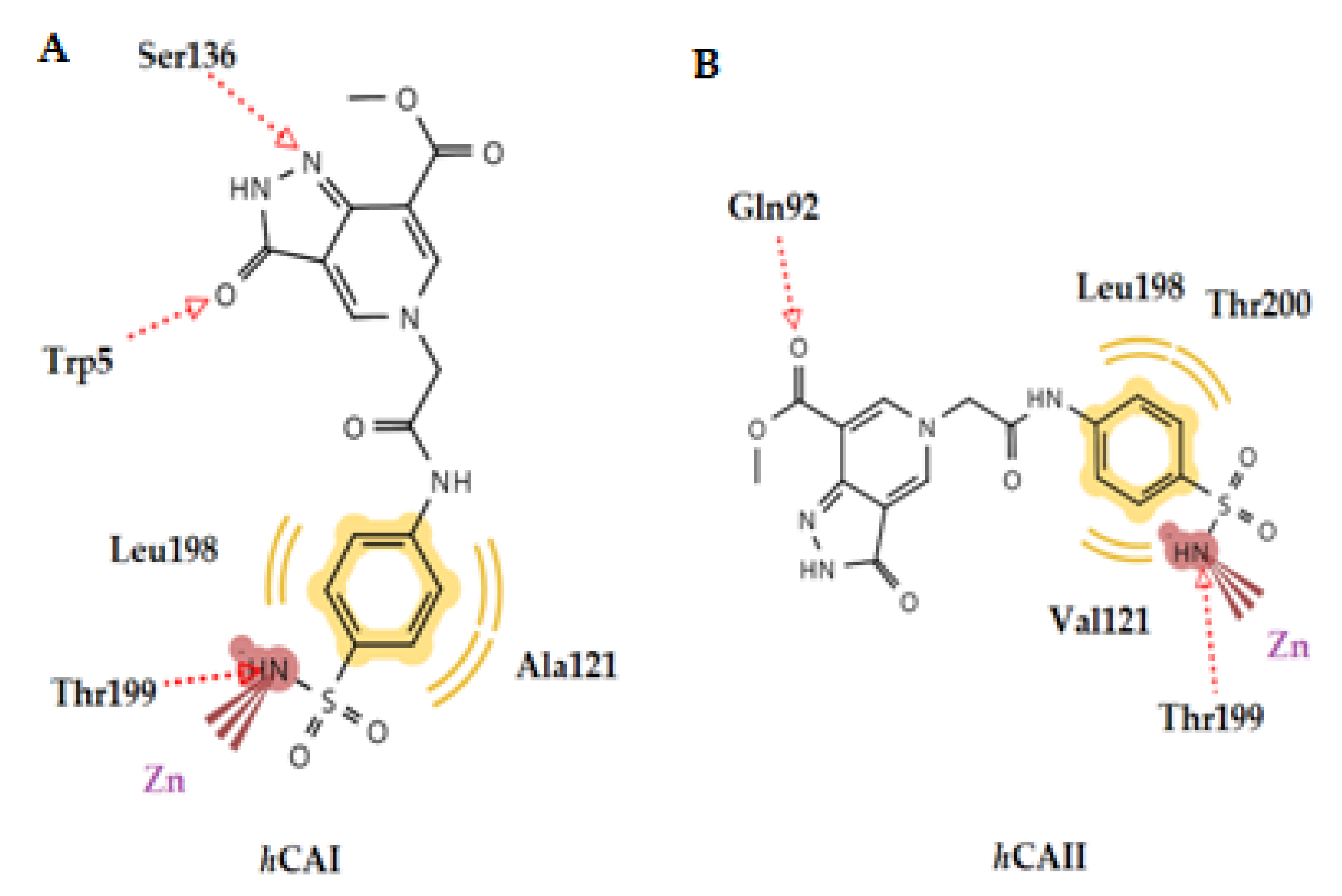
2.3.1. Molecular Docking Studies in Human CA Isoforms
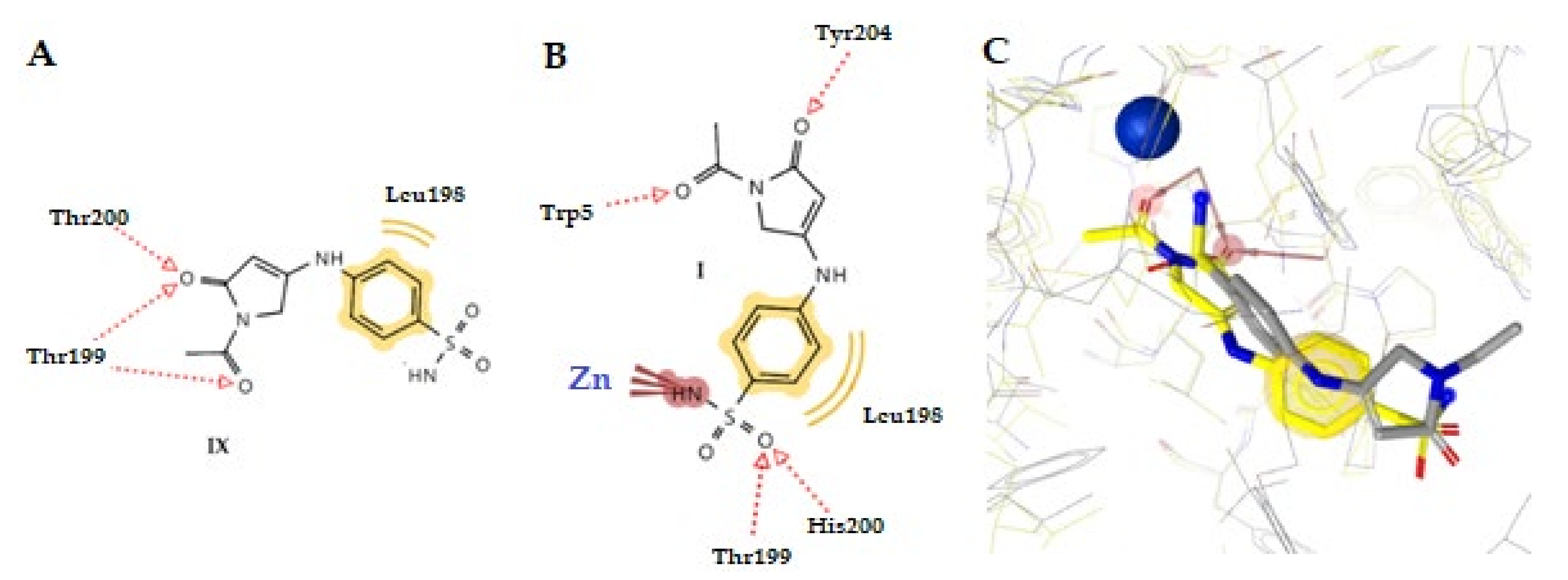
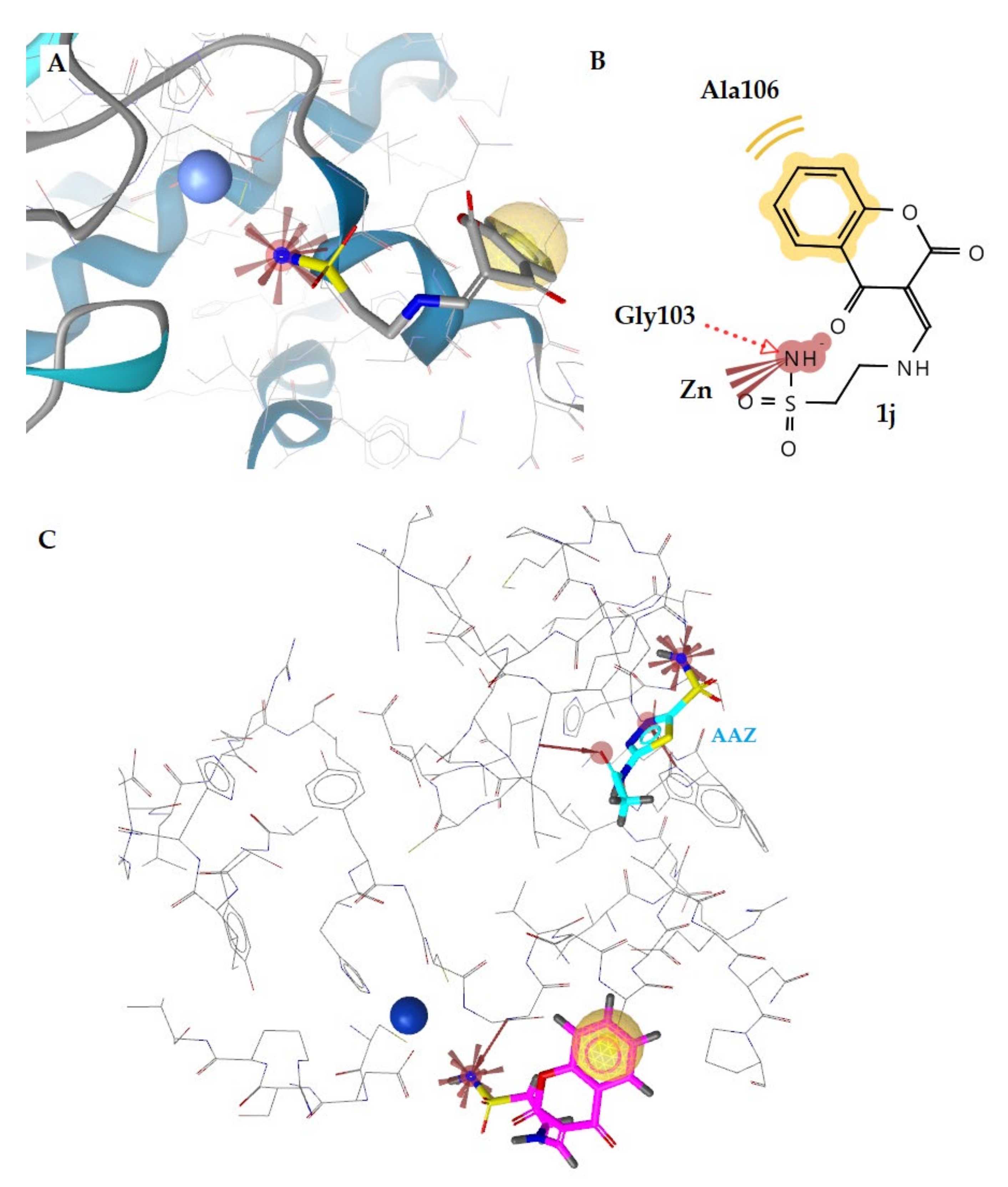
2.3.2. Molecular Docking Studies in β- and γ-CA Classes
2.4. In Silico Prediction Studies
Drug-Likeness
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of 5-Substituted Methyl 3-Oxo-3,5-dihydro-2H-pyrazolo[4,3-c]pyridine-7-carboxylates 1a–f (General Procedure)
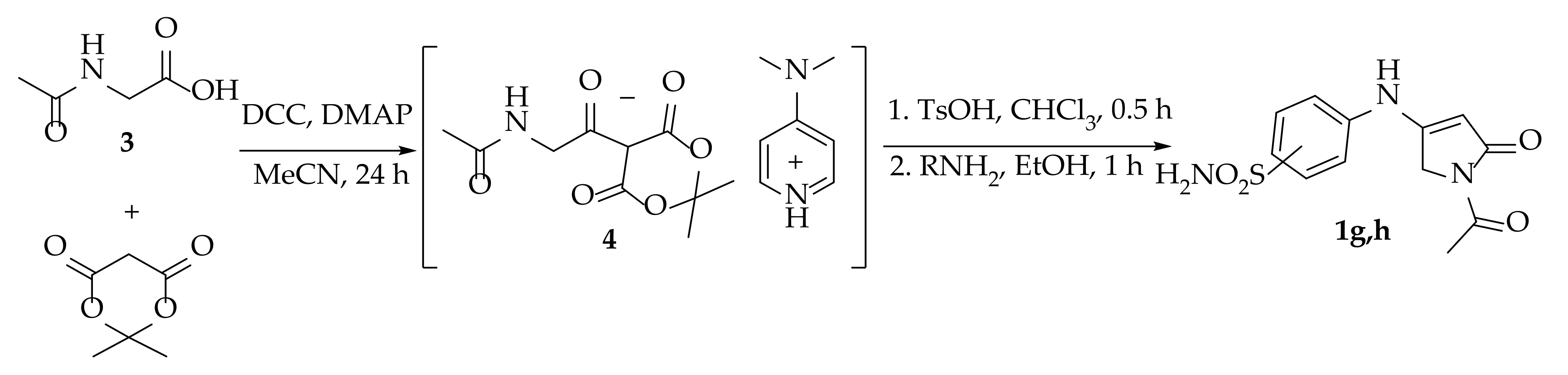
3.1.2. Synthesis of 1-Acetyl-4-(arylamino)-1,5-dihydro-2H-pyrrol-2-ones 1g,h (General Procedure)
3.1.3. Synthesis of 2-(4-Hydroxy-6-methyl-2-oxopyridin-1(2H)-yl)ethane-1-sulfonamide 1i
3.1.4. Synthesis of 2-(((2,4-Dioxochroman-3-ylidene)methyl)amino)ethane-1-sulfonamide 1j
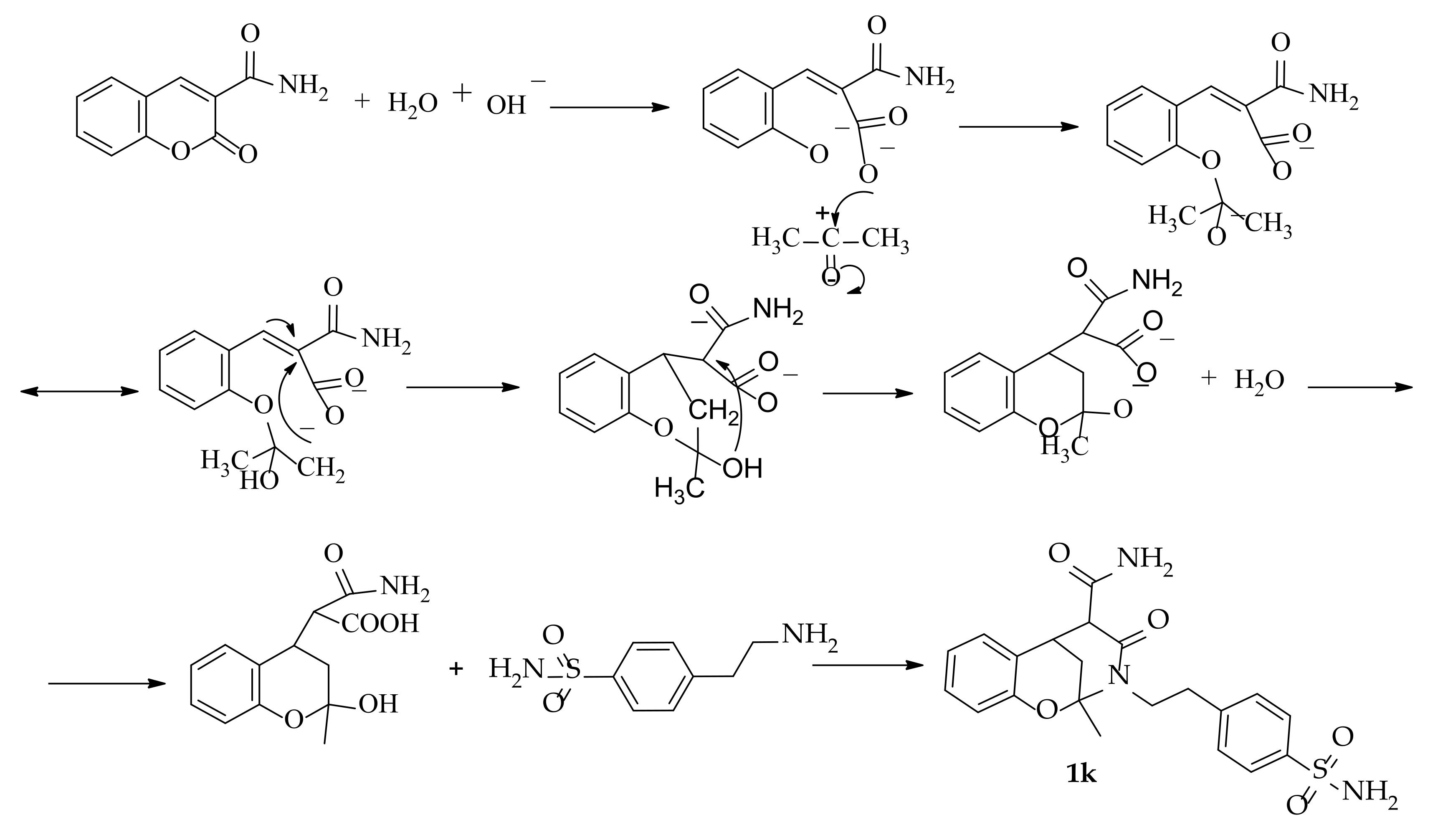
3.1.5. Synthesis of 2-Methyl-4-oxo-3-(4-sulfamoylphenethyl)-3,4,5,6-tetrahydro-2H-2,6-methanobenzo[g] [1,3]oxazocine-5-carboxamide 1k
3.2. Molecular Docking Studies
3.3. CA Inhibition Assay
3.4. Drug-Likness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Supuran, C.T.; Capasso, C. An Overview of the Bacterial Carbonic Anhydrases. Metabolites 2017, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Capasso, C. Antibacterial carbonic anhydrase inhibitors: An update on the recent literature. Expert Opin. Ther. Pat. 2020, 30, 963–982. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Emerging role of carbonic anhydrase inhibitors. Clin. Sci. 2021, 135, 1233–1249. [Google Scholar] [CrossRef]
- Alterio, V.; Di Fiore, A.; D’Ambrosio, K.; Supuran, C.T.; De Simone, G. Multiple binding modes of inhibitors to carbonic anhydrases: How to design specific drugs targeting 15 different isoforms? Chem. Rev. 2012, 112, 4421–4468. [Google Scholar] [CrossRef]
- Penning, T.D.; Talley, J.J.; Bertenshaw, S.R.; Carter, J.S.; Collins, P.W.; Docter, S.; Graneto, M.J.; Lee, L.F.; Malecha, J.W.; Miyashiro, J.M.; et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: Identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib). J. Med. Chem. 1997, 40, 1347–1365. [Google Scholar] [CrossRef]
- Fioravanti, R.; Bolasco, A.; Manna, F.; Rossi, F.; Orallo, F.; Ortuso, F.; Alcaro, S.; Cirilli, R. Synthesis and biological evaluation of N-substituted-3,5-diphenyl-2-pyrazoline derivatives as cyclooxygenase (COX-2) inhibitors. Eur. J. Med. Chem. 2010, 45, 6135–6138. [Google Scholar] [CrossRef]
- Devi, N.; Shankar, R.; Singh, V. 4-Formyl-Pyrazole-3-Carboxylate: A Useful Aldo-X Bifunctional Precursor for the Syntheses of Pyrazole-fused/Substituted Frameworks. J. Heterocycl. Chem. 2018, 55, 373–390. [Google Scholar] [CrossRef]
- Katz, R. Effects of zometapine, A structurally novel antidepressant, in an animal model of depression. Pharmacol. Biochem. Behav. 1984, 21, 487–490. [Google Scholar] [CrossRef]
- d’Aniello, F.; Santos, B.; Guglietta, A. Lorediplon: A New GABAA Modulator Drug for Treatment of Insomnia. Drug Treat. Sleep Disord. 2015, 49, 121–145. [Google Scholar] [CrossRef]
