Does Bariatric Surgery Reduce the Risk of Colorectal Cancer in Individuals with Morbid Obesity? A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Exclusion Criteria
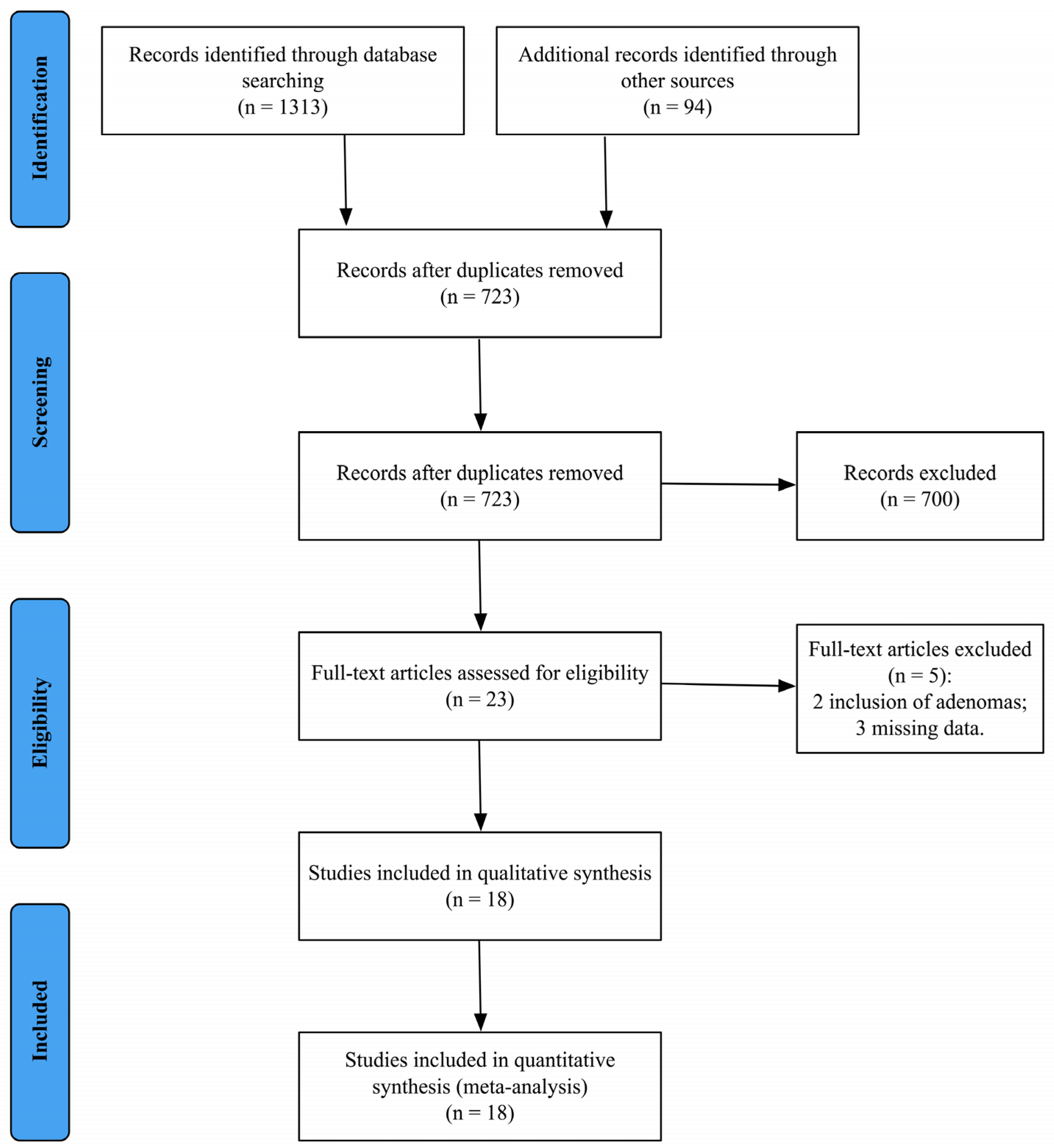
2.4. Systematic Review Process
2.5. Risk of Bias Assessment
2.6. Data Extraction and Assessment of Included Studies
2.7. Primary and Secondary Endpoints
2.8. Statistical Analysis
3. Results
3.1. Colorectal Cancer
3.2. Subgroup Analysis
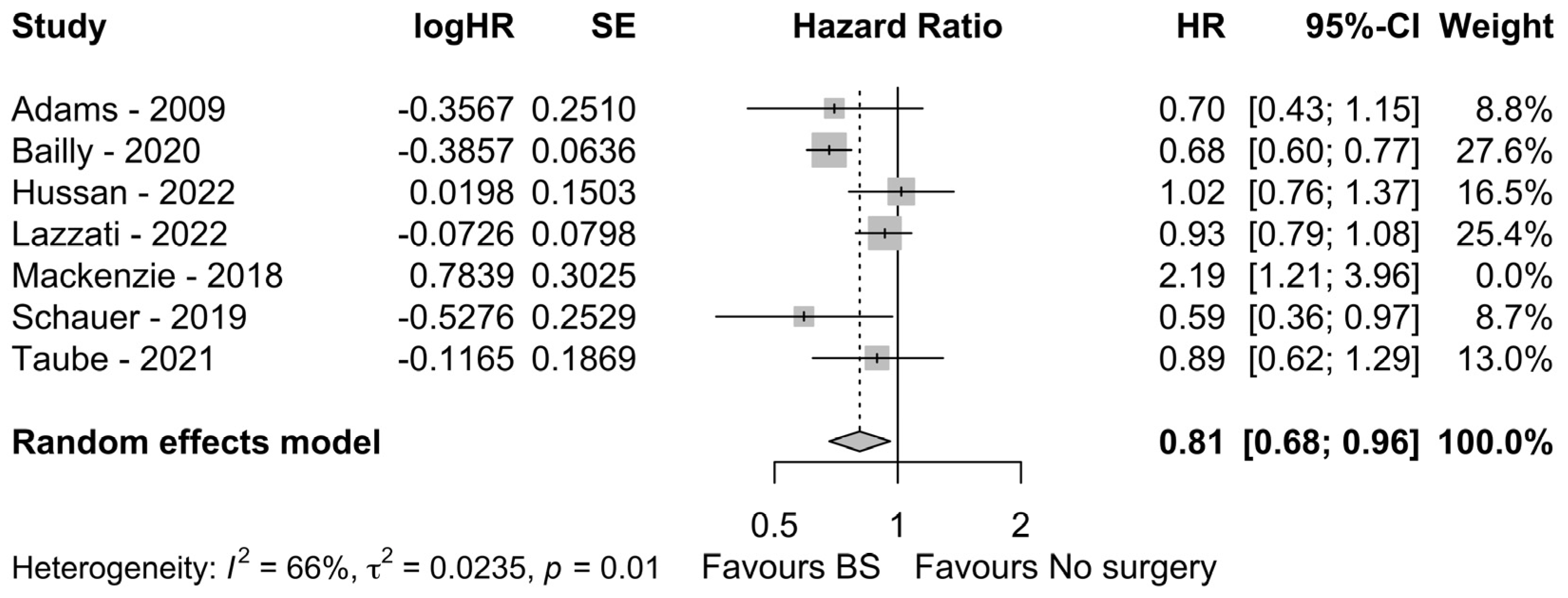
3.3. Meta-Analysis of HR
3.4. Assessment of Publication Bias and Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar]
- Tao, W.; Artama, M.; von Euler-Chelpin, M.; Hull, M.; Ljung, R.; Lynge, E.; Ólafsdóttir, G.H.; Pukkala, E.; Romundstad, P.; Talbäck, M.; et al. Colon and rectal cancer risk after bariatric surgery in a multicountry Nordic cohort study. Int. J. Cancer 2020, 147, 728–735. [Google Scholar] [CrossRef]
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef]
- Bardou, M.; Barkun, A.N.; Martel, M. Obesity and colorectal cancer. Gut 2013, 62, 933–947. [Google Scholar] [CrossRef]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Bailly, L.; Fabre, R.; Pradier, C.; Iannelli, A. Colorectal cancer risk following bariatric surgery in a nationwide study of French individuals with obesity. JAMA Surg. 2020, 155, 395–402. [Google Scholar] [CrossRef]
- Afshar, S.; Kelly, S.B.; Seymour, K.; Lara, J.; Woodcock, S.; Mathers, J.C. The Effects of Bariatric Surgery on Colorectal Cancer Risk: Systematic Review and Meta-analysis. Obes. Surg. 2014, 24, 1793–1799. [Google Scholar] [CrossRef]
- Almazeedi, S.; El-Abd, R.; Al-Khamis, A.; Albatineh, A.N.; Al-Sabah, S. Role of bariatric surgery in reducing the risk of colorectal cancer: A meta-analysis. Br. J. Surg. 2020, 107, 348–354. [Google Scholar] [CrossRef]
- Janik, M.R.; Clapp, B.; Sroczyński, P.; Ghanem, O. The Effect of Bariatric Surgery on Reducgin the Risk of Colorectal Cancer: A Meta-Analysis of 3,233,044 Patients. Surg. Obes. Relat. Dis. 2022. In press. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 105906. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, 4–10. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; McAleenan, A.; Reeves, B.C.; Higgins, J.P.T. Chapter 25: Assessing risk of bias in a non-randomized study. In Cochrane Handbook for Systematic Reviews of Interventions; Version, 6.2; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2021; Available online: www.training.cochrane.org/handbook (accessed on 31 August 2022).
