Prenatal Vitamin D, Multivitamin, and Folic Acid Supplementation and Brain Structure in Children with ADHD and ASD Traits: The Generation R Study
Abstract
1. Introduction
- We investigated the association between maternal vitamin D, folate levels, and multivitamins and both ASD and ADHD traits in children. Our hypothesis was that supplementation with these vitamins during pregnancy would be associated with lower ASD and ADHD traits in children.
- We investigated the association between maternal dietary supplements and structural brain morphometry in children. We expected that vitamin D, folate levels, and multivitamin use would all have significant associations with brain structure in the frontal–temporal and frontal–striatal structures, which are most associated with ASD and ADHD, respectively.
- We investigated whether ASD and ADHD traits in children are associated with brain structure morphometry. We expected to replicate earlier findings of lower cortical thickness in frontal–temporal areas in ASD and frontal–striatal/parietal areas in ADHD.
- Assuming that all the above stated effects were observed, we investigated whether the association between dietary supplements and ASD/ADHD traits is mediated through brain structure morphometry measures. We expected that part of the association between diet and neurodevelopmental disorders could be explained by differences in brain structure.
2. Methods
2.1. Sample Characteristics
2.2. Behavioral Outcome Variables
2.3. MRI Variables
2.4. Statistical Analyses
2.5. Exploratory Analyses of Additional Important Covariates
3. Results
3.1. Model 1: Dietary Supplements During Pregnancy and ADHD and ASD Traits in Children at Age 6 (Path C’)
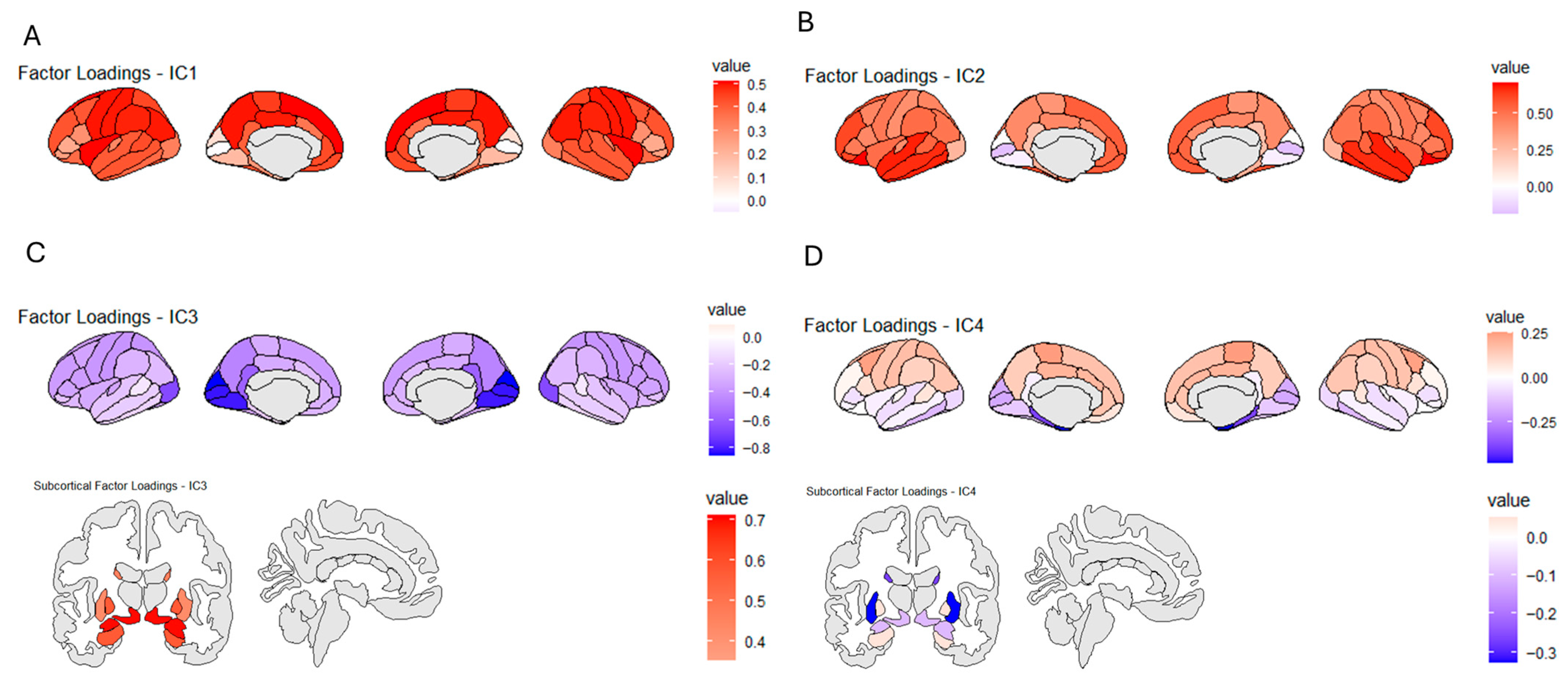
3.2. ICA Decomposition of Brain Segmentation
3.3. Model 2: Dietary Supplements During Pregnancy and Brain Volumes in Children at Age 10 (Path a)
3.4. Model 3: Associations Between Brain ICA Components and ADHD and ASD Traits in Children (Path b)
3.5. Model 4: Mediation of the Dietary Supplement Association with ADHD and ASD by Brain ICA Components (Path d)
3.6. Exploratory Analyses of Key Covariates
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nyaradi, A.; Li, J.; Hickling, S.; Foster, J.; Oddy, W.H. The role of nutrition in children’s neurocognitive development, from pregnancy through childhood. Front. Hum. Neurosci. 2013, 7, 97. [Google Scholar] [CrossRef]
- Steenweg-de Graaff, J.; Roza, S.J.; Steegers, E.A.; Hofman, A.; Verhulst, F.C.; Jaddoe, V.W.; Tiemeier, H. Maternal folate status in early pregnancy and child emotional and behavioral problems: The Generation R Study. Am. J. Clin. Nutr. 2012, 95, 1413–1421. [Google Scholar] [CrossRef]
- Parker, G.B.; Brotchie, H.; Graham, R.K. Vitamin D and depression. J. Affect. Disord. 2017, 208, 56–61. [Google Scholar] [CrossRef]