- Ervinna, N.; Mita, T.; Yasunari, E.; Azuma, K.; Tanaka, R.; Fujimura, S.; Sukmawati, D.; Nomiyama, T.; Kanazawa, A.; Kawamori, R.; et al. Anagliptin, a DPP-4 Inhibitor, Suppresses Proliferation of Vascular Smooth Muscles and Monocyte Inflammatory Reaction and Attenuates Atherosclerosis in Male apo E-Deficient Mice. Endocrinology 2013, 154, 1260–1270. [Google Scholar] [CrossRef]
- Marinescu, M. Synthesis of Antimicrobial Benzimidazole–Pyrazole Compounds and Their Biological Activities. Antibiotics 2021, 10, 1002. [Google Scholar] [CrossRef]
- Cetin, A.; Bildirici, I. A study on synthesis and antimicrobial activity of 4-acyl-pyrazoles. J. Saudi Chem. Soc. 2018, 22, 279–296. [Google Scholar] [CrossRef]
- Muhammad, Z.A.; Alshehrei, F.; Zayed, M.E.M.; Farghaly, T.A.; Abdallah, M.A. Synthesis of Novel Bis-pyrazole Derivatives as Antimicrobial Agents. Mini Rev. Med. Chem. 2019, 19, 1276–1290. [Google Scholar] [CrossRef]
- Da Costa, L.; Scheers, E.; Coluccia, A.; Casulli, A.; Roche, M.; Di Giorgio, C.; Neyts, J.; Terme, T.; Cirilli, R.; La Regina, G.; et al. Structure-Based Drug Design of Potent Pyrazole Derivatives against Rhinovirus Replication. J. Med. Chem. 2018, 61, 8402–8416. [Google Scholar] [CrossRef]
- Corona, A.; Onnis, V.; Deplano, A.; Bianco, G.; Demurtas, M.; Distinto, S.; Cheng, Y.-C.; Alcaro, S.; Esposito, F.; Tramontano, E. Design, synthesis and antiviral evaluation of novel heteroarylcarbothioamide derivatives as dual inhibitors of HIV-1 reverse transcriptase-associated RNase H and RDDP functions. Pathog. Dis. 2017, 75, ftx078. [Google Scholar] [CrossRef]
- Al-Zharani, M.; Al-Eissa, M.S.; Rudayni, H.A.; Ali, D.; Alkahtani, S.; Surendrakumar, R.; Idhayadhulla, A. Pyrazolo[3,4-b]pyridin-3(2H)-one derivatives: Synthesis and their investigation of mosquito larvicidal activity. J. King Saud Univ. Sci. 2022, 34, 101767. [Google Scholar] [CrossRef]
- Naim, M.J.; Alam, O.; Alam, M.J.; Hassan, M.Q.; Siddiqui, N.; Naidu, V.G.M.; Alam, M.I. Design, synthesis and molecular docking of thiazolidinedione based benzene sulphonamide derivatives containing pyrazole core as potential anti-diabetic agents. Bioorg. Chem. 2018, 76, 98–112. [Google Scholar] [CrossRef]
- Faidallah, H.M.; Al-Mohammadi, M.M.; Alamry, K.A.; Khan, K.A. Synthesis and biological evaluation of fluoropyrazolesulfonylurea and thiourea derivatives as possible antidiabetic agents. J. Enzyme Inhib. Med. Chem. 2016, 31, 157–163. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Tu, Z. Pyrazole Scaffold Synthesis, Functionalization, and Applications in Alzheimer’s Disease and Parkinson’s Disease Treatment (2011–2020). Molecules 2021, 26, 1202. [Google Scholar] [CrossRef]
- Lather, V.; Grewal, A.S.; Sharma, S.K.; Pandita, D. Synthesis, Docking and Evaluation of Novel Pyrazole Carboxamide Derivatives as Multifunctional Anti-Alzheimer’s Agents. J. Med. Chem. Toxicol. 2017, 2, 47–54. [Google Scholar] [CrossRef]
- Meta, E.; Brullo, C.; Tonelli, M.; Franzblau, S.G.; Wang, Y.; Ma, R.; Baojie, W.; Orena, B.S.; Pasca, M.R.; Bruno, O. Pyrazole and imidazo[1,2-b]pyrazole Derivatives as New Potential Antituberculosis Agents. Med. Chem. 2019, 15, 17–27. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C.; Ren, Q.-C.; Song, X.-F.; Feng, L.-S.; Lv, Z.-S. Recent advances of pyrazole-containing derivatives as anti-tubercular agents. Eur. J. Med. Chem. 2017, 139, 429–440. [Google Scholar] [CrossRef]