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2022; Available online: http://www.rstudio.com/ (accessed on 31 August 2022).
- Balduzzi, S.; Rücker, G.S.G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D.D. Companion R Package for the Guide “Doing Meta-Analysis in R”. 2019. Available online: http://dmetar.protectlab.org/ (accessed on 31 August 2022).
- Adams, T.D.; Hunt, S.C. Cancer and obesity: Effect of bariatric surgery. World J. Surg. 2009, 33, 2028–2033. [Google Scholar] [CrossRef]
- Aminian, A.; Wilson, R.; Al-Kurd, A.; Tu, C.; Milinovich, A.; Kroh, M.; Rosenthal, R.J.; Brethauer, S.A.; Schauer, P.R.; Kattan, M.W.; et al. Association of Bariatric Surgery with Cancer Risk and Mortality in Adults with Obesity. JAMA—J. Am. Med. Assoc. 2022, 327, 2423–2433. [Google Scholar] [CrossRef]
- Aravani, A.; Downing, A.; Thomas, J.D.; Lagergren, J.; Morris, E.J.A.; Hull, M.A. Obesity surgery and risk of colorectal and other obesity-related cancers: An English population-based cohort study. Cancer Epidemiol. 2018, 53, 99–104. [Google Scholar] [CrossRef]
- Christou, N.V.; Lieberman, M.; Sampalis, F.; Sampalis, J.S. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg. Obes. Relat. Dis. 2008, 4, 691–695. [Google Scholar] [CrossRef]
- Ciccioriccio, M.C.; Iossa, A.; Boru, C.E.; De Angelis, F.; Termine, P.; Giuffrè, M.; Silecchia, G.; Angrisani, L.; Balani, A.; Bellini, F.; et al. Colorectal cancer after bariatric surgery (Cric-Abs 2020): Sicob (Italian society of obesity surgery) endorsed national survey. Int. J. Obes. 2021, 45, 2527–2531. [Google Scholar] [CrossRef] [PubMed]
- Derogar, M.; Hull, M.A.; Kant, P.; Östlund, M.; Lu, Y.; Lagergren, J. Increased risk of colorectal cancer after obesity surgery. Ann. Surg. 2013, 258, 983–988. [Google Scholar] [CrossRef]
- Desai, D.; Singhal, S.; Koka, J. Evaluating the Correlation of Bariatric Surgery and the Prevalence of Cancers in Obese Patients: A Study of the National Inpatient Sample (NIS) Database. Cureus 2022, 14, e23976. [Google Scholar] [CrossRef]
