The Efficacy of S-Adenosyl Methionine and Probiotic Supplementation on Depression: A Synergistic Approach
Abstract
:1. Introduction
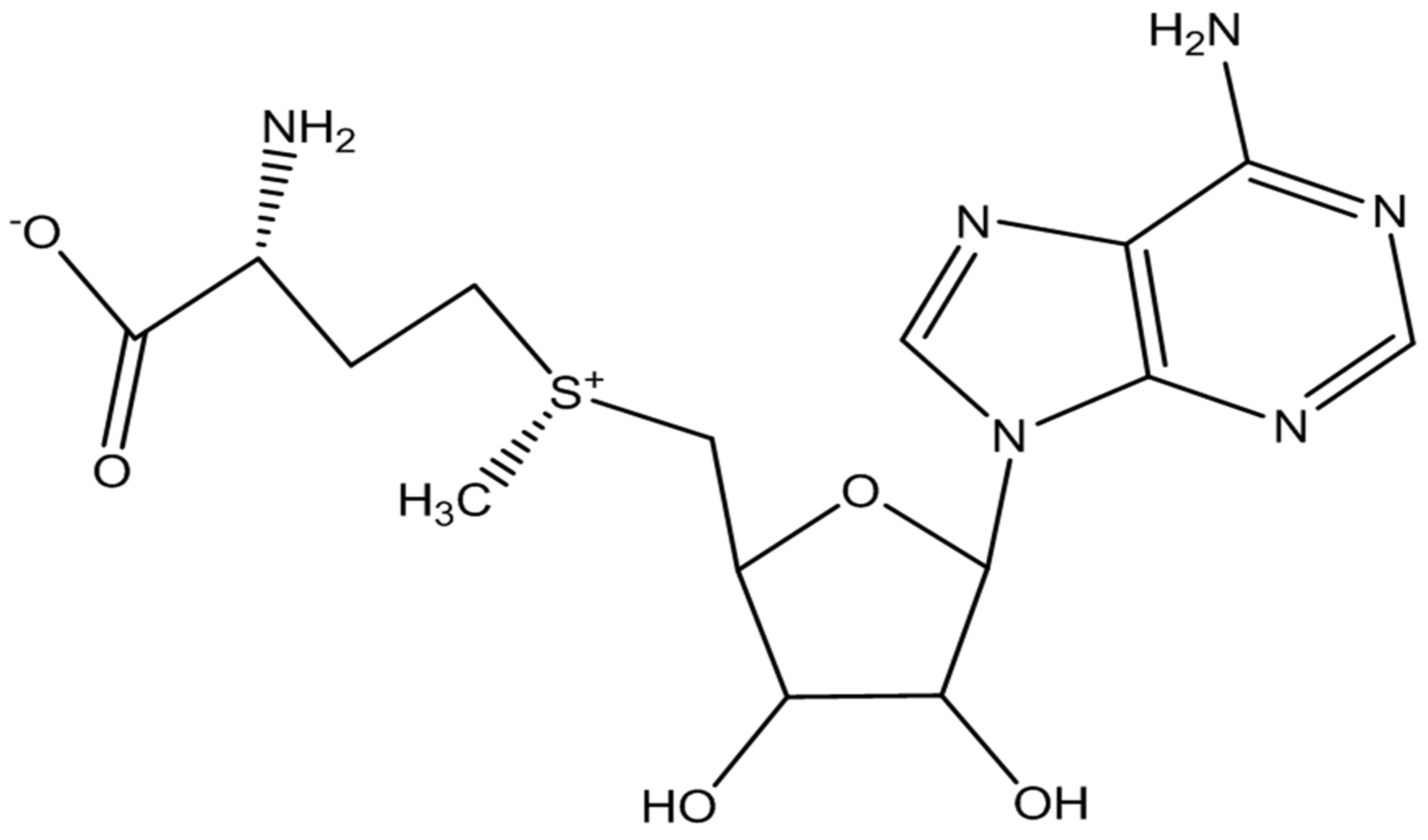
2. SAMe and Neurobiological Pathways Related to Depression
2.1. Antidepressant Efficacy of SAMe
| Mental Condition | Study Design | Intervention | Main Findings | References |
|---|---|---|---|---|
| MDD, with mild to moderate depression symptoms | Double-blind RCT. A total of 49 patients with no concurrent antidepressant therapy were recruited in the trial. | SAMe (800 mg) or placebo for 8 weeks. | Modest improvement of depressive symptoms in both SAMe and placebo groups. Increased folate concentrations in SAMe treated patients, correlated with improvement in symptoms. No change in one-carbon cycle biomarkers, BDNF, and SNPs. | [32] |
| Mild to moderate depression | Randomized observational controlled trial. A total of 46 subjects were enrolled in the trial. | SAMe (800 mg/day) (n = 23) or SAMe (750 mg/day + betaine 375 mg/day) (n = 23) for 90 days. | Improvement of depressive symptoms in both groups. Combination of SAMe and betaine demonstrated more effectiveness in the remission of symptoms, with mild to moderate depression. | [33] |
| MDD and cognitive deficits | Double-blind RCT. A total of 46 SRI nonresponders with MDD were selected. | Adjunctive SAMe (800 mg/day) or placebo for 6 weeks. | Significant improvement in memory-related cognitive symptoms. | [34] |
| MDD | Double-blind RCT. A total of 189 patients were recruited in the study for comparative analysis of SAMe and escitalopram. | SAMe (1600–3200 mg/day), escitalopram (10–20 mg/day), or placebo for 12 weeks. | No significant differences were noted in remission rates with SAMe and escitalopram. SAMe was more tolerable, with mild to moderate GI side effects. | [35] |
| MDD | Double-blind RCT. A total of 144 patients were recruited in the study for comparative analysis of SAMe and escitalopram. | SAMe (1600–3200 mg/day), escitalopram (10–20 mg/day), or placebo for 12 weeks. | SAMe was slightly more efficacious than escitalopram. The remission rates were 34% for SAMe, 23% for escitalopram, and 6% for placebo. | [36] |
| MDD | Double-blind RCT. A total of 73 SRI nonresponders (age: 18–80 years) were selected for the study. | Adjunctive oral SAMe with a target dose of 1600 mg/day or placebo for 6 weeks. | Responsive and remission rates with adjunctive SAMe were 36.1% and 25.8%, respectively, as compared with placebo (17.6% and 11. 7%). | [42] |
| Depression in patients with PD | Open-label clinical trial. A total of 13 patients were enrolled in the study. | SAMe (800–3600 mg/day). | A total of 11 patients completed the study, and 2 prematurely terminated because of increased anxiety. A total of 10 patients demonstrated at least 50% improvement on the HDRS-17 scale. | [37] |