- Ars, C.L.; Nijs, I.M.; Marroun, H.E.; Muetzel, R.; Schmidt, M.; Steenweg-De Graaff, J.; van der Lugt, A.; Jaddoe, V.W.; Hofman, A.; Steegers, E.A.; et al. Prenatal folate, homocysteine and vitamin B12 levels and child brain volumes, cognitive development and psychological functioning: The Generation R Study. Br. J. Nutr. 2019, 122, S1–S9. [Google Scholar] [CrossRef]
- Mazahery, H.; Camargo, C.A., Jr.; Conlon, C.; Beck, K.L.; Kruger, M.C.; von Hurst, P.R. Vitamin D and autism spectrum disorder: A literature review. Nutrients 2016, 8, 236. [Google Scholar] [CrossRef]
- O’Leary, O.F.; Felice, D.; Galimberti, D.; Savioz, A.; Shimshek, D.R. Advances in understanding the genetic basis of intellectual disability. F1000Research 2016, 5, 1–16. [Google Scholar] [CrossRef]
- Li, M.; Francis, E.; Hinkle, S.N.; Ajjarapu, A.S.; Zhang, C. Preconception and prenatal nutrition and neurodevelopmental disorders: A systematic review and meta-analysis. Nutrients 2019, 11, 1628. [Google Scholar] [CrossRef]
- Levine, S.Z.; Kodesh, A.; Viktorin, A.; Smith, L.; Uher, R.; Reichenberg, A.; Sandin, S. Association of Maternal Use of Folic Acid and Multivitamin Supplements in the Periods Before and During Pregnancy With the Risk of Autism Spectrum Disorder in Offspring. JAMA Psychiatry 2018, 75, 176–184. [Google Scholar] [CrossRef]
- DeVilbiss, E.A.; Magnusson, C.; Gardner, R.M.; Rai, D.; Newschaffer, C.J.; Lyall, K.; Dalman, C.; Lee, B.K. Antenatal nutritional supplementation and autism spectrum disorders in the Stockholm youth cohort: Population based cohort study. BMJ (Clin. Res. Ed.) 2017, 359, j4273. [Google Scholar] [CrossRef]
- Schmidt, R.J. Maternal folic acid supplements associated with reduced autism risk in the child. Evid.-Based Med. 2013, 18, e53. [Google Scholar] [CrossRef]
- Vinkhuyzen, A.A.; Eyles, D.W.; Burne, T.H.; Blanken, L.M.; Kruithof, C.J.; Verhulst, F.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Gestational vitamin D deficiency and autism-related traits: The Generation R Study. Mol. Psychiatry 2018, 23, 240–246. [Google Scholar] [CrossRef]
- Virk, J.; Liew, Z.; Olsen, J.; Nohr, E.A.; Catov, J.M.; Ritz, B. Pre-conceptual and prenatal supplementary folic acid and multivitamin intake, behavioral problems, and hyperkinetic disorders: A study based on the Danish National Birth Cohort (DNBC). Nutr. Neurosci. 2018, 21, 352–360. [Google Scholar] [CrossRef]
- Schlotz, W.; Jones, A.; Phillips, D.I.; Gale, C.R.; Robinson, S.M.; Godfrey, K.M. Lower maternal folate status in early pregnancy is associated with childhood hyperactivity and peer problems in offspring. J. Child Psychol. Psychiatry 2010, 51, 594–602. [Google Scholar] [CrossRef]
- Julvez, J.; Fortuny, J.; Mendez, M.; Torrent, M.; Ribas-Fitó, N.; Sunyer, J. Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. Paediatr. Perinat. Epidemiol. 2019, 23, 199–206. [Google Scholar] [CrossRef]