- Hussan, H.; Akinyeye, S.; Mihaylova, M.; McLaughlin, E.; Chiang, C.; Clinton, S.K.; Lieberman, D. Colorectal Cancer Risk Is Impacted by Sex and Type of Surgery After Bariatric Surgery. Obes. Surg. 2022, 32, 2880–2890. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.I.; Maasarani, S.; Wiegmann, J.; Wiegmann, A.L.; Becerra, A.Z.; Omotosho, P.; Torquati, A. Association of Bariatric Surgery and Risk of Cancer in PatientsWith Morbid Obesity. Ann. Surg. 2022, 275, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.; Mehaffey, J.H.; Hawkins, R.B.; Hedrick, T.L.; Slingluff, C.L.; Schirmer, B.; Hallowell, P.T.; Friel, C.M. Bariatric surgery is independently associated with a decrease in the development of colorectal lesions. Surgery 2019, 166, 322–326. [Google Scholar] [CrossRef]
- Lazzati, A.; Epaud, S.; Ortala, M.; Katsahian, S.; Lanoy, E. Effect of bariatric surgery on cancer risk: Results from an emulated target trial using population-based data. Br. J. Surg. 2022, 109, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, H.; Markar, S.R.; Askari, A.; Faiz, O.; Hull, M.; Purkayastha, S.; Møller, H.; Lagergren, J. Obesity surgery and risk of cancer. Br. J. Surg. 2018, 105, 1650–1657. [Google Scholar] [CrossRef]
- McCawley, G.M.; Ferriss, J.S.; Geffel, D.; Northup, C.J.; Modesitt, S.C. Cancer in Obese Women: Potential Protective Impact of Bariatric Surgery. J. Am. Coll. Surg. 2009, 208, 1093–1098. [Google Scholar] [CrossRef]
- Schauer, D.P.; Feigelson, H.S.; Koebnick, C.; Caan, B.; Weinmann, S.; Leonard, A.C.; Powers, J.D.; Yenumula, P.R.; Arterburn, D.E. Bariatric Surgery and the Risk of Cancer in a Large Multisite Cohort. Ann. Surg. 2019, 269, 95–101. [Google Scholar] [CrossRef]
- Taube, M.; Peltonen, M.; Sjöholm, K.; Palmqvist, R.; Andersson-Assarsson, J.C.; Jacobson, P.; Svensson, P.-A.; Carlsson, L.M.S. Long-term incidence of colorectal cancer after bariatric surgery or usual care in the Swedish Obese Subjects study. PLoS ONE 2021, 16, e0248550. [Google Scholar] [CrossRef] [PubMed]