| Antidepressants induced sexual dysfunction | Double-blind RCT. A total of 73 patients (age: 18–80 years) were randomized in two treatment groups (SAMe or placebo). | Augmentation of SSRI/SNRI with SAMe (800 mg/day) or placebo for 6 weeks. | Significant improvement in arousal and erectile dysfunctions in SAMe-augmented men at endpoint as compared with placebo-treated patients. | [39] |
2.2. Mechanisms of Action
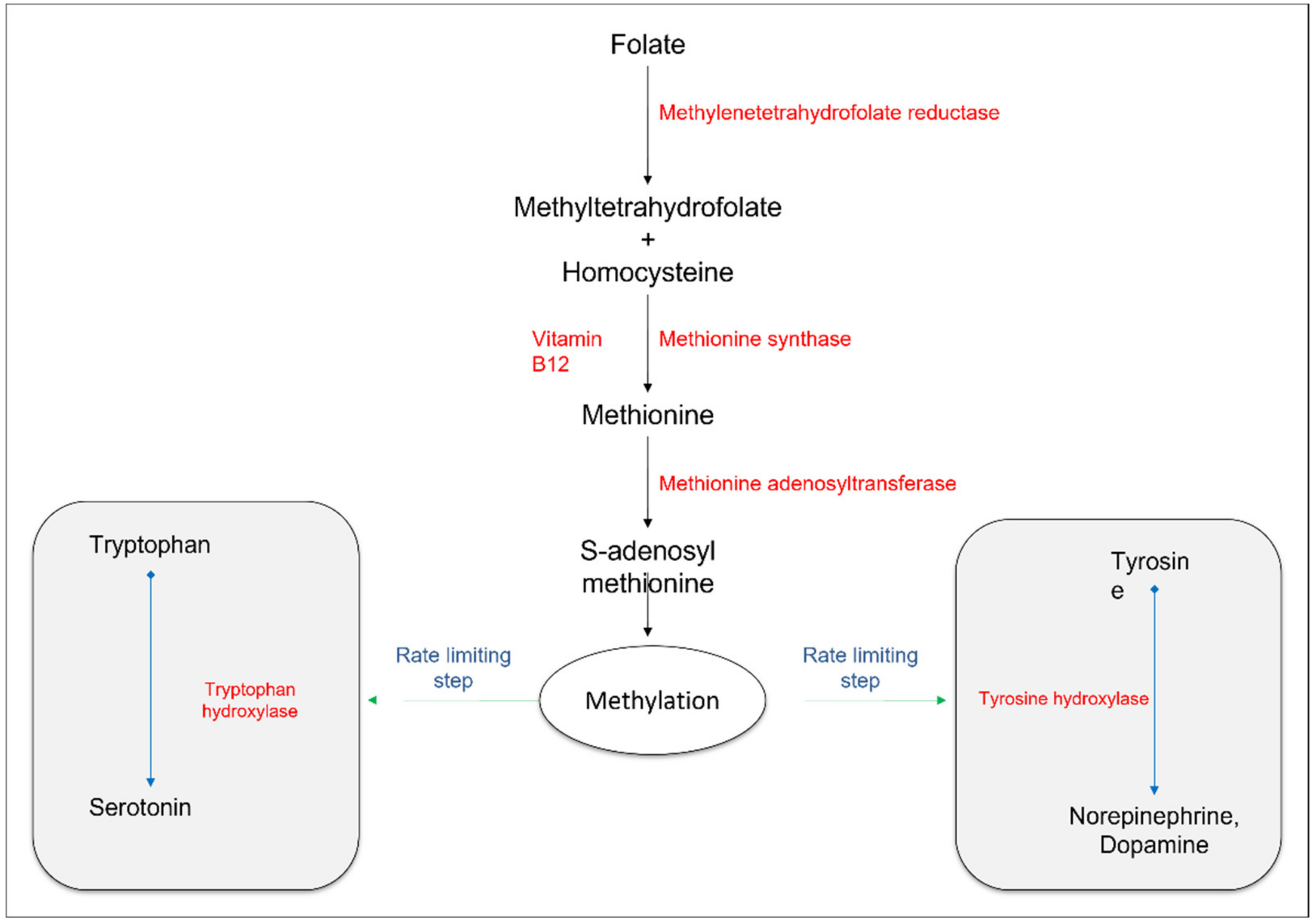
2.2.1. Transmethylation Pathways
2.2.2. Monoamine Neurotransmitters
2.3. Safety Concerns
2.4. The Possible Influence of New Dosage Forms
3. Gastrointestinal Microbiota and Neurobiological Pathways Related to Depression
3.1. Probiotics and Antidepressant Potential
| Mental Condition | Study Design | Intervention | Main Findings | References |
|---|---|---|---|---|
| IBS with mild to moderate depression and anxiety | Double-blind RCT. A total of 44 adult patients (age: 26–58 years) with IBS and mild to moderate depression and anxiety were selected. | B. longum (1 × 1010 CFU/g powder) (n = 22) or placebo (n = 22) for 6 weeks. | B. longum reduced depression scores but not anxiety scores with improvements in quality of life. | [101] |
| MDD | Double-blind RCT. A total of 110 patients were randomized to receive probiotic, prebiotic, or placebo. | Probiotic supplement (L. helveticus R0052 and B. longum R0175), prebiotic (galacto-oligosaccharide), or placebo for 8 weeks. | Probiotic supplementation decreased BDI scores, increased tryptophan/isoleucine ratio, and reduced kynurenine/tryptophan ratio, compared with placebo. No such effects were seen with prebiotics. | [102] |
| Healthy medical students | Double-blind placebo-controlled trial. Healthy medical students were recruited in the trial to evaluate the effects of probiotic species in the prevention of the onset of physical symptoms. | Fermented milk containing L. casei strain Shirota (n = 24) or placebo (n = 23) for 8 weeks. | Fermented milk reduced the onset of physical symptoms, with a significant elevation of fecal serotonin levels. No difference was found in HADS-anxiety, HADS-depression, SDS, and PSQI scores in both groups. | [103] |
| Healthy individuals | Triple-blind placebo-controlled randomized pre- and postintervention assessment design. A total of 40 healthy individuals participated in the evaluation of the effects of a multispecies probiotic supplement in the reduction of cognitive reactivity to sad mood. | Multispecies probiotic supplement (B. lactis W52, B. bifidum W23, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, L. lactis W19, and L. lactis W58) or placebo for 4 weeks. | LEIDS-r showed a significant reduction in the cognitive reactivity to depression, in particular, rumination and aggressive thoughts, in the probiotic-supplement-treated group. | [104] |