- Raghavan, R.; Selhub, J.; Paul, L.; Ji, Y.; Wang, G.; Hong, X.; Wang, X. A prospective birth cohort study on cord blood folate subtypes and risk of autism spectrum disorder. Am. J. Clin. Nutr. 2020, 112, 1304–1317. [Google Scholar] [CrossRef]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. 2018, 19, 464–484. [Google Scholar] [CrossRef]
- Zou, R.; El Marroun, H.; McGrath, J.J.; Muetzel, R.L.; Hillegers, M.; White, T.; Tiemeier, H. A prospective population-based study of gestational vitamin D status and brain morphology in preadolescents. Neuroimage 2020, 209, 116514. [Google Scholar] [CrossRef]
- Zou, R.; El Marroun, H.; Cecil, C.; Jaddoe, V.W.V.; Hillegers, M.; Tiemeier, H.; White, T. Maternal folate levels during pregnancy and offspring brain development in late childhood. Clin. Nutr. 2021, 40, 3391–3400. [Google Scholar] [CrossRef]
- Mou, Y.; Jansen, P.W.; Sun, H.; White, T.; Voortman, T. Diet quality during pregnancy, adolescent brain morphology, and cognitive performance in a population-based cohort. Am. J. Clin. Nutr. 2024, 120, 1125–1133. [Google Scholar] [CrossRef]
- van Rooij, D.; Schweren, L.; Shi, H.; Hartman, C.A.; Buitelaar, J.K. Cortical and subcortical brain volumes partially mediate the association between dietary composition and behavioral disinhibition: A UK Biobank study. Nutrients 2021, 13, 3542. [Google Scholar] [CrossRef]
- Bradshaw, J.L.; Enticott, P.G. Developmental Disorders of the Frontostriatal System: Neuropsychological, Neuropsychiatric and Evolutionary Perspectives; Psychology Press: London, UK, 2014. [Google Scholar]
- Jaddoe, V.W.V.; Mackenbach, J.P.; Moll, H.A.; Steegers, E.A.P.; Tiemeier, H.; Verhulst, F.C.; Witteman, J.C.M.; Hofman, A. The Generation R Study: Design and cohort profile. Eur. J. Epidemiol. 2006, 21, 475–484. [Google Scholar] [CrossRef]
- White, T.; Muetzel, R.L.; El Marroun, H.; Blanken, L.M.E.; Jansen, P.; Bolhuis, K.; Kocevska, D.; Mous, S.E.; Mulder, R.; Jaddoe, V.W.V.; et al. Paediatric population neuroimaging and the Generation R Study: The second wave. Eur. J. Epidemiol. 2018, 33, 99–125. [Google Scholar] [CrossRef]
- Jaddoe, V.W.; van Duijn, C.M.; Franco, O.H.; van der Heijden, A.J.; van IIzendoorn, M.H.; de Jongste, J.C.; van der Lugt, A.; Mackenbach, J.P.; Moll, H.A.; Raat, H.; et al. The Generation R Study: Design and cohort update 2012. Eur. J. Epidemiol. 2012, 27, 739–756. [Google Scholar] [CrossRef]
- Klipstein-Grobusch, K.; den Breeijen, J.H.; Goldbohm, R.A.; Geleijnse, J.M.; Hofman, A.; Grobbee, D.E.; Witteman, J.C.M. Dietary assessment in the elderly: Validation of a semiquantitative food frequency questionnaire. Eur. J. Clin. Nutr. 1998, 52, 588–596. [Google Scholar] [CrossRef]
- Nguyen, A.N.; de Barse, L.M.; Tiemeier, H.; Jaddoe, V.W.; Franco, O.H.; Jansen, P.W.; Voortman, T. Maternal history of eating disorders: Diet quality during pregnancy and infant feeding. Appetite 2017, 109, 108–114. [Google Scholar] [CrossRef]
