Two Gold Kiwifruit Daily for Effective Treatment of Constipation in Adults—A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
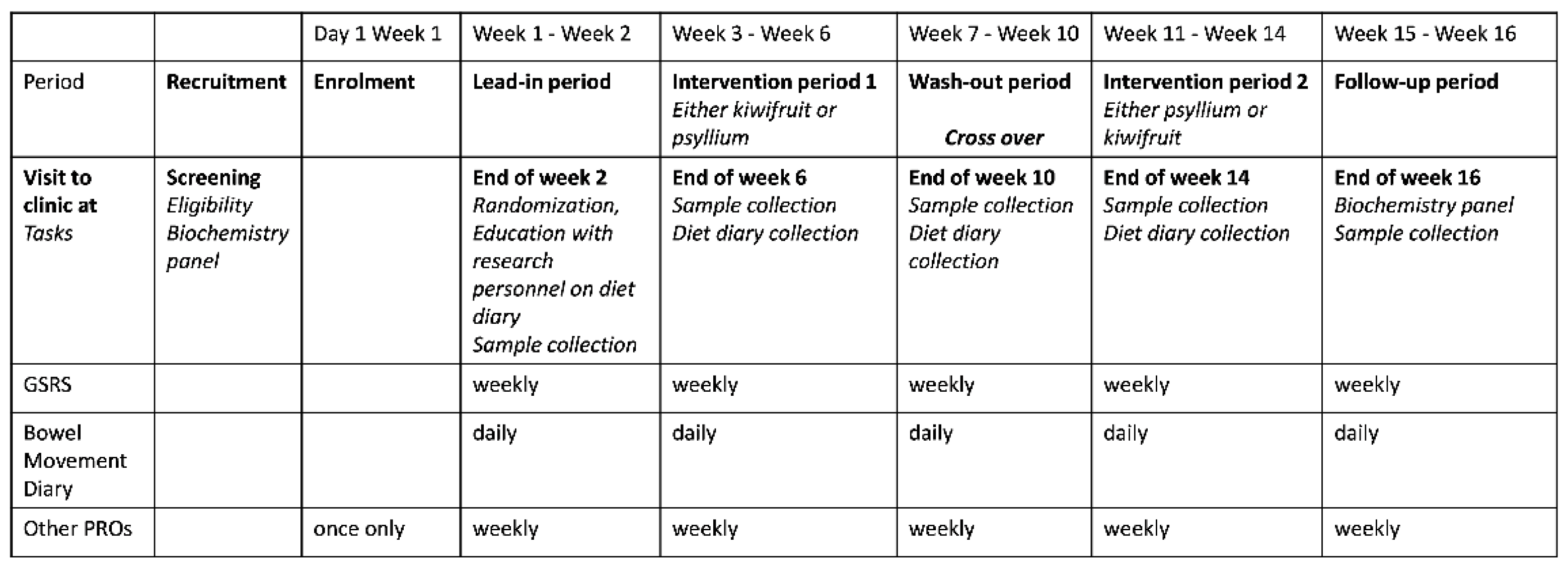
2.1. Study Protocol
2.2. Symptom Assessment
2.3. Clinical Endpoints
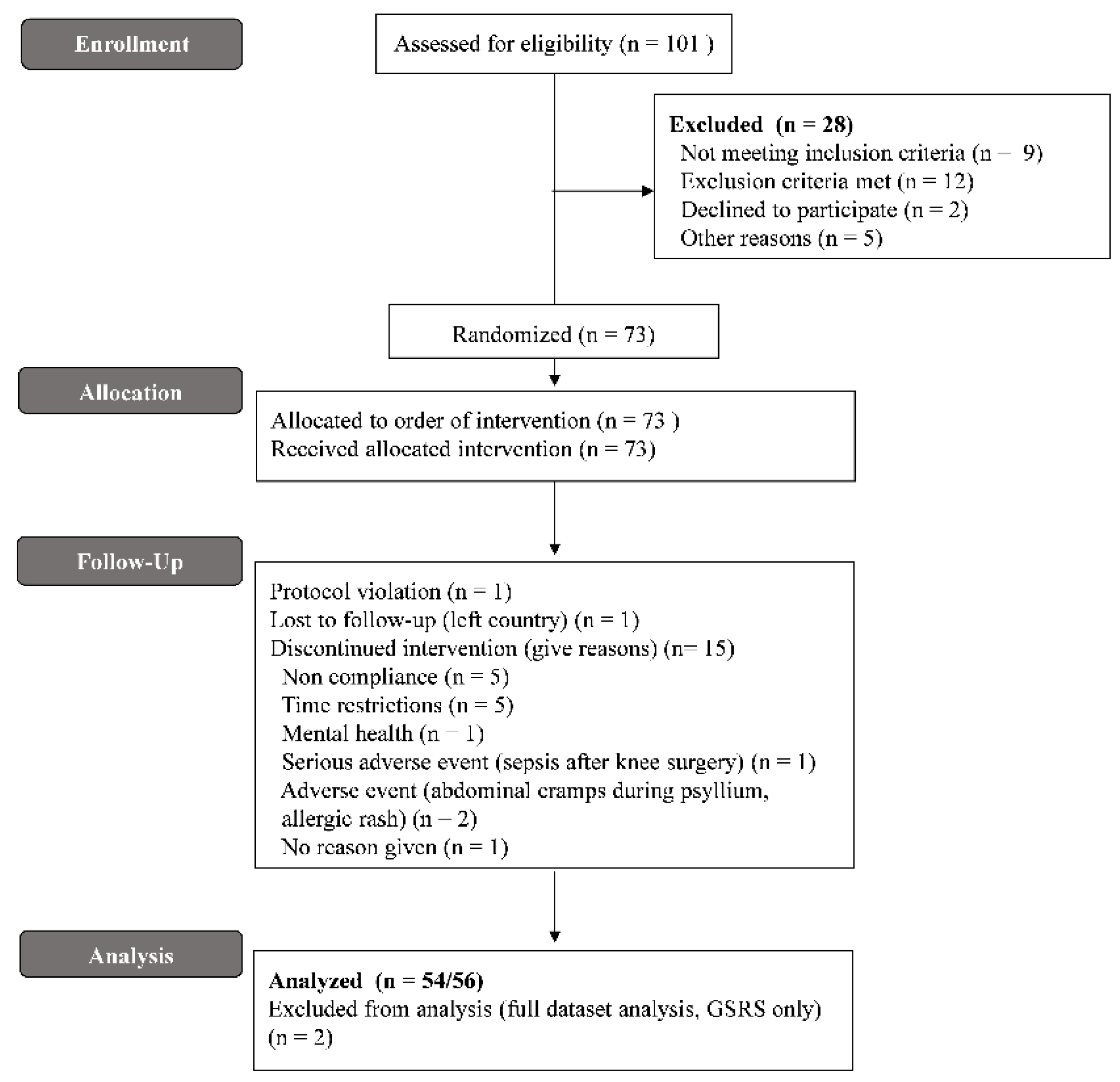
2.4. Protocol Deviation
2.5. Statistical Analysis
3. Results
3.1. Subject Characteristics
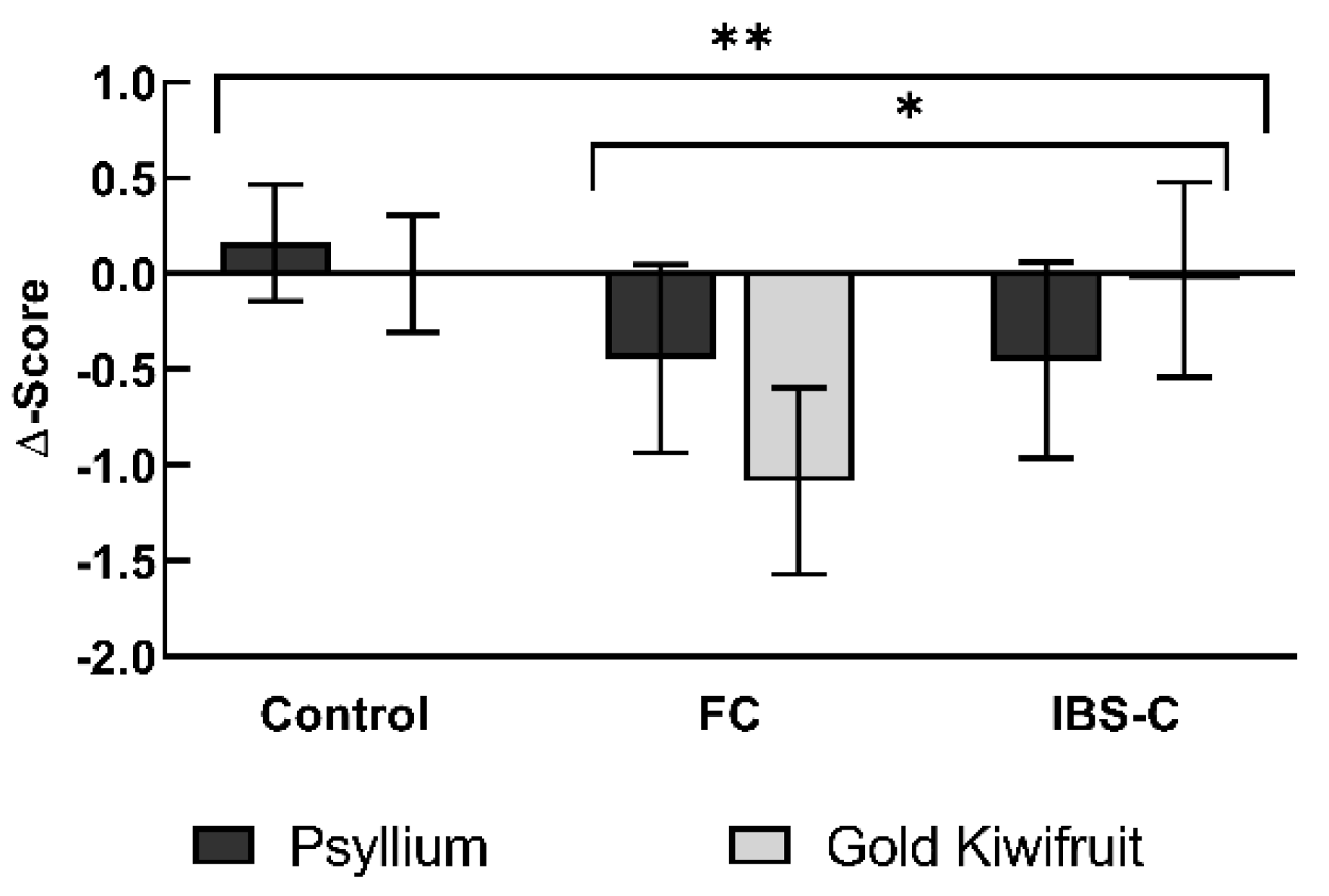
3.2. Primary Clinical Outcome—GSRS Constipation Domain Scores
3.3. Secondary Outcomes
3.3.1. CSBM and Laxative Use
3.3.2. Ease of Defecation
3.3.3. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.E3. [Google Scholar] [CrossRef] [PubMed]
- Barbezat, G.; Poulton, R.; Milne, B.; Howell, S.; Fawcett, J.P.; Talley, N. Prevalence and correlates of irritable bowel symptoms in a New Zealand birth cohort. N. Z. Med. J. 2002, 115, U220. [Google Scholar] [PubMed]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Moayyedi, P.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Quigley, E.M.; Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am. J. Gastroenterol. 2014, 109 (Suppl. 1), S2–S26, quiz S27. [Google Scholar] [CrossRef]