| Depressed mood | Double-blind randomized placebo-controlled trial. A total of 132 healthy individuals (mean age: 61.8 years) with poor mood voluntarily participated in the study. | Yoghurt containing L. casei Shirota (108/mL) or placebo for 3 weeks. | Improvement of mood with the consumption of a probiotic-containing yoghurt. | [105] |
| MDD | Double-blind placebo-controlled randomized clinical trial. A total of 110 patients with MDD (age: 18–50 years) were recruited in the trial. | Probiotic (L. helveticus R0052 and B. longum R0175), prebiotic (galacto-oligosaccharide), or placebo for 8 weeks. | No change in the level of inflammatory markers. Probiotic supplementation significantly decreased urinary cortisol levels and BDI scores. No antidepressant effects were seen with prebiotics. | [106] |
| MDD | Double-blind placebo-controlled randomized clinical trial. A total of 40 patients with MDD and in the age range of 20–55 years were recruited in the study. | Probiotic supplement containing L. acidophilus (2 × 109 CFU/g), L. casei (2 × 109 CFU/g), and B. bifidum (2 × 109 CFU/g) or placebo for 8 weeks. | Significant improvement in BDI scores, serum insulin concentration, HOMA-IR and hs-CRP, and glutathione concentrations with probiotic administration. | [107] |
| Depressive symptoms in mild to severe depression | Triple-blind placebo-controlled randomized clinical trial. A total of 71 patients with mild to severe depression were randomized in the intervention (mean age: 36.65 years) or placebo (mean age: 35.49 years) groups. | Ecologic® Barrier (2.5 × 109 CFU/g) containing B. bifidum W23, B. lactis W51, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, L. lactis W19, and L. lactis W58 (total cell count: 1 × 1010 CFU/day) or placebo for 8 weeks. | Significant improvement of cognitive symptoms in the intervention group as compared with the placebo group, especially in patients with mild to moderate depression. No significant effect on gut microbial alteration. | [108] |
| Inpatients diagnosed with depression | Randomized double-blind controlled trial. A total of 82 inpatients diagnosed with depression were randomized in the intervention group (n = 42) or placebo (n = 40). | OMNi-BiOTiC® Stress Repair (B. bifidum W23, B. lactis W51, B. lactis W52, L. acidophilus W22, L. casei W56, L. paracasei W20, L. plantarum W62, L. salivarius W24, and L. lactis W19) plus 125 mg D-biotin or placebo plus D-biotin for 4 weeks. | No significant differences between both groups in psychiatric symptoms. More abundance of Ruminococcus gauvreauii and Coprococcus 3 strains and elevation of inflammatory regulatory and metabolic pathways in the intervention group. | [110] |
3.2. Mechanistic Targets
4. The Possible Combination of Probiotics and S-Adenosyl Methionine
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- WHO Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 25 May 2022).