- Bekhit, A.A.; Saudi, M.N.; Hassan, A.M.; Fahmy, S.M.; Ibrahim, T.M.; Ghareeb, D.; El-Seidy, A.M.; Al-Qallaf, S.M.; Habib, H.J.; Bekhit, A.E.A.-D. Synthesis, molecular modeling and biological screening of some pyrazole derivatives as antileishmanial agents. Future Med. Chem. 2018, 10, 2325–2344. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, F.; Naureen, S.; Choudhry, S.; Huma, R.; Ashraf, M.; Al-Rashida, M.; Jahan, B.; Khan, M.H.; Iqbal, F.; Munawar, M.A.; et al. Evaluation of α-glucosidase inhibiting potentials with docking calculations of synthesized arylidene-pyrazolones. Bioorg. Chem. 2018, 77, 507–514. [Google Scholar] [CrossRef] [PubMed]
- El-Gohary, N.; Shaaban, M. New pyrazolopyridine analogs: Synthesis, antimicrobial, antiquorum-sensing and antitumor screening. Eur. J. Med. Chem. 2018, 152, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.S.; Ali, M.A.M. Novel Pyrazolo[3,4-b]pyridine Derivatives: Synthesis, Characterization, Antimicrobial and Antiproliferative Profile. Biol. Pharm. Bull. 2016, 39, 473–483. [Google Scholar] [CrossRef] [PubMed]
- El-Borai, M.A.; Rizk, H.F.; Beltagy, D.M.; El-Deeb, I.Y. Microwave-assisted synthesis of some new pyrazolopyridines and their antioxidant, antitumor and antimicrobial activities. Eur. J. Med. Chem. 2013, 66, 415–422. [Google Scholar] [CrossRef]
- Bare, T.M.; McLaren, C.D.; Campbell, J.B.; Firor, J.W.; Resch, J.F.; Walters, C.P.; Salama, A.I.; Meiners, B.A.; Patel, J.B. Synthesis and structure-activity relationships of a series of anxioselective pyrazolopyridine ester and amide anxiolytic agents. J. Med. Chem. 1989, 32, 2561–2573. [Google Scholar] [CrossRef]
- Gu, X.; Ma, S. Recent Advances in the Development of Pyrazolopyridines as Anticancer Agents. Anti Cancer Agents Med. Chem. 2021. [Google Scholar] [CrossRef]
- Mor, S.; Khatri, M.; Punia, R.; Sindhu, S. Recent Progress in Anticancer Agents Incorporating Pyrazole Scaffold. Mini Rev. Med. Chem. 2022, 22, 115–163. [Google Scholar] [CrossRef]
- Gavriil, E.-S.; Lougiakis, N.; Pouli, N.; Marakos, P.; Skaltsounis, A.-L.; Nam, S.; Jove, R.; Horne, D.; Gioti, K.; Pratsinis, H.; et al. Synthesis and antiproliferative activity of new pyrazolo[3,4-c]pyridines. Med. Chem. 2017, 13, 365–374. [Google Scholar] [CrossRef]
- Giannouli, V.; Lougiakis, N.; Kostakis, I.K.; Pouli, N.; Marakos, P.; Skaltsounis, A.-L.; Nam, S.; Jove, R.; Horne, D.; Tenta, R.; et al. The discovery of new cytotoxic pyrazolopyridine derivatives. Bioorganic Med. Chem. Lett. 2016, 26, 5229–5233. [Google Scholar] [CrossRef]
- Anand, D.; Yadav, P.K.; Patel, O.P.S.; Parmar, N.; Maurya, R.K.; Vishwakarma, P.; Raju, K.S.R.; Taneja, I.; Wahajuddin, M.; Kar, S.; et al. Antileishmanial Activity of Pyrazolopyridine Derivatives and Their Potential as an Adjunct Therapy with Miltefosine. J. Med. Chem. 2017, 60, 1041–1059. [Google Scholar] [CrossRef]