- Bergen, N.; Jaddoe, V.; Timmermans, S.; Hofman, A.; Lindemans, J.; Russcher, H.; Raat, H.; Steegers-Theunissen, R.P.M.; Steegers, E.A.P. Homocysteine and folate concentrations in early pregnancy and the risk of adverse pregnancy outcomes: The Generation R Study. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 739–751. [Google Scholar] [CrossRef]
- Vinkhuyzen, A.A.; Eyles, D.W.; Burne, T.H.; Blanken, L.M.; Kruithof, C.J.; Verhulst, F.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Prevalence and predictors of vitamin D deficiency based on maternal mid-gestation and neonatal cord bloods: The Generation R Study. J. Steroid Biochem. Mol. Biol. 2016, 164, 161–167. [Google Scholar] [CrossRef]
- Achenbach, T.M.; Rescorla, L.A. Child Behavior Checklist for Ages 6–18; University of Vermont: Burlington, VT, USA, 2001; pp. 1–6. [Google Scholar]
- Constantino, J.N.; Gruber, C.P. Social Responsiveness Scale Second Edition (SRS-2): Manual; Western Psychological Services (WPS): Torrance, CA, USA, 2012. [Google Scholar]
- Roza, S.J.; Verburg, B.O.; Jaddoe, V.W.; Hofman, A.; Mackenbach, J.P.; Steegers, E.A.; Witteman, J.C.; Verhulst, F.C.; Tiemeier, H. Effects of maternal smoking in pregnancy on prenatal brain development. Gener. R Study Eur. J. Neurosci. 2007, 25, 611–617. [Google Scholar] [CrossRef]
- Jaddoe, V.W.; Bakker, R.; Hofman, A.; Mackenbach, J.P.; Moll, H.A.; Steegers, E.A.; Witteman, J.C. Moderate alcohol consumption during pregnancy and the risk of low birth weight and preterm birth. Gener. R Study Ann. Epidemiol. 2007, 17, 834–840. [Google Scholar] [CrossRef]
- Jaddoe, V.W.V.; van Duijn, C.M.; van der Heijden, A.J.; Mackenbach, J.P.; Moll, H.A.; Steegers, E.A.P.; Tiemeier, H.; Uitterlinden, A.G.; Verhulst, F.C.; Hofman, A. The Generation R Study: Design and cohort update 2010. Eur. J. Epidemiol. 2010, 25, 823–841. [Google Scholar] [CrossRef]
- Jawad, A.; Patel, D.; Brima, N.; Stephenson, J. Alcohol, smoking, folic acid and multivitamin use among women attending maternity care in London: A cross-sectional study. Sex. Reprod. Healthc. 2019, 22, 100461. [Google Scholar] [CrossRef]
- van Soelen, I.L.; Brouwer, R.M.; Peper, J.S.; van Beijsterveldt, T.C.; van Leeuwen, M.; de Vries, L.S.; Kahn, R.S.; Hulshoff Pol, H.E.; Boomsma, D.I. Effects of gestational age and birth weight on brain volumes in healthy 9 year-old children. J. Pediatr. 2010, 156, 896–901. [Google Scholar] [CrossRef]
- Turrell, G.; Kavanagh, A.M. Socio-economic pathways to diet: Modelling the association between socio-economic position and food purchasing behaviour. Public Health Nutr. 2006, 9, 375–383. [Google Scholar] [CrossRef]
- Noble, K.G.; Houston, S.M.; Kan, E.; Sowell, E.R. Neural correlates of socioeconomic status in the developing human brain. Dev. Sci. 2012, 15, 516–527. [Google Scholar] [CrossRef]
