Greater Glycemic Burden Is Associated with Further Poorer Glycemic Control in Newly-Diagnosed Type 2 Diabetes Mellitus Patients
Abstract
:1. Introduction
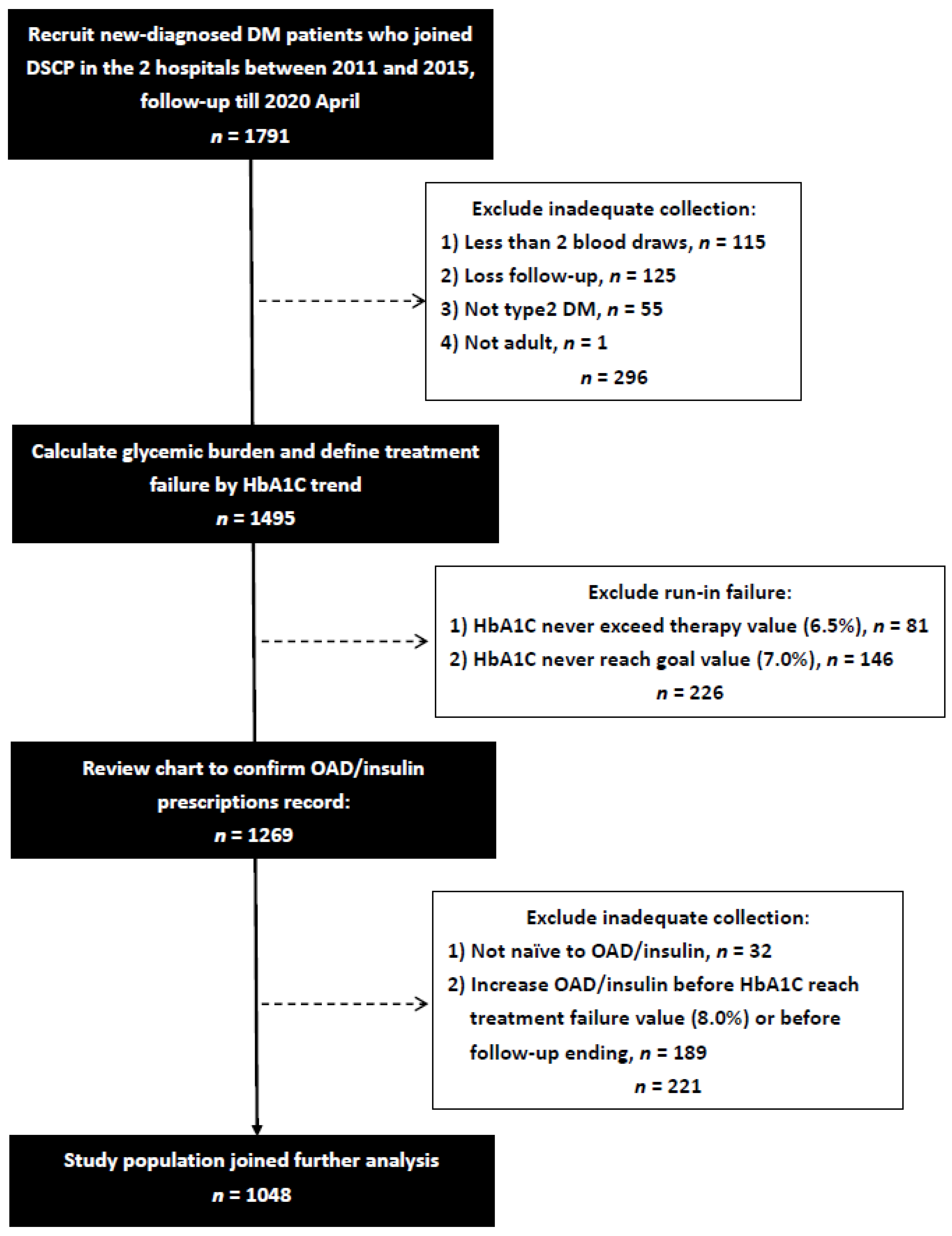
2. Materials and Methods
2.1. Study Population and Setting
2.2. Definition of the Glycemic Burden, Glycemic Durability, and Initially Prescribed OAD/Insulin
2.3. Other Covariates and Divided Subgroups
2.4. Statistical Analyses
3. Results
3.1. Patients Baseline Characteristics
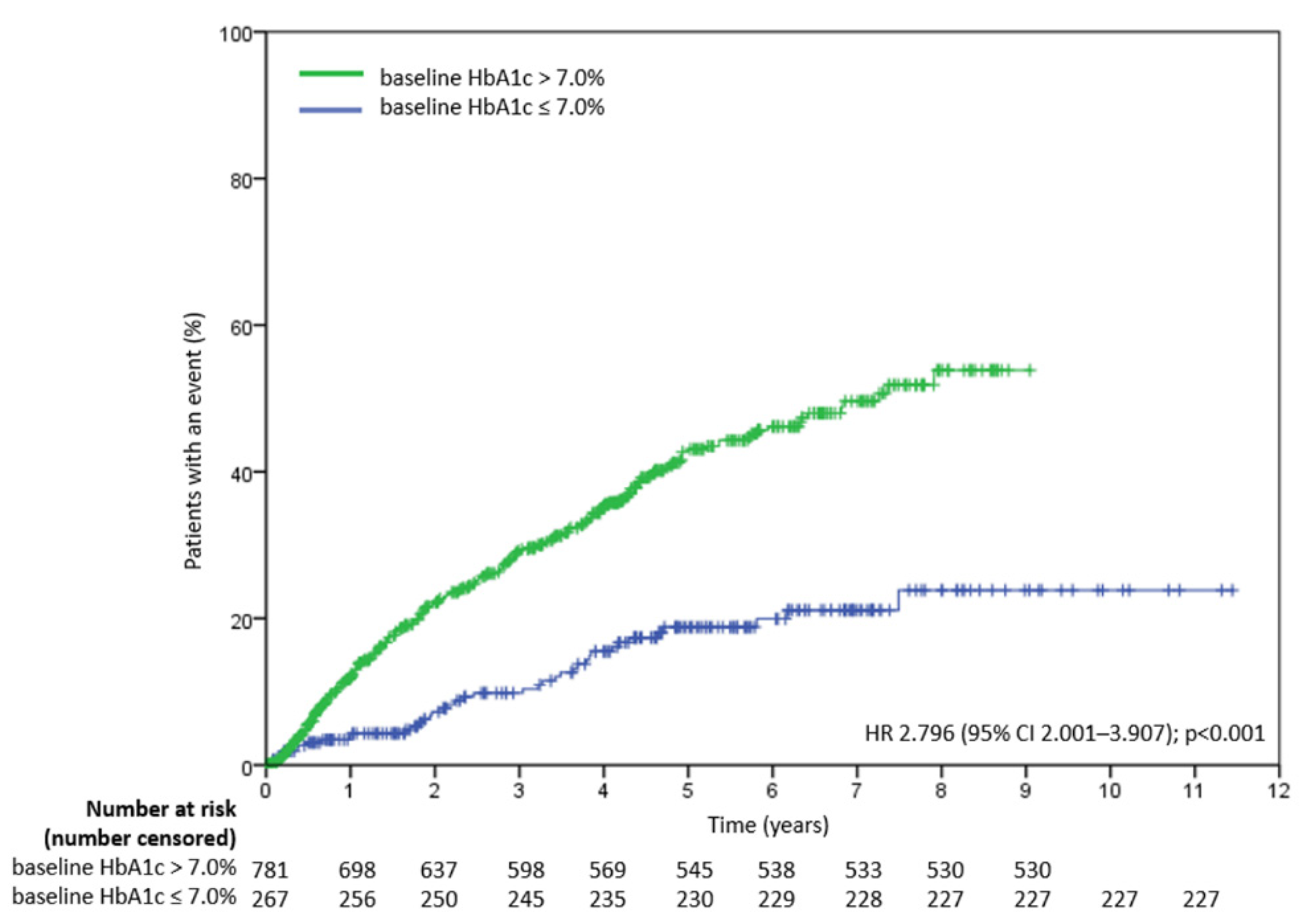
3.2. Determinants of Treatment Failure in all Study Patients
3.3. Determinants of Treatment Failure in Study Patients with Baseline HbA1c > 7.0%
3.4. Determinants of Treatment Failure in Study Patients with Baseline HbA1c ≤ 7.0%
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Prato, S.; Marchetti, P.; Bonadonna, R.C. Phasic insulin release and metabolic regulation in type 2 diabetes. Diabetes 2002, 51 (Suppl. 1), S109–S116. [Google Scholar] [CrossRef] [Green Version]
- Gleason, C.E.; Gonzalez, M.; Harmon, J.S.; Robertson, R.P. Determinants of glucose toxicity and its reversibility in the pancreatic islet beta-cell line, HIT-T15. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E997–E1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyzogopoulou, E.V.; Kalfarentzos, F.; Vagenakis, A.G.; Alexandrides, T.K. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes 2003, 52, 1098–1103. [Google Scholar] [CrossRef] [Green Version]