- Juruena, M.F. Understanding subthreshold depression. Shanghai Arch. Psychiatry 2012, 24, 292. [Google Scholar] [PubMed]
- Rodríguez, M.R.; Nuevo, R.; Chatterji, S.; Ayuso-Mateos, J.L. Definitions and factors associated with subthreshold depressive conditions: A systematic review. BMC Psychiatry 2012, 12, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, S.K.; Rapaport, M.H. Classification and treatment of sub-threshold depression. Curr. Opin. Psychiatry 2006, 19, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.S.; Jin, A.; Nyunt, M.S.Z.; Feng, L.; Ng, T.P. Mortality rates in major and subthreshold depression: 10-year follow-up of a Singaporean population cohort of older adults. Postgrad. Med. 2016, 128, 642–647. [Google Scholar] [CrossRef]
- Biella, M.M.; Borges, M.K.; Strauss, J.; Mauer, S.; Martinelli, J.E.; Aprahamian, I. Subthreshold depression needs a prime time in old age psychiatry? A narrative review of current evidence. Neuropsychiatr. Dis. Treat. 2019, 15, 2763–2772. [Google Scholar] [CrossRef] [Green Version]
- Spijker, J.; Nolen, W.A. An algorithm for the pharmacological treatment of depression: Clinical overview. Acta Psychiatr. Scand. 2010, 121, 180–189. [Google Scholar] [CrossRef]
- Jeuring, H.W.; Huisman, M.; Comijs, H.C.; Stek, M.L.; Beekman, A.T.F. The long-term outcome of subthreshold depression in later life. Psychol. Med. 2016, 46, 2855–2865. [Google Scholar] [CrossRef]
- Williams, J.W.; Barrett, J.; Oxman, T.; Frank, E.; Katon, W.; Sullivan, M.; Cornell, J.; Sengupta, A. Treatment of dysthymia and minor depression in primary care: A randomized controlled trial in older adults. J. Am. Med. Assoc. 2000, 284, 1519–1526. [Google Scholar] [CrossRef] [Green Version]
- Barbui, C.; Cipriani, A.; Patel, V.; Ayuso-Mateos, J.L.; van Ommeren, M. Efficacy of antidepressants and benzodiazepines in minor depression: Systematic review and meta-analysis. Br. J. Psychiatry 2011, 198, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Stimpson, N.; Agrawal, N.; Lewis, G. Randomised controlled trials investigating pharmacological and psychological interventions for treatment-refractory depression. Br. J. Psychiatry 2002, 181, 284–294. [Google Scholar] [CrossRef] [Green Version]
- Rayner, L.; Price, A.; Evans, A.; Valsraj, K.; Higginson, I.J.; Hotopf, M. Antidepressants for depression in physically Ill People. Cochrane Database Syst. Rev. 2010, 17, CD007503. [Google Scholar] [CrossRef] [PubMed]
- Kapczinski, F.F.; Lima, M.S.; Souza, J.S.; Schmitt, R. Antidepressant for generalized anxiety disorder (Review). Cochrane Database Syst. Rev. 2016, 3, CD003592. [Google Scholar] [PubMed] [Green Version]
- Gabriel, M.; Sharma, V. Antidepressant discontinuation syndrome. CMAJ 2017, 189, E747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacka, F.N.; Berk, M. Depression, diet and exercise. Med. J. Aust. 2013, 199, S21–S23. [Google Scholar] [CrossRef]
- Bamber, D.J.; Stokes, C.S.; Stephen, A.M. The role of diet in the prevention and management of adolescent depression. Nutr. Bull. 2007, 32, 90–99. [Google Scholar] [CrossRef]
- Khalid, S.; Williams, C.M.; Reynolds, S.A. Is there an association between diet and depression in children and adolescents? A systematic review. Br. J. Nutr. 2016, 116, 2097–2108. [Google Scholar] [CrossRef]
- Hoffmann, K.; Emons, B.; Brunnhuber, S.; Karaca, S.; Juckel, G. The role of dietary supplements in depression and anxiety-a narrative review. Pharmacopsychiatry 2019, 52, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Andrade, C. N-acetylcysteine augmentation for patients with major depressive disorder and bipolar depression. J. Clin. Psychiatry 2021, 82, 25943. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Cassiello, C.F.; Iovieno, N. Folates and S-adenosylmethionine for major depressive disorder. Can. J. Psychiatry 2012, 57, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef]
- Shayganfard, M. Are essential trace elements effective in modulation of mental disorders? Update and perspectives. Biol. Trace Elem. Res. 2022, 200, 1032–1059. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, G.L. The nature of the active methyl donor formed enzymatically from L-methionine and adenosinetriphosphate. J. Am. Chem. Soc. 1952, 74, 2942–2943. [Google Scholar] [CrossRef]
- Shaw, S. S-adenosylmethionine (SAMe). In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 11–17. [Google Scholar]
- Grillo, M.A.; Colombatto, S. S-adenosylmethionine and its products. Amino Acids 2008, 34, 187–193. [Google Scholar] [CrossRef]