- Lee, B.K.; Eyles, D.W.; Magnusson, C.; Newschaffer, C.J.; McGrath, J.J.; Kvaskoff, D.; Ko, P.; Dalman, C.; Karlsson, H.; Gardner, R.M. Developmental vitamin D and autism spectrum disorders: Findings from the Stockholm Youth Cohort. Mol. Psychiatry 2021, 26, 1578–1588. [Google Scholar] [CrossRef]
- Sable, P.; Kale, A.; Joshi, A.; Joshi, S. Maternal micronutrient imbalance alters gene expression of BDNF, NGF, TrkB and CREB in the offspring brain at an adult age. Int. J. Dev. Neurosci. 2014, 34, 24–32. [Google Scholar] [CrossRef]
- Hawes, J.E.; Tesic, D.; Whitehouse, A.J.; Zosky, G.R.; Smith, J.T.; Wyrwoll, C.S. Maternal vitamin D deficiency alters fetal brain development in the BALB/c mouse. Behav. Brain Res. 2015, 286, 192–200. [Google Scholar] [CrossRef]
- Eyles, D.W. Vitamin D: Brain and behavior. J. Bone Miner. Res. Plus 2021, 5, e10419. [Google Scholar] [CrossRef]
- Bourre, J.M. Effects of nutrients (in food) on the structure and function of the nervous system: Update on dietary requirements for brain. Part 2: Macronutrients. J. Nutr. Health Aging 2006, 10, 386. [Google Scholar]
- Kesby, J.P.; Turner, K.M.; Alexander, S.; Eyles, D.W.; McGrath, J.J.; Burne, T.H. Developmental vitamin D deficiency alters multiple neurotransmitter systems in the neonatal rat brain. Int. J. Dev. Neurosci. 2017, 62, 1–7. [Google Scholar] [CrossRef]
- Eyles, D.; Burne, T.; McGrath, J. Vitamin D in fetal brain development. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2011; Volume 22, pp. 629–636. [Google Scholar]
- Van De Rest, O.; Van Hooijdonk, L.W.; Doets, E.; Schiepers, O.J.; Eilander, A.; De Groot, L.C. B Vitamins and n–3 fatty acids for brain development and function: Review of human studies. Ann. Nutr. Metab. 2012, 60, 272–292. [Google Scholar] [CrossRef]
- Kurtys, E.; Eisel, U.; Verkuyl, J.; Broersen, L.; Dierckx, R.; de Vries, E.F. The combination of vitamins and omega-3 fatty acids has an enhanced anti-inflammatory effect on microglia. Neurochem. Int. 2016, 99, 206–214. [Google Scholar] [CrossRef]
- Uberti, F.; Morsanuto, V.; Molinari, C. Vitamin D in Oxidative Stress and Diseases; IntechOpen: Vienna, Austria, 2017. [Google Scholar]
- Borge, T.C.; Aase, H.; Brantsaeter, A.L.; Biele, G. The importance of maternal diet quality during pregnancy on cognitive and behavioural outcomes in children: A systematic review and meta-analysis. BMJ Open 2017, 7, e016777. [Google Scholar] [CrossRef]
- Mahmassani, H.A.; Switkowski, K.M.; Scott, T.M.; Johnson, E.J.; Rifas-Shiman, S.L.; Oken, E.; Jacques, P.F. Maternal diet quality during pregnancy and child cognition and behavior in a US cohort. Am. J. Clin. Nutr. 2022, 115, 128–141. [Google Scholar] [CrossRef]