- Poulsen, C.H.; Eplov, L.F.; Hjorthøj, C.; Hastrup, L.H.; Eliasen, M.; Dantoft, T.M.; Schröder, A.; Jørgensen, T. Irritable bowel symptoms, use of healthcare, costs, sickness and disability pension benefits: A long-term population-based study. Scand. J. Public Health 2019, 47, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Maxion-Bergemann, S.; Thielecke, F.; Abel, F.; Bergemann, R. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics 2006, 24, 21–37. [Google Scholar] [CrossRef]
- Talley, N.J.; Gabriel, S.E.; Harmsen, W.S.; Zinsmeister, A.R.; Evans, R.W. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology 1995, 109, 1736–1741. [Google Scholar] [CrossRef]
- Koloski, N.A.; Talley, N.J.; Boyce, P.M. The impact of functional gastrointestinal disorders on quality of life. Am. J. Gastroenterol. 2000, 95, 67–71. [Google Scholar] [CrossRef]
- Lee, C.; Doo, E.; Choi, J.M.; Jang, S.H.; Ryu, H.S.; Lee, J.Y.; Oh, J.H.; Park, J.H.; Kim, Y.S.; Brain-Gut Axis Research Group of Korean Society of Neurogastroenterology and Motility. The Increased Level of Depression and Anxiety in Irritable Bowel Syndrome Patients Compared with Healthy Controls: Systematic Review and Meta-Analysis. J. Neurogastroenterol. Motil. 2017, 23, 349–362. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Irritable Bowel Syndrome and Stress-Related Psychiatric Co-Morbidities: Focus on Early Life Stress. Handb. Exp. Pharmacol. 2017, 239, 219–246. [Google Scholar] [CrossRef]
- Bayer, S.B.; Gearry, R.B.; Drummond, L.N. Putative mechanisms of kiwifruit on maintenance of normal gastrointestinal function. Crit. Rev. Food Sci. Nutr. 2018, 58, 2432–2452. [Google Scholar] [CrossRef]
- Defrees, D.N.; Bailey, J. Irritable Bowel Syndrome: Epidemiology, Pathophysiology, Diagnosis, and Treatment. Prim. Care Clin. Off. Pract. 2017, 44, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Weiser, K.; Noddin, L.; Robertson, D.J.; Crowell, M.D.; Parratt-Engstrom, C.; Grau, M.V. Irritable bowel syndrome: Patients’ attitudes, concerns and level of knowledge. Aliment. Pharmacol. Ther. 2007, 25, 1329–1341. [Google Scholar] [CrossRef]
- Bohn, L.; Storsrud, S.; Tornblom, H.; Bengtsson, U.; Simren, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, Y.T.; Lu, Y.T.; Liu, Y.S.; Liu, J.F. Kiwifruit improves bowel function in patients with irritable bowel syndrome with constipation. Asia Pac. J. Clin. Nutr. 2010, 19, 451–457. [Google Scholar]