- Chu, J.; Qian, J.; Zhuang, Y.; Zhang, S.; Li, Y. Progress in the research of S-adenosyl-l-methionine production. Appl. Microbiol. Biotechnol. 2013, 97, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gerbarg, P.; Bottiglieri, T.; Massoumi, L.; Carpenter, L.L.; Lavretsky, H.; Muskin, P.R.; Brown, R.P.; Mischoulon, D. S-adenosylmethionine (SAMe) for neuropsychiatric disorders: A clinician-oriented review of research. J. Clin. Psychiatry 2017, 78, 656. [Google Scholar] [CrossRef] [Green Version]
- Obeid, R.; Herrmann, W. Homocysteine and lipids: S-adenosyl methionine as a key intermediate. FEBS Lett. 2009, 583, 1215–1225. [Google Scholar] [CrossRef] [Green Version]
- Mischoulon, D.; Fava, M. Role of S-adenosyl-l-methionine in the treatment of depression: A review of the evidence. Am. J. Clin. Nutr. 2002, 76, 1158S–1161S. [Google Scholar] [CrossRef] [Green Version]
- Papakostas, G.I.; Alpert, J.E.; Fava, M. S-adenosyl-methionine in depression: A comprehensive review of the literature. Curr. Psychiatry Rep. 2003, 5, 460–466. [Google Scholar] [CrossRef]
- Sarris, J.; Murphy, J.; Stough, C.; Mischoulon, D.; Bousman, C.; MacDonald, P.; Adams, L.; Nazareth, S.; Oliver, G.; Cribb, L.; et al. S-adenosylmethionine (SAMe) monotherapy for depression: An 8-week double-blind, randomised, controlled trial. Psychopharmacology 2020, 237, 209–218. [Google Scholar] [CrossRef]
- Di Pierro, F.; Orsi, R.; Settembre, R. Role of betaine in improving the antidepressant effect of S-adenosyl-methionine in patients with mild-to-moderate depression. J. Multidiscip. Healthc. 2015, 8, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Levkovitz, Y.; Alpert, J.E.; Brintz, C.E.; Mischoulon, D.; Papakostas, G.I. Effects of S-adenosylmethionine augmentation of serotonin-reuptake inhibitor antidepressants on cognitive symptoms of major depressive disorder. J. Affect. Disord. 2012, 136, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Mischoulon, D.; Price, L.H.; Carpenter, L.L.; Tyrka, A.R.; Papakostas, G.I.; Baer, L.; Dording, C.M.; Clain, A.J.; Durham, K.; Walker, R.; et al. A double-blind, randomized, placebo-controlled clinical trial of S-adenosyl-l-methionine (SAMe) versus escitalopram in major depressive disorder. J. Clin. Psychiatry 2014, 75, 370–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarris, J.; Papakostas, G.I.; Vitolo, O.; Fava, M.; Mischoulon, D. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: Efficacy and effects of histamine and carnitine as moderators of response. J. Affect. Disord. 2014, 64, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, A.; Rogers, J.D.; Brown, R.; Werner, P.; Bottiglieri, T. S-adenosyl-methionine improves Depression in Patients with Parkinson’s Disease in an Open-Label Clinical Trial. Mov. Disord. 2000, 15, 1225–1229. [Google Scholar] [CrossRef]
- Healy, D. Antidepressants and sexual dysfunction: A history. J. R. Soc. Med. 2020, 113, 133–135. [Google Scholar] [CrossRef]
- Dording, C.M.; Mischoulon, D.; Shyu, I.; Alpert, J.E.; Papakostas, G.I. SAMe and sexual functioning. Eur. Psychiatry 2012, 27, 451–454. [Google Scholar] [CrossRef]
- Cuomo, A.; Crescenzi, B.B.; Bolognesi, S.; Goracci, A.; Koukouna, D.; Rossi, R.; Fagiolini, A. Adenosylmethionine (SAMe) in major depressive disorder (mdd): A clinician-oriented systematic review. Ann. Gen. Psychiatry 2020, 19, 1–7. [Google Scholar] [CrossRef]
- Galizia, I.; Oldani, L.; Macritchie, K.; Amari, E.; Dougall, D.; Jones, T.N.; Lam, R.W.; Massei, G.J.; Yatham, L.N.; Young, A.H. S-adenosyl methionine (SAMe) for depression in adults. Cochrane Database Syst. Rev. 2016, 10, CD011286. [Google Scholar] [CrossRef] [Green Version]
- Papakostas, G.I.; Mischoulon, D.; Shyu, I.; Alpert, J.E.; Fava, M. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: A double-blind, randomized clinical trial. Am. J. Psychiatry 2010, 167, 942–948. [Google Scholar] [CrossRef]
- Gao, J.; Cahill, C.M.; Huang, X.; Roffman, J.L.; Lamon-Fava, S.; Fava, M.; Mischoulon, D.; Rogers, J.T. S-adenosyl methionine and transmethylation pathways in neuropsychiatric diseases throughout life. Neurotherapeutics 2018, 15, 156–175. [Google Scholar] [CrossRef] [Green Version]