- Tsui, S.T.; Yang, J.; Zhang, X.; Docimo, S.; Spaniolas, K.; Talamini, M.A.; Sasson, A.R.; Pryor, A.D. Development of cancer after bariatric surgery. Surg. Obes. Relat. Dis. 2020, 16, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Pandeya, N.; Byrnes, G.; Renehan, A.G.; A Stevens, G.; Ezzati, M.; Ferlay, J.; Miranda, J.J.; Romieu, I.; Dikshit, R.; et al. Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol. 2015, 16, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, V.K.; Li, Y.; Gupta, K.; Minacapelli, C.D.; Bhurwal, A.; Catalano, C.; Elsaid, M.I. Bariatric Surgery Reduces Cancer Risk in Adults With Nonalcoholic Fatty Liver Disease and Severe Obesity. Gastroenterology 2021, 161, 171–184.e10. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, S.; Sofia, M.; Agosta, M.; Litrico, G.; Sarvà, I.; La Greca, G.; Latteri, S. The impact of bariatric surgery on colorectal cancer risk. Surg. Obes. Relat. Dis. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E.; Makowski, L.; DiGiovanni, J.; Kolonin, M.G. Cancer as a matter of fat: The crosstalk between adipose tissue and tumors. Trends Cancer 2018, 4, 374–384. [Google Scholar] [CrossRef]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Ramos, A.; Shikora, S.; Kow, L. Bariatric Surgery Survey 2018: Similarities and Disparities among the 5 IFSO Chapters. Obes. Surg. 2021, 31, 1937–1948. [Google Scholar] [CrossRef]


| Author | Year | Country | BS * | Control * | Follow-Up (y) | Risk Estimate † (95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| Total | Events | Total | Events | |||||
| Adams et al. | 2008 | USA | 6596 | 25 (0.004) | 9442 | 52 (0.006) | 12.3 | HR: 0.7 (0.43–1.15) |
| Aminian et al. | 2022 | USA | 5053 | 16 (0.003) | 25,265 | 86 (0.003) | 6.1 | - |
| Aravani et al. | 2018 | UK | 39,747 | 43 (0.001) | 962,860 | 3237 (0.003) | 3 | - |
| Bailly et al. | 2020 | FR | 74,131 | 423 (0.006) | 971,217 | 12629 (0.013) | 5.7 | HR: 0.68 (0.6–0.77) |
| Christou et al. | 2008 | CA | 1035 | 2 (0.002) | 5746 | 35 (0.006) | 5 | RR: 0.32 (0.076–1.313) |
| Ciccioriccio et al. | 2021 | IT | 20,571 | 22 (0.001) | - | - | 4.3 | - |
| Derogar et al. | 2013 | SE | 15,095 | 70 (0.005) | 62,016 | 373 (0.006) | 10 | - |
| Desai et al. | 2022 | USA | 279,145 | 19 (0.0001) | 7,398,104 | 32,276 (0.004) | - | OR: 0.06 (0.04–0.1) |