- Chan, A.O.; Leung, G.; Tong, T.; Wong, N.Y. Increasing dietary fiber intake in terms of kiwifruit improves constipation in Chinese patients. World J. Gastroenterol. 2007, 13, 4771–4775. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition Novel Foods Food Allergens; Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Green kiwifruit (lat. Actinidia deliciosa var. Hayward) and maintenance of normal defecation: Evaluation of a health claim pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2021, 19, e06641. [Google Scholar] [CrossRef]
- Bayer, S.B.; Frampton, C.M.; Gearry, R.B.; Barbara, G. Habitual Green Kiwifruit Consumption Is Associated with a Reduction in Upper Gastrointestinal Symptoms: A Systematic Scoping Review. Adv. Nutr. 2022, 13, 846–856. [Google Scholar] [CrossRef]
- Eady, S.L.; Wallace, A.J.; Butts, C.A.; Hedderley, D.; Drummond, L.; Ansell, J.; Gearry, R.B. The effect of ‘Zesy002’ kiwifruit (Actinidia chinensis var. chinensis) on gut health function: A randomised cross-over clinical trial. J. Nutr. Sci. 2019, 8, e18. [Google Scholar] [CrossRef]
- Ministry of Health. The Four Food Groups. Available online: https://www.health.govt.nz/your-health/healthy-living/food-activity-and-sleep/healthy-eating/four-food-groups (accessed on 1 April 2022).