- Karas Kuželički, N. S-adenosyl methionine in the therapy of depression and other psychiatric disorders. Drug Dev. Res. 2016, 77, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Meng, L.; Pei, F.; Zheng, Y.; Leng, J. A review of DNA methylation in depression. J. Clin. Neurosci. 2017, 43, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.S.; Deth, R.C. Role of a redox-based methylation switch in Mrna life cycle (Pre and post-transcriptional maturation) and protein turnover: Implications in neurological disorders. Front. Neurosci. 2012, 6, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boks, M.P.; de Jong, N.M.; Kas, M.J.H.; Vinkers, C.H.; Fernandes, C.; Kahn, R.S.; Mill, J.; Ophoff, R.A. Current status and future prospects for epigenetic psychopharmacology. Epigenetics 2012, 7, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Pfalzer, A.C.; Choi, S.W.; Tammen, S.A.; Park, L.K.; Bottiglieri, T.; Parnell, L.D.; Lamon-Fava, S. S-adenosylmethionine mediates inhibition of inflammatory response and changes in DNA methylation in human macrophages. Physiol. Genom. 2014, 46, 617–623. [Google Scholar] [CrossRef]
- Gobejishvili, L.; Avila, D.V.; Barker, D.F.; Ghare, S.; Henderson, D.; Brock, G.N.; Kirpich, I.A.; Joshi-Barve, S.; Mokshagundam, S.P.L.; McClain, C.J. S-adenosylmethionine decreases lipopolysaccharide-induced phosphodiesterase 4B2 and attenuates tumor necrosis factor expression via CAMP/Protein kinase A pathway. J. Pharmacol. Exp. Ther. 2011, 337, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Peedicayil, J. Role of epigenetics in pharmacotherapy, psychotherapy and nutritional management of mental disorders. J. Clin. Pharm. Ther. 2012, 37, 499–501. [Google Scholar] [CrossRef]
- Brummelte, S.; Mc Glanaghy, E.; Bonnin, A.; Oberlander, T.F. Developmental changes in serotonin signaling: Implications for early brain function, behavior and adaptation. Neuroscience 2017, 342, 212–231. [Google Scholar] [CrossRef] [Green Version]
- May, J.M.; Qu, Z.-C.; Nazarewicz, R.; Dikalov, S. Ascorbic acid efficiently enhances neuronal synthesis of norepinephrine from dopamine. Brain Res. Bull. 2013, 90, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Haleem, D.J. Improving therapeutics in anorexia nervosa with tryptophan. Life Sci. 2017, 178, 87–93. [Google Scholar] [CrossRef]
- Moat, S.J.; Clarke, Z.L.; Madhavan, A.K.; Lewis, M.J.; Lang, D. Folic acid reverses endothelial dysfunction induced by inhibition of tetrahydrobiopterin biosynthesis. Eur. J. Pharmacol. 2006, 530, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Sumi-Ichinose, C.; Urano, F.; Kuroda, R.; Ohye, T.; Kojima, M.; Tazawa, M.; Shiraishi, H.; Hagino, Y.; Nagatsu, T.; Nomura, T.; et al. Catecholamines and serotonin are differently regulated by tetrahydrobiopterin: A study from 6-pyruvoyltetrahydropterin synthase knockout mice. J. Biol. Chem. 2001, 276, 41150–41160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottiglieri, T.; Laundy, M.; Crellin, R.; Toone, B.K.; Carney, M.W.P.; Reynolds, E.H. Homocysteine, folate, methylation, and monoamine metabolism in depression. J. Neurol. Neurosurg. Psychiatry 2000, 69, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.; Liu, T.; Peter, I.; Buel, J.; Arsenault, L.; Scott, T.; Qiu, W.W. The homocysteine hypothesis of depression. Am. J. Psychiatry 2007, 164, 861–867. [Google Scholar] [CrossRef]
- Gören, J.L.; Stoll, A.L.; Damico, K.E.; Sarmiento, I.A.; Cohen, B.M. Bioavailability and lack of toxicity of S-adenosyl-l-methionine (SAMe) in humans. Pharmacotherapy 2004, 24, 1501–1507. [Google Scholar] [CrossRef]
- Abeysundera, H.; Gill, R. Possible SAMe-induced mania. BMJ Case Rep. 2018, 2018, bcr2018224338. [Google Scholar] [CrossRef]
- Targum, S.D.; Cameron, B.R.; Ferreira, L.; MacDonald, I.D. An augmentation study of MSI-195 (S-adenosylmethionine) in major depressive disorder. J. Psychiatr. Res. 2018, 107, 86–96. [Google Scholar] [CrossRef]
- Sakurai, H.; Carpenter, L.L.; Tyrka, A.R.; Price, L.H.; Papakostas, G.I.; Dording, C.M.; Yeung, A.S.; Cusin, C.; Ludington, E.; Bernard-Negron, R.; et al. Dose increase of S-adenosyl-methionine and escitalopram in a randomized clinical trial for major depressive disorder. J. Affect. Disord. 2020, 262, 118–125. [Google Scholar] [CrossRef]
- Green, T.; Steingart, L.; Frisch, A.; Zarchi, O.; Weizman, A.; Gothelf, D. The feasibility and safety of S-adenosyl-l-methionine (SAMe) for the treatment of neuropsychiatric symptoms in 22q11.2 deletion syndrome: A double-blind placebo-controlled trial. J. Neural Transm. 2012, 119, 1417–1423. [Google Scholar] [CrossRef]
- Bottiglieri, T. S-adenosyl-l-methionine (SAMe): From the bench to the bedside-molecular basis of a pleiotrophic molecule. Am. J. Clin. Nutr. 2002, 76, 1151S–1157S. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, I.D.; Harrison, N.; Takacs-Cox, A.; Purac, A.; Blazek-Welsh, A. S-Adenosylmethionine Formulations with Enhanced Bioavailability 2013, 772. Available online: https://patents.google.com/patent/EP2512490A4/en (accessed on 13 March 2022).