- Lecorguillé, M.; Teo, S.; Phillips, C.M. Maternal dietary quality and dietary inflammation associations with offspring growth, placental development, and DNA methylation. Nutrients 2021, 13, 3130. [Google Scholar] [CrossRef]
- Roza, S.J.; van Batenburg-Eddes, T.; Steegers, E.A.P.; Jaddoe, V.W.V.; Mackenbach, J.P.; Hofman, A.; Verhulst, F.C.; Tiemeier, H. Maternal folic acid supplement use in early pregnancy and child behavioural problems: The Generation R Study. Br. J. Nutr. 2010, 103, 445–452. [Google Scholar] [CrossRef]
- Amiri, M.; Lamballais, S.; Geenjaar, E.; Blanken, L.M.; El Marroun, H.; Tiemeier, H.; White, T. Environment-Wide Association Study (EnWAS) of Prenatal and Perinatal Factors Associated With Autistic Traits: A Population-Based Study. Autism Res. 2020, 13, 1582–1600. [Google Scholar] [CrossRef]

| N Available | Mean or % | SD | Min. | Max. | |
| Child age during MRI scan (years) | 3737 (1951 males) | 10.12 | 0.59 | 8.50 | 12.00 |
| ASD traits (SRS overall score) | 3070 | 4.93 | 3.83 | 0 | 46.00 |
| ADHD traits (CBCL ADHD subscale T-score) | 3334 | 52.99 | 5.03 | 50 | 80 |
| Folate serum concentration during pregnancy (nmol/L) | 2571 | 19.21 | 9.10 | 3.70 | 45.30 |
| Vitamin D serum concentration during pregnancy (nmol/L) | 3072 | 56.35 | 31.53 | 1.50 | 161.00 |
| Multivitamin use during pregnancy (self-reported) | 2930 | 34% yes | - | - | |
| Diet quality score during pregnancy (FFQ) | 2709 | 7.75 | 1.57 | 1.55 | 12.99 |
| ADHD Traits | ASD Traits | |||||
| Beta (β) | t Value | p Value | Beta (β) | t Value | p Value | |
| Vitamin D (serum) | −0.03 | −1.09 | 0.27 | −0.07 | −2.64 | <0.008 |
| Folate (serum) | −0.01 | −0.91 | 0.36 | −0.03 | −0.50 | 0.35 |
| Multivitamin use | −0.47 * | 1.81 | 0.07 | −0.39 * | 1.76 | 0.08 |
| Diet score pregnancy | −0.09 | −3.68 | <0.001 | −0.05 | −2.09 | <0.04 |
| Child age (year) | 0.01 | 0.55 | 0.577 | 0.00 | 0.32 | 0.975 |
| Sex (1 = male; 2 = female) | −0.34 * | −1.44 | 0.150 | −0.70 * | −3.35 | <0.001 |
| IC1: | Temporal/Parietal | IC2: | Frontal–Temporal | |||||
|---|---|---|---|---|---|---|---|---|
| Beta (β) | Std error | t value | p value | Beta (β) | Std error | t value | p value | |
| Vitamin D | 0.06 | 0.00 | 2.23 | 0.03 | −0.08 | 0.00 | −3.05 | <0.001 |
| Folate | 0.01 | 0.00 | 0.42 | 0.68 | 0.01 | 0.00 | 0.49 | 0.62 |
| Multivitamin use | 0.04 * | 0.05 | 1.46 | 0.14 | −0.04 * | 0.05 | −1.52 | 0.13 |
| Diet score pregnancy | 0.06 | 0.02 | 2.29 | 0.02 | −0.08 | 0.02 | −3.44 | <0.001 |
| Child age (year) | 0.03 | 0.04 | 1.16 | 0.25 | 0.09 | 0.04 | 3.89 | <0.001 |
| Sex (1 = male; 2 = female) | −0.14 * | 0.05 | −5.91 | <0.001 | 0.23 * | 0.05 | 9.82 | <0.001 |
| IC3: | Subcortical | IC4: | Hippocampal | |||||
| Beta (β) | Std error | t value | p value | Beta (β) | Std error | t value | p value | |
| Vitamin D | 0.02 | 0.00 | 0.71 | 0.48 | −0.04 | 0.00 | −1.56 | 0.12 |
| Folate | 0.06 | 0.00 | 2.19 | 0.03 | 0.04 | 0.00 | 1.67 | 0.10 |