- Drummond, L. The composition and nutritional value of kiwifruit. Adv. Food Nutr. Res. 2013, 68, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Sivakumaran, S.; Huffman, L.; Sivakumaran, S.; Drummond, L. The nutritional composition of Zespri® SunGold Kiwifruit and Zespri® Sweet Green Kiwifruit. Food Chem. 2018, 238, 195–202. [Google Scholar] [CrossRef]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef]
- Chao, D. Actinidin: The Predominant Protease in Kiwifruit. Master’s Thesis, Massey University, Palmerston North, New Zealand, 2016. [Google Scholar]
- Chey, S.W.; Chey, W.D.; Jackson, K.; Eswaran, S. Exploratory Comparative Effectiveness Trial of Green Kiwifruit, Psyllium, or Prunes in US Patients with Chronic Constipation. ACG Am. J. Gastroenterol. 2021, 116, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Heenan, P.; Creemers, R.H.; Sharma, S.; Keenan, J.; Bayer, S.; Young, W.; Cooney, J.; Armstrong, K.; Fraser, K.; Skidmore, P.M.; et al. Cohort Profile: The Christchurch IBS cOhort to investigate Mechanisms FOr gut Relief and improved Transit (COMFORT). Inflamm. Intest. Dis. 2020, 5, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Svedlund, J.; Sjodin, I.; Dotevall, G. GSRS—A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- Wright-McNaughton, M.; Ten Bokkel Huinink, S.; Frampton, C.M.A.; McCombie, A.M.; Talley, N.J.; Skidmore, P.M.L.; Gearry, R.B. Measuring Diet Intake and Gastrointestinal Symptoms in Irritable Bowel Syndrome: Validation of the Food and Symptom Times Diary. Clin. Transl. Gastroenterol. 2019, 10, e00103. [Google Scholar] [CrossRef]
- Koloski, N.A.; Jones, M.; Hammer, J.; von Wulffen, M.; Shah, A.; Hoelz, H.; Kutyla, M.; Burger, D.; Martin, N.; Gurusamy, S.R.; et al. The Validity of a New Structured Assessment of Gastrointestinal Symptoms Scale (SAGIS) for Evaluating Symptoms in the Clinical Setting. Dig. Dis. Sci. 2017, 62, 1913–1922. [Google Scholar] [CrossRef]
- Cella, D.; Riley, W.; Stone, A.; Rothrock, N.; Reeve, B.; Yount, S.; Amtmann, D.; Bode, R.; Buysse, D.; Choi, S.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010, 63, 1179–1194. [Google Scholar] [CrossRef]
- Spiegel, B.M.; Hays, R.D.; Bolus, R.; Melmed, G.Y.; Chang, L.; Whitman, C.; Khanna, P.P.; Paz, S.H.; Hays, T.; Reise, S.; et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am. J. Gastroenterol. 2014, 109, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Schunemann, H. How can quality of life researchers make their work more useful to health workers and their patients? Qual. Life Res. 2007, 16, 1097–1105. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Osoba, D.; Wu, A.W.; Wyrwich, K.W.; Norman, G.R.; Clinical Significance Consensus Meeting Group. Methods to explain the clinical significance of health status measures. Mayo Clin. Proc. 2002, 77, 371–383. [Google Scholar] [CrossRef]
- Chan, L.; Mulgaonkar, S.; Walker, R.; Arns, W.; Ambuhl, P.; Schiavelli, R. Patient-reported gastrointestinal symptom burden and health-related quality of life following conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium. Transplantation 2006, 81, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- The New Zealand Institute for Plant and Food Research Limited and New Zealand Ministry of Health. New Zealand Food Composition Database 2022. Available online: https://www.foodcomposition.co.nz (accessed on 19 March 2022).