| Hussan et al. | 2022 | USA | 88,630 | 88 (0.001) | 327,734 | 325 (0.001) | 3 | HR: 1.02 (0.76–1.37) |
| Khalid et al. | 2019 | USA | 19,272 | 66 (0.003) | 9636 | 55 (0.006) | 5 | - |
| Kwak et al. | 2019 | USA | 2231 | 5 (0.002) | 2231 | 6 (0.002) | 7.8 | OR: 0.62 (0.42–0.91) § |
| Lazzati et al. | 2022 | FR | 288,604 | 329 (0.001) | 851,743 | 4434 (0.005) | 5.7 | HR: 0.93 (0.79–1.08) |
| Mackenzie et al. | 2018 | SE/UK | 8794 | 16 (0.002) | 8794 | 35 (0.004) | 4.6 | HR: 2.19 (1.21–3.96) |
| McCawley et al. | 2009 | USA | 1482 | 1 (0.0007) | 3495 | 11 (0.003) | - | - |
| Schauer et al. | 2019 | USA | 22,198 | 105 (0.005) | 66,427 | 533 (0.008) | 3.5 | HR: 0.59 (0.36–0.97) |
| Tao et al. | 2019 | DK/SE/NO/FI/IS | 49,931 | 155 (0.003) | 492,427 | 3158 (0.006) | 3.1 | - |
| Taube et al. | 2021 | SE | 2006 | 58 (0.03) | 2038 | 67 (0.03) | 22.2 | HR: 0.89 (0.62–1.28) |
| Tsui et al. | 2020 | USA | 71,000 | 340 (0.005) | 323,197 | 1334 (0.004) | - | - |
| 995,521 | 11,522,372 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chierici, A.; Amoretti, P.; Drai, C.; De Fatico, S.; Barriere, J.; Schiavo, L.; Iannelli, A. Does Bariatric Surgery Reduce the Risk of Colorectal Cancer in Individuals with Morbid Obesity? A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 467. https://doi.org/10.3390/nu15020467
Chierici A, Amoretti P, Drai C, De Fatico S, Barriere J, Schiavo L, Iannelli A. Does Bariatric Surgery Reduce the Risk of Colorectal Cancer in Individuals with Morbid Obesity? A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(2):467. https://doi.org/10.3390/nu15020467
Chicago/Turabian StyleChierici, Andrea, Paolo Amoretti, Céline Drai, Serena De Fatico, Jérôme Barriere, Luigi Schiavo, and Antonio Iannelli. 2023. "Does Bariatric Surgery Reduce the Risk of Colorectal Cancer in Individuals with Morbid Obesity? A Systematic Review and Meta-Analysis" Nutrients 15, no. 2: 467. https://doi.org/10.3390/nu15020467
APA StyleChierici, A., Amoretti, P., Drai, C., De Fatico, S., Barriere, J., Schiavo, L., & Iannelli, A. (2023). Does Bariatric Surgery Reduce the Risk of Colorectal Cancer in Individuals with Morbid Obesity? A Systematic Review and Meta-Analysis. Nutrients, 15(2), 467. https://doi.org/10.3390/nu15020467