| Multivitamin use | −0.01 * | 0.05 | −0.34 | 0.73 | 0.00 * | 0.05 | −0.20 | 0.84 |
| Diet score pregnancy | 0.05 | 0.01 | 2.15 | 0.03 | 0.04 | 0.02 | 1.46 | 0.14 |
| Child age (year) | 0.05 | 0.04 | 2.02 | 0.04 | −0.08 | 0.04 | −3.50 | <0.001 |
| Sex (1 = male; 2 = female) | −0.36 * | 0.04 | −15.83 | <0.001 | 0.12 * | 0.05 | 5.13 | <0.001 |
| ADHD | ASD | |||||
| Beta (β) | t Value | p Value | Beta (β) | t Value | p Value | |
| IC1 (temporal/parietal) | −0.07 | −3.78 | <0.001 | −0.05 | −2.79 | 0.01 |
| IC2 (frontal–temporal) | −0.05 | −2.72 | <0.001 | −0.08 | −4.26 | <0.001 |
| IC3 (subcortical) | 0.10 | 5.79 | <0.001 | 0.06 | 3.09 | <0.001 |
| IC4 (hippocampal) | −0.01 | −0.58 | 0.56 | −0.01 | −0.63 | 0.53 |
| Child age (year) | −0.11 | −5.49 | <0.001 | −0.12 | −6.09 | <0.001 |
| Sex (1 = male; 2 = female) | 0.00 * | −0.11 | 0.92 | 0.02 * | 1.07 | 0.28 |
| Mediation Model 1 | |||||
| Vitamin D > ADHD | Effect (path) | Estimate (b) | std. error | z value | p value (fdr) |
| IC 3: subcortical | Indirect 1 (d1) | 0 | 0 | −1.26 | 0.20 |
| IC2: frontal–temporal | Indirect 2 (d2) | 0 | 0 | −1.31 | 0.19 |
| IC1: temporal/parietal | Indirect 3 (d3) | −0.001 | 0 | −2.67 | 0.01 |
| IC4: hippocampal | Indirect 4 (d4) | 0 | 0 | −0.43 | 0.66 |
| Total (c) | −0.006 | 0.003 | −1.66 | 0.09 | |
| Mediation model 2 | |||||
| Folic acid > ADHD | Effect (path) | Estimate (b) | std. error | z value | p value (fdr) |
| IC 3: subcortical | Indirect 1 (d1) | 0.00 | 0.00 | −1.11 | 0.27 |
| IC2: frontal–temporal | Indirect 2 (d2) | 0.00 | 0.00 | −1.66 | 0.10 |
| IC1: temporal/parietal | Indirect 3 (d3) | 0.00 | 0.00 | −1.81 | 0.07 |
| IC4: hippocampal | Indirect 4 (d4) | 0.00 | 0.00 | −0.68 | 0.50 |
| Total (c) | −0.03 | 0.01 | −2.27 | 0.02 | |
| Mediation model 3 | |||||
| Multivitamin use > ADHD | Effect (path) | Estimate (b) | std. error | z value | p value (fdr) |
| IC 3: subcortical | Indirect 1 (d1) | −0.01 | 0.011 | −0.96 | 0.34 |
| IC2: frontal–temporal | Indirect 2 (d2) | −0.02 | 0.015 | −1.38 | 0.17 |
| IC1: temporal/parietal | Indirect 3 (d3) | −0.04 | 0.02 | −2.18 | 0.03 |
| IC4: hippocampal | Indirect 4 (d4) | −0.004 | 0.008 | −0.44 | 0.66 |
| Total (c) | 0.05 | 0.21 | 0.25 | 0.79 | |
| Mediation model 4 | |||||
| Diet score pregnancy > ADHD | Effect (path) | Estimate (b) | std. error | z value | p value (fdr) |
| IC 3: subcortical | Indirect 1 (d1) | −0.006 | 0.00 | −1.52 | 0.127 |
| IC2: frontal–temporal | Indirect 2 (d2) | −0.008 | 0.00 | −1.50 | 0.133 |
| IC1: temporal/parietal | Indirect 3 (d3) | −0.015 | 0.00 | −2.51 | 0.012 |
| IC4: hippocampal | Indirect 4 (d4) | −0.001 | 0.00 | −0.45 | 0.648 |
| Total (c) | −0.25 | 0.06 | −4.10 | 0 |
| Mediation model 5. | |||||
| Vitamin D > ASD | Effect (path) | Estimate (b) | std. error | z value | p value (fdr) |
| IC 3: subcortical | Indirect 1 (d1) | 0 | 0 | −0.21 | 0.83 |