- de Mello, H.; Echevarria, A.; Bernardino, A.M.R.; Canto-Cavalheiro, A.M.; Leon, L. Antileishmanial Pyrazolopyridine Derivatives: Synthesis and Structure−Activity Relationship Analysis. J. Med. Chem. 2004, 47, 5427–5432. [Google Scholar] [CrossRef]
- Pinheiro, L.C.S.; Feitosa, L.M.; Gandi, M.O.; Silveira, F.F.; Boechat, N. The Development of Novel Compounds Against Malaria: Quinolines, Triazolpyridines, Pyrazolopyridines and Pyrazolopyrimidines. Molecules 2019, 24, 4095. [Google Scholar] [CrossRef]
- Hamblin, J.N.; Angell, T.D.; Ballantine, S.P.; Cook, C.M.; Cooper, A.W.; Dawson, J.; Delves, C.J.; Jones, P.S.; Lindvall, M.; Lucas, F.S.; et al. Pyrazolopyridines as a novel structural class of potent and selective PDE4 inhibitors. Bioorganic Med. Chem. Lett. 2008, 18, 4237–4241. [Google Scholar] [CrossRef]
- Sklepari, M.; Lougiakis, N.; Papastathopoulos, A.; Pouli, N.; Marakos, P.; Myrianthopoulos, V.; Robert, T.; Bach, S.; Mikros, E.; Ruchaud, S. Synthesis, Docking Study and Kinase Inhibitory Activity of a Number of New Substituted Pyrazolo[3,4-c]pyridines. Chem. Pharm. Bull. 2017, 65, 66–81. [Google Scholar] [CrossRef]
- Michailidou, M.; Giannouli, V.; Kotsikoris, V.; Papadodima, O.; Kontogianni, G.; Kostakis, I.K.; Lougiakis, N.; Chatziioannou, A.; Kolisis, F.N.; Marakos, P.; et al. Novel pyrazolopyridine derivatives as potential angiogenesis inhibitors: Synthesis, biological evaluation and transcriptome-based mechanistic analysis. Eur. J. Med. Chem. 2016, 121, 143–157. [Google Scholar] [CrossRef]
- Abbas, H.-A.; El-Karim, S.A.; Abdelwahed, N.A.M. Synthesis and biological evaluation of sulfonamide derivatives as antimicrobial agents. Acta Pol. Pharm. Drug Res. 2017, 74, 849–860. [Google Scholar]
- Shahzad, S.; Qadir, M.A.; Ahmed, M.; Ahmad, S.; Khan, M.J.; Gulzar, A.; Muddassar, M. Folic acid-sulfonamide conjugates as antibacterial agents: Design, synthesis and molecular docking studies. RSC Adv. 2020, 10, 42983–42992. [Google Scholar] [CrossRef]
- Akili, A.; Hadda, D.; Bitar, Y.; Balash, A.; Chehna, A. Design, Synthesis and Characterization of Novel Sulfonamides Derivatives as Anticancer Agent Targeting EGFR TK, and Development of New Methods of Synthesis by Microwave Irradiation. Int. J. Org. Chem. 2021, 11, 199–223. [Google Scholar] [CrossRef]
- Pingaew, R.; Mandi, P.; Prachayasittikul, V.; Thongnum, A.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Investigations on Anticancer and Antimalarial Activities of Indole-Sulfonamide Derivatives and In Silico Studies. ACS Omega 2021, 6, 31854–31868. [Google Scholar] [CrossRef]
- Alshibl, H.M.; Al-Abdullah, E.S.; Haiba, M.E.; Alkahtani, H.M.; Awad, G.E.; Mahmoud, A.H.; Ibrahim, B.M.; Bari, A.; Villinger, A. Synthesis and Evaluation of New Coumarin Derivatives as Antioxidant, Antimicrobial, and Anti-Inflammatory Agents. Molecules 2020, 25, 3251. [Google Scholar] [CrossRef]
- Akgul, O.; Di Cesare Mannelli, L.; Vullo, D.; Angeli, A.; Ghelardini, C.; Bartolucci, G.; Altamimi, A.S.A.; Scozzafava, A.; Supuran, C.T.; Carta, F. Discovery of Novel Nonsteroidal Anti-Inflammatory Drugs and Carbonic Anhydrase Inhibitors Hybrids (NSAIDs–CAIs) for the Management of Rheumatoid Arthritis. J. Med. Chem. 2018, 61, 4961–4977. [Google Scholar] [CrossRef]