- Black, C.J.; Yiannakou, Y.; Houghton, L.A.; Ford, A.C. Epidemiological, Clinical, and Psychological Characteristics of Individuals with Self-reported Irritable Bowel Syndrome Based on the Rome IV vs Rome III Criteria. Clin. Gastroenterol. Hepatol. 2020, 18, 392–398.e2. [Google Scholar] [CrossRef]
- Aziz, I.; Tornblom, H.; Palsson, O.S.; Whitehead, W.E.; Simren, M. How the Change in IBS Criteria From Rome III to Rome IV Impacts on Clinical Characteristics and Key Pathophysiological Factors. Am. J. Gastroenterol. 2018, 113, 1017–1025. [Google Scholar] [CrossRef]
- Johanson, J.F.; Wald, A.; Tougas, G.; Chey, W.D.; Novick, J.S.; Lembo, A.J.; Fordham, F.; Guella, M.; Nault, B. Effect of tegaserod in chronic constipation: A randomized, double-blind, controlled trial. Clin. Gastroenterol. Hepatol. 2004, 2, 796–805. [Google Scholar] [CrossRef]
- Rush, E.C.; Patel, M.; Plank, L.D.; Ferguson, L.R. Kiwifruit promotes laxation in the elderly. Asia Pac. J. Clin. Nutr. 2002, 11, 164–168. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Li, T.Y.; Joshipura, K.J.; Hu, F.B. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 2008, 31, 1311–1317. [Google Scholar] [CrossRef]


| Gold Kiwifruit | Psyllium Preparation | |
|---|---|---|
| Daily serving size | 2 fruits, ~118 g per fruit | 1.5 teaspoons, 7.5 g |
| Ingredients | SunGold kiwifruit | Husk powder, sugar, natural orange flavor/color, citric acid |
| Total fiber | 1.4 g dietary fiber per 100 g 1 | 33.6 g dietary fiber per 100 g |
| Vitamin C | 161.3 mg per 100 g fruit 1 | nil |
| Sugar | 12.3 g per 100 g fruit 1 | 40 g sucrose per 100 g 2 |
| Attribute | Control (n = 32) 1 | FC (n = 11) 1 | IBS-C (n = 13) 1 |
|---|---|---|---|
| Gender: | |||
| Female | 26 (81%) | 10 (91%) | 12 (92%) |
| Male | 6 (19%) | 1 (9%) | 1 (8%) |
| Ethnicity (self-reported): | |||
| NZ European | 27 (84%) | 9 (82%) | 9 (69%) |
| Māori | 0 (0%) | 0 (0%) | 2 (15%) |
| Other 2 | 5 (16%) | 2 (18%) | 2 (15%) |
| Age (years) | 35.0 ± 2.7 | 40.2 ± 4.1 | 44.2 ± 3.2 |
| BMI (kg/m2) | 23.9 ± 0.6 | 24.9 ± 1.3 | 24.8 ± 1.2 |
| Weight (kg) | 67.2 ± 1.8 | 68.7 ± 2.8 | 71.3 ± 3.2 |
| Height (m) | 1.68 ± 0.01 | 1.66 ± 0.02 | 1.70 ± 0.09 |
| IBS-SSI * | 25.7 ± 6.6 | 136.7 ± 21.6 | 252.9 ± 10.9 |
| p-Value Factor (n = 54) | ||||||
|---|---|---|---|---|---|---|
| GSRS-Domain 2 (MID [37]) | Participant Group | Gold Kiwifruit 1 | Psyllium 1 | Effect of Interventions 3 | Effects of Participant Group 3 | Gold Kiwifruit vs. Psyllium 3 |
| Constipation | Controls | 0.00 (0.30, −0.30) | 0.16 (0.47, −0.14) | p = 0.004 ** | p = 0.044 * | p = 0.407 |
| (±0.6) | FC | −1.08 (−0.60, −1.57) | −0.44 (0.05, −0.94) | |||
| IBS-C | −0.03 (0.48, −0.54) | −0.45 (0.06, −0.97) | ||||
| Diarrhea | Controls | 0.18 (0.44, −0.07) | 0.08 (0.31, −0.16) | p = 0.722 | p = 0.679 | p = 0.281 |
| (±0.4) | FC | 0.00 (0.41, −0.41) | −0.03 (0.35, −0.41) | |||
| IBS-C | 0.15 (0.58, −0.28) | −0.24 (0.15, −0.64) | ||||
| Indigestion | Controls | −0.02 (0.20, −0.25) | 0.04 (0.21, −0.13) | p = 0.008 ** | p = 0.457 | p = 0.056 |
| (±0.7) | FC | −0.54 (−0.18, −0.90) | −0.33 (−0.06, −0.61) | |||
| IBS-C | −0.27 (0.10, −0.65) | 0.11 (0.40, −0.17) | ||||
| Pain | Controls | 0.03 (0.34, −0.28) | 0.00 (0.15, −0.15) | p = 0.290 | p = 0.750 | p = 0.581 |