| IC2: frontal–temporal | Indirect 2 (d2) | 0 | 0 | −1.86 | 0.06 |
| IC1: temporal/parietal | Indirect 3 (d3) | 0 | 0 | −0.11 | 0.91 |
| IC4: hippocampal | Indirect 4 (d4) | 0 | 0 | −0.28 | 0.77 |
| Total (c) | −0.01 | 0.00 | −4.76 | 0 | |
| Mediation model 6 | |||||
| Folic acid > ASD | Effect (path) | Estimate (b) | std. error | z value | p value (fdr) |
| IC 3: subcortical | Indirect 1 (d1) | 0.00 | 0.00 | −0.07 | 0.95 |
| IC2: frontal–temporal | Indirect 2 (d2) | 0.00 | 0.00 | −1.60 | 0.11 |
| IC1: temporal/parietal | Indirect 3 (d3) | 0.00 | 0.00 | −0.45 | 0.65 |
| IC4: hippocampal | Indirect 4 (d4) | 0.00 | 0.00 | −1.33 | 0.18 |
| Total (c) | −0.03 | 0.01 | −3.11 | <0.001 | |
| Mediation model 7 | |||||
| Multivitamin use > ASD | Effect (path) | Estimate (b) | std. error | z value | p value (fdr) |
| IC 3: subcortical | Indirect 1 (d1) | −0.001 | 0.00 | −0.27 | 0.78 |
| IC2: frontal–temporal | Indirect 2 (d2) | −0.03 | 0.01 | −2.00 | 0.04 |
| IC1: temporal/parietal | Indirect 3 (d3) | −0.018 | 0.01 | −1.26 | 0.21 |
| IC4: hippocampal | Indirect 4 (d4) | 0 | 0.00 | −0.06 | 0.95 |
| Total (c) | 0.093 | 0.19 | 0.46 | 0.64 | |
| Mediation model 8 | |||||
| Diet score pregnancy > ASD | Effect (path) | Estimate (b) | std. error | z value | p value (fdr) |
| IC3: subcortical | Indirect 1 (d1) | 0 | 0.00 | 0.02 | 0.98 |
| IC2: frontal–temporal | Indirect 2 (d2) | −0.008 | 0.00 | −1.56 | 0.11 |
| IC1: temporal/parietal | Indirect 3 (d3) | −0.004 | 0.00 | −1.06 | 0.29 |
| IC4: hippocampal | Indirect 4 (d4) | −0.001 | 0.00 | −0.56 | 0.57 |
| Total (c) | −0.19 | 0.05 | −3.87 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Rooij, D.; Mou, Y.; White, T.; Voortman, T.; Jansen, P.W.; Buitelaar, J.K. Prenatal Vitamin D, Multivitamin, and Folic Acid Supplementation and Brain Structure in Children with ADHD and ASD Traits: The Generation R Study. Nutrients 2025, 17, 2979. https://doi.org/10.3390/nu17182979
van Rooij D, Mou Y, White T, Voortman T, Jansen PW, Buitelaar JK. Prenatal Vitamin D, Multivitamin, and Folic Acid Supplementation and Brain Structure in Children with ADHD and ASD Traits: The Generation R Study. Nutrients. 2025; 17(18):2979. https://doi.org/10.3390/nu17182979
Chicago/Turabian Stylevan Rooij, Daan, Yuchan Mou, Tonya White, Trudy Voortman, Pauline W. Jansen, and Jan K. Buitelaar. 2025. "Prenatal Vitamin D, Multivitamin, and Folic Acid Supplementation and Brain Structure in Children with ADHD and ASD Traits: The Generation R Study" Nutrients 17, no. 18: 2979. https://doi.org/10.3390/nu17182979
APA Stylevan Rooij, D., Mou, Y., White, T., Voortman, T., Jansen, P. W., & Buitelaar, J. K. (2025). Prenatal Vitamin D, Multivitamin, and Folic Acid Supplementation and Brain Structure in Children with ADHD and ASD Traits: The Generation R Study. Nutrients, 17(18), 2979. https://doi.org/10.3390/nu17182979