- Salve, M.; Jadhav, S., Sr. Synthesis, Characterization and Antidiabetic Evaluation of Sulfonamide in Corporated with 1,3,4-Oxadiazole Derivatives. IJPER 2021, 55, 1145–1150. [Google Scholar] [CrossRef]
- Azzam, R.A.; Elsayed, R.E.; Elgemeie, G.H. Design, Synthesis, and Antimicrobial Evaluation of a New Series of N-Sulfonamide 2-Pyridones as Dual Inhibitors of DHPS and DHFR Enzymes. ACS Omega 2020, 5, 10401–10414. [Google Scholar] [CrossRef] [PubMed]
- Gokcen, T.; Gulcin, I.; Ozturk, T.; Goren, A.C. A class of sulfonamides as carbonic anhydrase I and II inhibitors. J. Enzyme Inhib. Med. Chem. 2016, 31, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Giovannuzzi, S.; D’Ambrosio, M.; Luceri, S.; Osman, S.M.; Pallecchi, M.; Bartolucci, G.; Nocentini, A.; Supuran, C.T. Aromatic Sulfonamides including a Sulfonic Acid Tail: New Membrane Impermeant Carbonic Anhydrase Inhibitors for Targeting Selectively the Cancer-Associated Isoforms. Int. J. Mol. Sci. 2022, 23, 461. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, A.; Nocentini, A.; Bua, S.; Combs, J.; Lomelino, C.; Andring, J.; Lucarini, L.; Sgambellone, S.; Masini, E.; McKenna, R.; et al. Sulfonamide Inhibitors of Human Carbonic Anhydrases Designed through a Three-Tails Approach: Improving Ligand/Isoform Matching and Selectivity of Action. J. Med. Chem. 2020, 63, 7422–7444. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Kartsev, V.; Petrou, A.; Pinteala, M.; Brovarets, V.; Vydzhak, R.; Panchishin, S.; Geronikaki, A.; Supuran, C.T. Carbonic Anhydrase Inhibition with Sulfonamides Incorporating Pyrazole- and Pyridazinecarboxamide Moieties Provides Examples of Isoform-Selective Inhibitors. Molecules 2021, 26, 7023. [Google Scholar] [CrossRef]
- Angeli, A.; Kartsev, V.; Petrou, A.; Pinteala, M.; Vydzhak, R.M.; Panchishin, S.Y.; Brovarets, V.; De Luca, V.; Capasso, C.; Geronikaki, A.; et al. New Sulfanilamide Derivatives Incorporating Heterocyclic Carboxamide Moieties as Carbonic Anhydrase Inhibitors. Pharmaceuticals 2021, 14, 828. [Google Scholar] [CrossRef]
- Angeli, A.; Kartsev, V.; Petrou, A.; Pinteala, M.; Brovarets, V.; Slyvchuk, S.; Pilyo, S.; Geronikaki, A.; Supuran, C. Chromene-Containing Aromatic Sulfonamides with Carbonic Anhydrase Inhibitory Properties. Int. J. Mol. Sci. 2021, 22, 5082. [Google Scholar] [CrossRef]
- Bevk, D.; Svete, J.; Stanovnik, B. Synthesis of 2-unsubstituted 2,3,5,6,7,8-Hexahydropyrazolo[4,3-d][1,2]diazepinone-8-carboxylates. Heterocycles 2007, 71, 657–668. [Google Scholar] [CrossRef]
- Hamilakis, S.; Kontonassios, D.; Sandris, C. Acylaminoacetyl derivatives of active methylene compounds. 3.C-Acylation Reactionsviathe Hippuric Acid Azlactone. J. Heterocycl. Chem. 1994, 31, 1145–1150. [Google Scholar] [CrossRef]
- Hiersemann, M.; Tymann, D.; Tymann, D.C.; Benedix, L.; Iovkova, L.; Pallach, R.; Henke, S. Photochemical Approach to the Cyclohepta[b]indole Scaffold by Annulative Two-Carbon Ring-Expansion. Chem. A Eur. J. 2020, 26, 11974–11978. [Google Scholar] [CrossRef]