- Cameron, B.R.; Ferreira, L.; MacDonald, I.D. Pharmacokinetic study of a novel oral formulation of S-adenosylmethionine (MSI-195) in healthy subjects: Dose escalation, food effect and comparison to a commercial nutritional supplement product. BMC Pharmacol. Toxicol. 2020, 21, 88. [Google Scholar] [CrossRef] [PubMed]
- Francioso, A.; Fanelli, S.; D’Erme, M.; Lendaro, E.; Miraglia, N.; Fontana, M.; Cavallaro, R.A.; Mosca, L. Pharmacokinetic properties of a novel formulation of S-adenosyl-l-methionine phytate. Amino Acids 2021, 53, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Caetano M Antunes, L.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lederberg, J.; McCray, A. ’Ome Sweet ’Omics—A Genealogical Treasury of Words | The Scientist Magazine®. Scientist 2001, 15, 8. [Google Scholar]
- Sommer, F.; Bäckhed, F. The gut microbiota-masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Icaza-Chávez, M.E. Gut Microbiota in health and disease. Rev. Gastroenterol. Mex. 2013, 78, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Roy Sarkar, S.; Banerjee, S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 2019, 328, 98–104. [Google Scholar] [CrossRef]
- Xu, H.; Liu, M.; Cao, J.; Li, X.; Fan, D.; Xia, Y.; Lu, X.; Li, J.; Ju, D.; Zhao, H.; et al. The dynamic interplay between the gut microbiota and autoimmune diseases. J. Immunol. Res. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Van Ameringen, M.; Turna, J.; Patterson, B.; Pipe, A.; Mao, R.Q.; Anglin, R.; Surette, M.G. The gut microbiome in psychiatry: A primer for clinicians. Depress. Anxiety 2019, 36, 1004–1025. [Google Scholar] [CrossRef] [PubMed]
- Miller, I. The gut–brain axis: Historical reflections. Microb. Ecol. Health Dis. 2018, 29, 1542921. [Google Scholar] [CrossRef] [PubMed]
- Manning, A.; Thompson, W.G.; Heaton, K.; Morris, A. Towards positive diagnosis of the irritable bowel. Br. Med. J. 1978, 2, 653–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zyoud, S.H.; Smale, S.; Waring, W.S.; Sweileh, W.M.; Al-Jabi, S.W. Global research trends in microbiome-gut-brain axis during 2009–2018: A bibliometric and visualized study. BMC Gastroenterol. 2019, 19, 158. [Google Scholar] [CrossRef] [Green Version]
- Chu, F.; Shi, M.; Lang, Y.; Shen, D.; Jin, T.; Zhu, J.; Cui, L. Gut microbiota in multiple sclerosis and experimental autoimmune encephalomyelitis: Current applications and future perspectives. Mediat. Inflamm. 2018, 2018, 1–17. [Google Scholar] [CrossRef]
- Foster, J.A.; Neufeld, K.-A.M. Gut—Brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut-brain axis in 2016: Brain-gut-microbiota axis-mood, metabolism and behaviour. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 69–70. [Google Scholar] [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, N.C.; Frissen, M.N.; Groen, A.K.; Nieuwdorp, M. Gut microbiota and the gut-brain axis: New insights in the pathophysiology of metabolic syndrome. Psychosom. Med. 2017, 79, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Park, A.J.; Collins, J.; Blennerhassett, P.A.; Ghia, J.E.; Verdu, E.F.; Bercik, P.; Collins, S.M. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol. Motil. 2013, 25, 733-e575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellavance, M.A.; Rivest, S. The HPA—Immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front. Immunol. 2014, 5, 136. [Google Scholar] [CrossRef] [Green Version]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Bazin, T.; Pellissier, S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [Green Version]
- Douglas-Escobar, M.; Elliott, E.; Neu, J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013, 167, 374–379. [Google Scholar] [CrossRef]
- Kim, N.; Yun, M.; Oh, Y.J.; Choi, H.J. Mind-altering with the gut: Modulation of the gut-brain axis with probiotics. J. Microbiol. 2018, 56, 172–182. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Vetulani, J. Early maternal separation: A rodent model of depression and a prevailing human condition. Pharmacol. Rep. 2013, 65, 1451–1461. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligezka, A.N.; Sonmez, A.I.; Corral-Frias, M.P.; Golebiowski, R.; Lynch, B.; Croarkin, P.E.; Romanowicz, M. A systematic review of microbiome changes and impact of probiotic supplementation in children and adolescents with neuropsychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 108, 110187. [Google Scholar] [CrossRef] [PubMed]
- de Filippis, A.; Ullah, H.; Baldi, A.; Dacrema, M.; Esposito, C.; Garzarella, E.U.; Santarcangelo, C.; Tantipongpiradet, A.; Daglia, M. Gastrointestinal disorders and metabolic syndrome: Dysbiosis as a key link and common bioactive dietary components useful for their treatment. Int. J. Mol. Sci. 2020, 21, 4929. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, G.V.; Meneghin, F.; Raimondi, C.; Dilillo, D.; Agostoni, C.; Riva, E.; Giovannini, M. Probiotics in clinical practice: An overview. J. Int. Med. Res. 2008, 36, 1A–53A. [Google Scholar] [CrossRef]
- Ng, Q.X.; Peters, C.; Ho, C.Y.X.; Lim, D.Y.; Yeo, W.S. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord. 2018, 228, 13–19. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology 2017, 153, 448–459. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Suda, K.; Kawai, M.; Shimizu, K.; Kushiro, A.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef. Microbes 2016, 7, 153–156. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Djafarian, K. Effect of prebiotic and probiotic supplementation on circulating pro-inflammatory cytokines and urinary cortisol levels in patients with major depressive disorder: A double-blind, placebo-controlled randomized clinical trial. J. Funct. Foods 2019, 52, 596–602. [Google Scholar] [CrossRef]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Chahwan, B.; Kwan, S.; Isik, A.; van Hemert, S.; Burke, C.; Roberts, L. Gut feelings: A randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef]