| (±0.6) | FC | −0.31 (0.19, −0.80) | −0.25 (−0.01, −0.49) | |||
| IBS-C | −0.09 (0.43, −0.61) | 0.09 (0.34, −0.16) | ||||
| Reflux | Controls | −0.05 (0.17, −0.27) | 0.03 (0.21, −0.15) | p = 0.071 | p = 0.120 | p = 0.261 |
| (±0.8) | FC | −0.08 (0.27, −0.43) | −0.25 (0.04, −0.54) | |||
| IBS-C | −0.41 (−0.04, −0.77) | 0.05 (0.35, −0.26) |
| p-Value Factor (n = 54) | ||||||
|---|---|---|---|---|---|---|
| Daily BM Diary 2 | Participant Group | Gold Kiwifruit 1 | Psyllium 1 | Effect of Interventions 3 | Effects of Participant Group 3 | Gold Kiwifruit vs. Psyllium 3 |
| Total BMs | Controls | −0.22 (1.14, −1.58) | 0.25 (1.45, −0.95) | p = 0.906 | p = 0.747 | p = 0.134 |
| FC | −0.81 (1.37, −2.99) | 0.47 (2.40, −1.45) | ||||
| IBS-C | −0.56 (1.53, −2.66) | 1.13 (2.98, −0.72) | ||||
| Complete BM | Controls | −0.03 (1.19, −1.24) | −0.01 (1.28, −1.29) | p = 0.006 ** | p = 0.981 | p = 0.820 |
| FC | 1.36 (3.31, −0.60) | 1.49 (3.55, −0.58) | ||||
| IBS-C | 1.44 (3.32, −0.44) | 1.78 (3.77, −0.20) | ||||
| Spontaneous BM | Controls | −0.25 (1.10, −1.60) | 0.45 (1.70, −0.80) | p = 0.962 | p = 0.913 | p = 0.154 |
| FC | −0.87 (1.29, −3.04) | 0.39 (2.40, −1.62) | ||||
| IBS-C | −0.56 (1.52, −2.65) | 0.74 (2.67, −1.19) | ||||
| CSBM | Controls | 0.04 (1.26, −1.17) | −0.04 (1.23, −1.30) | p = 0.014 * | p = 0.886 | p = 0.632 |
| FC | 1.08 (3.03, −0.87) | 1.57 (3.60, −0.47) | ||||
| IBS-C | 0.99 (2.86, −0.88) | 1.63 (3.58, −0.33) | ||||
| Manual Evacuation | Controls | 0.00 (0.26, −0.25) | 0.17 (0.67, −0.32) | p = 0.725 | p = 0.312 | p = 0.359 |
| Techniques | FC | 0.25 (0.66, −0.16) | 0.08 (0.89, −0.72) | |||
| IBS-C | 0.21 (0.60, −0.19) | −0.51 (0.26, −1.28) | ||||
| Straining | Controls | −0.50 (0.36, −1.36) | 0.23 (1.17, −0.71) | p = 0.151 | p = 0.623 | p = 0.021 * |
| FC | −1.10 (0.29, −2.48) | 0.81 (2.32, −0.70) | ||||
| IBS-C | −1.37 (−0.04, −2.70) | −0.34 (1.11, −1.79) | ||||
| BSFS | Controls | 0.34 (0.75, −0.07) | 0.08 (0.57, −0.40) | p = 0.027 * | p = 0.643 | p = 0.842 |
| FC | 0.29 (0.95, −0.37) | 0.46 (1.24, −0.32) | ||||
| IBS-C | 0.12 (0.75, −0.52) | 0.37 (1.12, −0.38) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayer, S.B.; Heenan, P.; Frampton, C.; Wall, C.L.; Drummond, L.N.; Roy, N.C.; Gearry, R.B. Two Gold Kiwifruit Daily for Effective Treatment of Constipation in Adults—A Randomized Clinical Trial. Nutrients 2022, 14, 4146. https://doi.org/10.3390/nu14194146
Bayer SB, Heenan P, Frampton C, Wall CL, Drummond LN, Roy NC, Gearry RB. Two Gold Kiwifruit Daily for Effective Treatment of Constipation in Adults—A Randomized Clinical Trial. Nutrients. 2022; 14(19):4146. https://doi.org/10.3390/nu14194146
Chicago/Turabian StyleBayer, Simone B., Phoebe Heenan, Chris Frampton, Catherine L. Wall, Lynley N. Drummond, Nicole C. Roy, and Richard B. Gearry. 2022. "Two Gold Kiwifruit Daily for Effective Treatment of Constipation in Adults—A Randomized Clinical Trial" Nutrients 14, no. 19: 4146. https://doi.org/10.3390/nu14194146
APA StyleBayer, S. B., Heenan, P., Frampton, C., Wall, C. L., Drummond, L. N., Roy, N. C., & Gearry, R. B. (2022). Two Gold Kiwifruit Daily for Effective Treatment of Constipation in Adults—A Randomized Clinical Trial. Nutrients, 14(19), 4146. https://doi.org/10.3390/nu14194146