- Kraus, G.A.; Wanninayake, U.K.; Bottoms, J. Triacetic acid lactone as a common intermediate for the synthesis of 4-hydroxy-2-pyridones and 4-amino-2-pyrones. Tetrahedron Lett. 2016, 57, 1293–1295. [Google Scholar] [CrossRef][Green Version]
- Hamdi, M.; Granier, P.; Sakellariou, R.; Spéziale, V. Reaction of amines on 3-ureidomethylenecoumarins. A new route to N-(methylene-4-oxocoumarinyl)amines. J. Heterocycl. Chem. 1993, 30, 1155–1157. [Google Scholar] [CrossRef]
- O’Callaghan, C.N.; Mcmurry, T.B.H.; O’Brien, J.E. The Formation of Polyheterocyclic Systems by the Reaction of 2-Oxo-2H- 1-benzopyran-3-carboxamide and Related Compounds with Active Methylene Compounds. J. Chem. Res. 1995, 12, 3001–3017. [Google Scholar] [CrossRef]
- Alterio, V.; Hilvo, M.; Di Fiore, A.; Supuran, C.T.; Pan, P.; Parkkila, S.; Scaloni, A.; Pastorek, J.; Pastorekova, S.; Scozzafava, A.; et al. Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc. Natl. Acad. Sci. USA 2009, 106, 16233–16238. [Google Scholar] [CrossRef]
- Di Fiore, A.; Truppo, E.; Supuran, C.T.; Alterio, V.; Dathan, N.; Bootorabi, F.; Parkkila, S.; Monti, S.M.; De Simone, G. Crystal structure of the C183S/C217S mutant of human CA VII in complex with acetazolamide. Bioorgan. Med. Chem. Lett. 2010, 20, 5023–5026. [Google Scholar] [CrossRef]
- Whittington, D.A.; Waheed, A.; Ulmasov, B.; Shah, G.N.; Grubb, J.H.; Sly, W.S.; Christianson, D.W. Crystal structure of the dimeric extracellular domain of human carbonic anhydrase XII, a bitopic membrane protein overexpressed in certain cancer tumor cells. Proc. Natl. Acad. Sci. USA 2001, 98, 9545–9550. [Google Scholar] [CrossRef]
- Supuran, C.T. Structure-based drug discovery of carbonic anhydrase inhibitors. J. Enzyme Inhib. Med. Chem. 2012, 27, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. An Overview of the Carbonic Anhydrases from Two Pathogens of the Oral Cavity: Streptococcus mutans and Porphyromonas gingivalis. Curr. Top. Med. Chem. 2016, 16, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. Sulfa and trimethoprim-like drugs-antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J. Enzyme Inhib. Med. Chem. 2014, 29, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Pinard, M.A.; Lotlikar, S.R.; Boone, C.D.; Vullo, D.; Supuran, C.T.; Patrauchan, M.A.; McKenna, R. Structure and inhibition studies of a type II beta-carbonic anhydrase psCA3 from Pseudomonas aeruginosa. Bioorgan. Med. Chem. 2015, 23, 4831–4838. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Isik, S.; Del Prete, S.; De Luca, V.; Carginale, V.; Scozzafava, A.; Supuran, C.T.; Capasso, C. Anion inhibition studies of the α-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Bioorgan. Med. Chem. Lett. 2013, 23, 1636–1638. [Google Scholar] [CrossRef] [PubMed]
- Iverson, T.M.; Alber, B.E.; Kisker, C.; Ferry, J.G.; Rees, D.C. A closer look at the active site of gamma-class carbonic anhydrases: High-resolution crystallographic studies of the carbonic anhydrase from Methanosarcina thermophila. Biochemistry 2000, 39, 9222–9231. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- A Structural View of Biology. Available online: http://www.rcsb.org/ (accessed on 30 May 2020).