- Saxelin, M.; Pessi, T.; Salminen, S. Fecal recovery following oral administration of Lactobacillus strain GG (ATCC 53103) in gelatine capsules to healthy volunteers. Int. J. Food Microbiol. 1995, 25, 199–203. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Platzer, M.; Kohlhammer-Dohr, A.; Hamm, C.; Mörkl, S.; Bengesser, S.A.; Fellendorf, F.T.; Lahousen-Luxenberger, T.; Leitner-Afschar, B.; Schöggl, H.; et al. Provit: Supplementary probiotic treatment and vitamin b7 in depression—A randomized controlled trial. Nutrients 2020, 12, 3422. [Google Scholar] [CrossRef]
- Isolauri, E.; Yelda, S.; Pasi, K.; Heikki, A.; Seppo, S. Probiotics: Effects on immunity. Am. J. Clin. Nutr. 2001, 73, 444s–450s. [Google Scholar] [CrossRef] [Green Version]
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014, 26, 510–520. [Google Scholar] [CrossRef]
- Dhakal, R.; Bajpai, V.K.; Baek, K.H. Production of GABA (γ-aminobutyric acid) by microorganisms: A review. Braz. J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef] [Green Version]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Logan, A.C.; Katzman, M. Major depressive disorder: Probiotics may be an adjuvant therapy. Med. Hypotheses 2005, 64, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Birmann, P.T.; Casaril, A.M.; Pesarico, A.P.; Caballero, P.S.; Smaniotto, T.Â.; Rodrigues, R.R.; Moreira, Â.N.; Conceição, F.R.; Sousa, F.S.S.; Collares, T.; et al. Komagataella pastoris KM71H modulates neuroimmune and oxidative stress parameters in animal models of depression: A proposal for a new probiotic with antidepressant-like effect. Pharmacol. Res. 2021, 171, 105740. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J.; Tong, T.; Chew, J.; Lim, W.L. Antidepressive mechanisms of probiotics and their therapeutic potential. Front. Neurosci. 2020, 13, 1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luca, M.; di Mauro, M.; di Mauro, M.; Luca, A. Gut microbiota in Alzheimer’s disease, depression, and type 2 diabetes mellitus: The role of oxidative stress. Oxid. Med. Cell. Longev. 2019, 2019, 4730539. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- Sackeim, H.A.; Rush, A.J.; George, M.S.; Marangell, L.B.; Husain, M.M.; Nahas, Z.; Johnson, C.R.; Seidman, S.; Giller, C.; Haines, S.; et al. Vagus nerve stimulation (VNSTM) for treatment-resistant depression: Efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001, 25, 713–728. [Google Scholar] [CrossRef] [Green Version]
- Dhaliwal, J.; Singh, D.P.; Singh, S.; Pinnaka, A.K.; Boparai, R.K.; Bishnoi, M.; Kondepudi, K.K.; Chopra, K. Lactobacillus plantarum MTCC 9510 supplementation protects from chronic unpredictable and sleep deprivation-induced behaviour, biochemical and selected gut microbial aberrations in mice. J. Appl. Microbiol. 2018, 125, 257–269. [Google Scholar] [CrossRef]
- Matt, S.M.; Allen, J.M.; Lawson, M.A.; Mailing, L.J.; Woods, J.A.; Johnson, R.W. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front. Immunol. 2018, 9, 1832. [Google Scholar] [CrossRef]
- Lee, H.J.; Yeonghoon Son, M.L.; Moon, C.; Kim, S.H.; Shin, I.S.; Yang, M.; Bae, S.; Kim, J.S. Sodium butyrate prevents radiation-induced cognitive impairment by restoring PCREB/BDNF expression. Neural Regen. Res. 2019, 14, 1530–1535. [Google Scholar]
- Liu, Y.W.; Liu, W.H.; Wu, C.C.; Juan, Y.C.; Wu, Y.C.; Tsai, H.P.; Wang, S.; Tsai, Y.C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016, 1631, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Jansen, K.; Titus, S.; Carvalho, A.F.; Gabbay, V.; Quevedo, J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: Evidences from animal and human studies. J. Psychiatr. Res. 2015, 68, 316–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.L.; Wang, S.; Yen, J.T.; Cheng, Y.F.; Liao, C.L.; Hsu, C.C.; Wu, C.C.; Tsai, Y.C. Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res. 2019, 1711, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Q.; Wang, Y.; Sun, A.; Lin, Y.; Jin, Y.; Li, X. Oral probiotics ameliorate the behavioral deficits induced by chronic mild stress in mice via the gut microbiota-inflammation axis. Front. Behav. Neurosci. 2018, 12, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, B.B. The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders. Pediatr. Res. 2019, 85, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Vitetta, L.; Bambling, M.; Alford, H. The gastrointestinal tract microbiome, probiotics, and mood. Inflammopharmacology 2014, 22, 333–339. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Koppel, N.; Rekdal, V.M.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science 2017, 356, 1–11. [Google Scholar] [CrossRef]
- Kim, J.Y.; Suh, J.W.; Ji, G.E. Evaluation of S-adenosyl-l-methionine production by Bifidobacterium bifidum BGN4. Food Sci. Biotechnol. 2008, 17, 184–187. [Google Scholar]
- Saccarello, A.; Montarsolo, P.; Massardo, I.; Picciotto, R.; Pedemonte, A.; Castagnaro, R.; Brasesco, P.C.; Guida, V.; Picco, P.; Fioravanti, P.; et al. Oral administration of S-adenosylmethionine (SAMe) and Lactobacillus plantarum HEAL9 improves the mild-to-moderate symptoms of depression: A randomized, double-blind, placebo-controlled study. Prim. Care Companion CNS Disord. 2020, 22, 19m02578. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, H.; Khan, A.; Rengasamy, K.R.R.; Di Minno, A.; Sacchi, R.; Daglia, M. The Efficacy of S-Adenosyl Methionine and Probiotic Supplementation on Depression: A Synergistic Approach. Nutrients 2022, 14, 2751. https://doi.org/10.3390/nu14132751
Ullah H, Khan A, Rengasamy KRR, Di Minno A, Sacchi R, Daglia M. The Efficacy of S-Adenosyl Methionine and Probiotic Supplementation on Depression: A Synergistic Approach. Nutrients. 2022; 14(13):2751. https://doi.org/10.3390/nu14132751
Chicago/Turabian StyleUllah, Hammad, Ayesha Khan, Kannan R. R. Rengasamy, Alessandro Di Minno, Roberto Sacchi, and Maria Daglia. 2022. "The Efficacy of S-Adenosyl Methionine and Probiotic Supplementation on Depression: A Synergistic Approach" Nutrients 14, no. 13: 2751. https://doi.org/10.3390/nu14132751