- Stefanucci, A.; Angeli, A.; Dimmito, M.P.; Luisi, G.; Del Prete, S.; Capasso, C.; Donald, W.A.; Mollica, A.; Supuran, C.T. Activation of β- and γ-carbonic anhydrases from pathogenic bacteria with tripeptides. J. Enzyme Inhib. Med. Chem. 2018, 33, 945–950. [Google Scholar] [CrossRef]
- Angeli, A.; Mannelli, L.d.C.; Lucarini, E.; Peat, T.S.; Ghelardini, C.; Supuran, C.T. Design, synthesis and X-ray crystallography of selenides bearing benzenesulfonamide moiety with neuropathic pain modulating effects. Eur. J. Med. Chem. 2018, 154, 210–219. [Google Scholar] [CrossRef]
- Angeli, A.; Pinteala, M.; Maier, S.S.; Simionescu, B.C.; Milaneschi, A.; Abbas, G.; Del Prete, S.; Capasso, C.; Capperucci, A.; Tanini, D.; et al. Evaluation of Thio- and Seleno-Acetamides Bearing Benzenesulfonamide as Inhibitor of Carbonic Anhydrases from Different Pathogenic Bacteria. Int. J. Mol. Sci. 2020, 21, 598. [Google Scholar] [CrossRef]

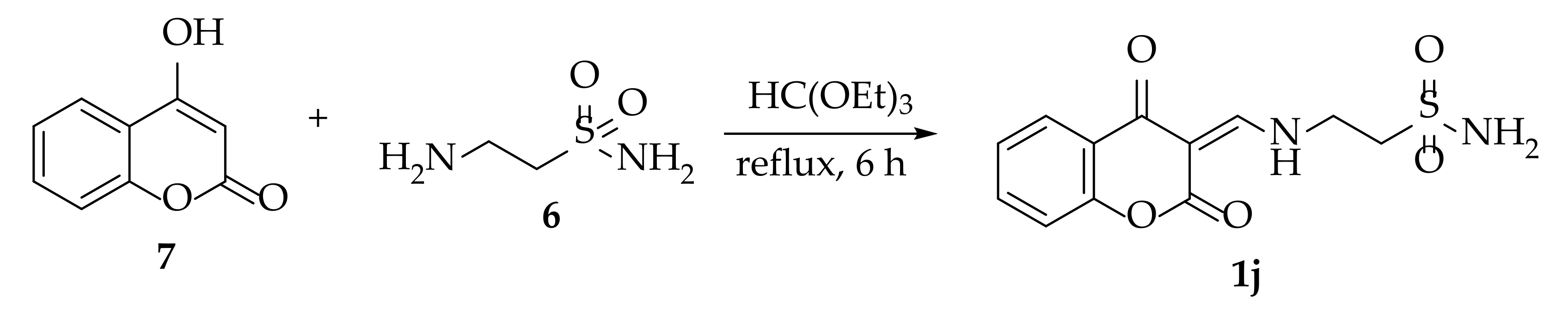
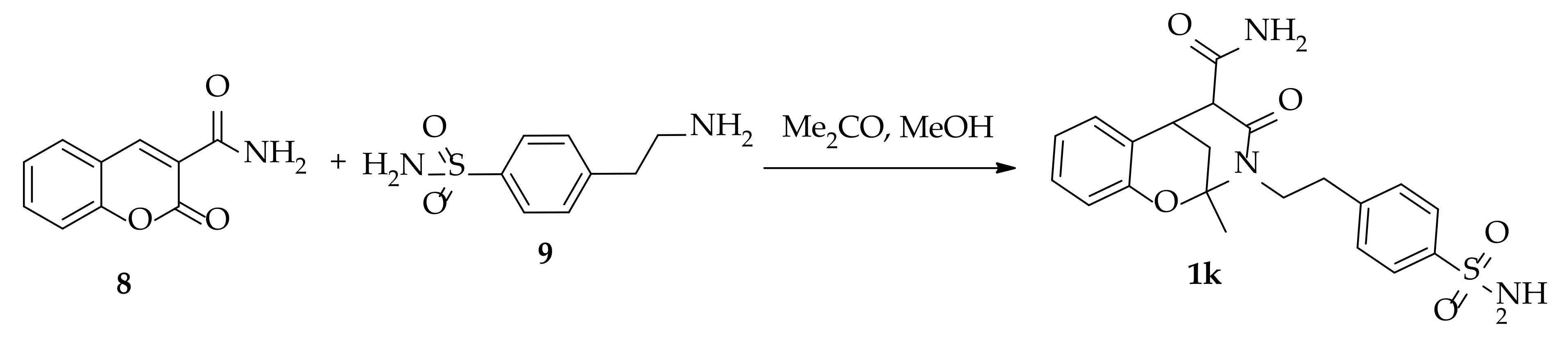


| Compound | Structure | Compound | Structure |
|---|---|---|---|
| 1a | 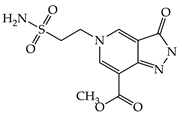 | 1g | 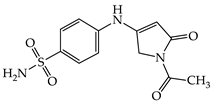 |
| 1b | 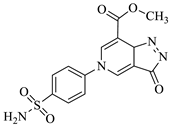 | 1h | 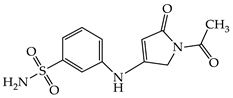 |
| 1c | 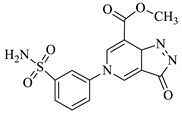 | 1i | 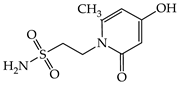 |
| 1d | 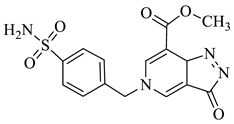 | 1j |  |
| 1e | 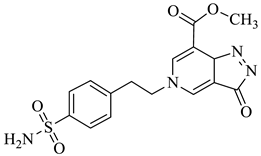 | 1k | 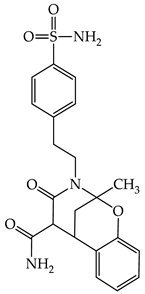 |
| 1f | 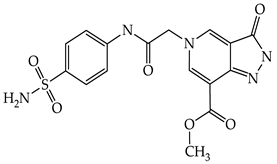 |
| KI (nM) * | ||||
|---|---|---|---|---|
| Cmp | hCA I | hCAII | hCA IX | hCA XII |
| 1a | 8010 | 7329 | 97.9 | 282.3 |
| 1b | 156.8 | 51.4 | 319.1 | 358.2 |
| 1c | 1443 | 247.4 | 589.5 | 143.2 |
| 1d | 847.7 | 779.3 | 644.7 | 262.4 |
| 1e | 864.2 | 658.3 | 848.8 | 397.4 |
| 1f | 58.8 | 6.6 | 907.5 | 474.8 |
| 1g | 66.8 | 41.7 | 294.2 | 508.5 |
| 1h | 135.8 | 61.7 | 94.3 | 713.6 |
| 1i | 5439 | 6791 | 79.6 | 104.8 |
| 1j | 3865 | 5712 | 97.8 | 285.1 |
| 1k | 88.3 | 5.6 | 421.4 | 34.5 |
| AAZ | 250.0 | 12.1 | 25.8 | 5.7 |
| KI (nM) * | ||||||
|---|---|---|---|---|---|---|
| Cmp | E.coli β | E.coli γ | BpsCAβ | BpsCAγ | PgiCAγ | VhCAβ |
| 1a | 861.9 | 61.8 | 654.3 | 912.8 | 783.0 | 2334 |
| 1b | 3457 | 57.8 | 229.1 | 513.2 | 91.0 | 844.2 |
| 1c | 3836 | 79.1 | 785.4 | 613.5 | 637.1 | 913.3 |
| 1d | 5027 | 189.7 | 644.4 | 805.1 | 848.9 | 670.7 |
| 1e | 3136 | 58.1 | 682.9 | 1341 | 96.1 | 840.0 |
| 1f | 3650 | 66.8 | 236.3 | 2179 | 667.8 | 466.6 |
| 1g | 453.8 | 204.7 | 664.3 | 97.1 | 83.1 | 1449 |
| 1h | 711.9 | 524.3 | 2961 | 833.2 | 90.0 | 2617 |
| 1i | 3048 | 92.7 | 96.4 | 191.5 | 95.6 | 2241 |
| 1j | 94.9 | 67.1 | 788.8 | 625.4 | 84.3 | 642.3 |
| 1k | 3864 | 63.5 | 212.5 | 952.3 | 201.6 | 355.8 |
| AAZ | 227 | 248 | 745 | 149 | 324 | 451 |
| No | hCA Isoform | Estimated Free Binding Energy (Kcal/mol) | Chelating the Zn (II) Ion | Residues Involved in H-Bond Interactions | Residues Involved in Hydrophobic Interactions |
|---|---|---|---|---|---|
| 1c | hCA I | −4.70 | No | - | - |
| hCA II | −5.03 | No | - | Ile91, Phe131 | |
| hCA IX | −6.06 | Yes | Thr199 | Val121, Leu198 | |
| hCA XII | −5.92 | Yes | - | Leu198 | |
| 1g | hCA I | −10.42 | Yes | Trp5, Thr199, His200 | Leu198, His200 |
| hCA II | −6.89 | Yes | Thr199 | Val121, Leu198 | |
| hCA IX | −7.65 | Yes | Thr199, Thr200 | Leu198 | |
| hCA XII | 6.11 | Yes | Thr200 | Trp5, Leu198 | |
| 1f | hCA I | −11.37 | Yes | Trp5, Ser136, Thr199 | Ala121, Leu198 |
| hCA II | −10.12 | Yes | Gln92, Thr199 | Val121, Leu198, Thr200 | |
| hCA IX | −4.29 | Yes | - | Val121, Leu198 | |
| hCA XII | −5.50 | Yes | Gln92 | Val121, Leu198 | |
| 1k | hCA I | −9.25 | Yes | Thr199, His200 | Leu198, His200 |
| hCA II | −10.53 | Yes | Gln92, Thr199 (2) | Val121, Phe131, Val135, Leu198 | |
| hCA IX | −6.17 | Yes | - | Val121, Leu198 | |
| hCA XII | −6.79 | Yes | Thr199 | Val121, Leu198, Trp209 | |
| AAZ | hCA I | −8.28 | Yes | Gln92 | Leu198, Thr199, His200, Pro201, Trp209 |
| hCA II | −8.87 | Yes | Thr199, Thr200 | Val121, Phe131, Leu198, Trp209 | |
| hCA IX | −9.02 | Yes | Thr199, Thr200 | Val121, Val143, Val131, Leu198, Trp209 | |
| hCA XII | −9.14 | Yes | Thr199, Thr200 | Val121, Val143, Leu198, Trp209 |
| No | hCA Isoform | Estimated Free Binding Energy (Kcal/mol) | Chelating The Zn (II) Ion | Residues Involved in H-Bond Interactions | Residues Involved in Hydrophobic Interactions |
|---|---|---|---|---|---|
| 1a | E. coli β | −3.15 | No | - | - |
| γ | −5.18 | No | - | Leu80, Ala82 | |
| 1b | E. coli β | −1.07 | No | - | - |
| γ | −10.86 | Yes | Gln120, H2O | Val79 | |
| 1c | E. coli β | −2.40 | No | - | - |
| γ | −7.52 | Yes | Ser57 | Val78 | |
| 1d | E. coli β | −1.66 | No | - | - |
| γ | - | No | - | - | |
| 1e | E. coli β | −2.71 | No | - | - |
| γ | −10.57 | Yes | H2O | Val78, Val79 | |
| 1f | E. coli β | - | No | - | - |
| γ | −9.16 | Yes | Ser57, Arg59 | Val79, Leu83 | |
| 1g | E. coli β | −3.16 | No | - | Ala106 |
| γ | −2.55 | No | - | Val78 | |
| 1h | E. coli β | −3.02 | No | - | Ile126 |
| γ | −2.61 | No | - | Val78 | |
| 1i | E. coli β | −1.28 | No | - | - |
| γ | −7.43 | Yes | Glu62 | Val79 | |
| 1j | E. coli β | −8.61 | Yes | Gly103 | Ala106 |
| γ | −10.35 | Yes | Arg59, H2O | Val79 | |
| 1k | E. coli β | −2.58 | No | - | - |
| γ | −10.59 | Yes | Arg59, H2O | Val78 | |
| AAZ | E. coli β | −3.46 | No | - | Ala106, Val198 |
| γ | −4.27 | No | Glu140 | - |
| Cmp | MW | Number of HBA a | Number of HBD b | Log Po/w (iLOGP) c | Log S d | TPSA e | Lipinski Violations | Bioavailability Score | Drug-Likeness Model Score |
|---|---|---|---|---|---|---|---|---|---|
| 1a | 300.29 | 7 | 2 | −0.01 | Very soluble | 145.52 | 0 | 0.55 | −0.43 |
| 1b | 348.33 | 7 | 2 | 1.36 | Soluble | 145.52 | 0 | 0.55 | −0.47 |
| 1c | 348.33 | 7 | 2 | 0.92 | Soluble | 145.52 | 0 | 0.55 | −0.94 |
| 1d | 362.36 | 7 | 2 | 1.45 | Moderately soluble | 145.52 | 0 | 0.55 | −0.08 |
| 1e | 376.39 | 7 | 2 | 1.74 | Moderately soluble | 145.52 | 0 | 0.55 | −0.04 |
| 1f | 405.39 | 8 | 3 | 0.57 | Moderately soluble | 174.62 | 1 * | 0.55 | 0.83 |
| 1g | 295.31 | 5 | 2 | 1.29 | Very soluble | 117.95 | 0 | 0.55 | 0.90 |
| 1h | 294.31 | 6 | 1 | −2.99 | Very Soluble | 105.92 | 0 | 0.55 | −0.13 |
| 1i | 232.26 | 5 | 2 | 0.16 | Very soluble | 105.92 | 0 | 0.55 | −0.08 |
| 1j | 296.30 | 6 | 2 | 0.57 | Soluble | 123.94 | 0 | 0.55 | 0.01 |
| 1k | 429.49 | 6 | 2 | 1.09 | Moderately soluble | 141.17 | 0 | 0.55 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angeli, A.; Kartsev, V.; Petrou, A.; Lichitsky, B.; Komogortsev, A.; Pinteala, M.; Geronikaki, A.; Supuran, C.T. Pyrazolo[4,3-c]pyridine Sulfonamides as Carbonic Anhydrase Inhibitors: Synthesis, Biological and In Silico Studies. Pharmaceuticals 2022, 15, 316. https://doi.org/10.3390/ph15030316
Angeli A, Kartsev V, Petrou A, Lichitsky B, Komogortsev A, Pinteala M, Geronikaki A, Supuran CT. Pyrazolo[4,3-c]pyridine Sulfonamides as Carbonic Anhydrase Inhibitors: Synthesis, Biological and In Silico Studies. Pharmaceuticals. 2022; 15(3):316. https://doi.org/10.3390/ph15030316
Chicago/Turabian StyleAngeli, Andrea, Victor Kartsev, Anthi Petrou, Boris Lichitsky, Andrey Komogortsev, Mariana Pinteala, Athina Geronikaki, and Claudiu T. Supuran. 2022. "Pyrazolo[4,3-c]pyridine Sulfonamides as Carbonic Anhydrase Inhibitors: Synthesis, Biological and In Silico Studies" Pharmaceuticals 15, no. 3: 316. https://doi.org/10.3390/ph15030316
APA StyleAngeli, A., Kartsev, V., Petrou, A., Lichitsky, B., Komogortsev, A., Pinteala, M., Geronikaki, A., & Supuran, C. T. (2022). Pyrazolo[4,3-c]pyridine Sulfonamides as Carbonic Anhydrase Inhibitors: Synthesis, Biological and In Silico Studies. Pharmaceuticals, 15(3), 316. https://doi.org/10.3390/ph15030316