- McInnes, N.; Smith, A.; Otto, R.; Vandermey, J.; Punthakee, Z.; Sherifali, D.; Balasubramanian, K.; Hall, S.; Gerstein, H.C. Piloting a Remission Strategy in Type 2 Diabetes: Results of a Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2017, 102, 1596–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aminian, A.; Vidal, J.; Salminen, P.; Still, C.D.; Nor Hanipah, Z.; Sharma, G.; Tu, C.; Wood, G.C.; Ibarzabal, A.; Jimenez, A.; et al. Late Relapse of Diabetes After Bariatric Surgery: Not Rare, but Not a Failure. Diabetes Care 2020, 43, 534–540. [Google Scholar] [CrossRef]
- McInnes, N.; Hall, S.; Hramiak, I.; Sigal, R.J.; Goldenberg, R.; Gupta, N.; Rabasa-Lhoret, R.; Braga, M.; Woo, V.; Sultan, F.; et al. Remission of Type 2 Diabetes Following a Short-term Intensive Intervention with Insulin Glargine, Sitagliptin, and Metformin: Results of an Open-label Randomized Parallel-Design Trial. Diabetes Care 2021, 105, 2532–2540. [Google Scholar] [CrossRef]
- Stolar, M. Glycemic control and complications in type 2 diabetes mellitus. Am. J. Med. 2010, 123, S3–S11. [Google Scholar] [CrossRef]
- Sasso, F.C.; Rinaldi, L.; Lascar, N.; Marrone, A.; Pafundi, P.C.; Adinolfi, L.E.; Marfella, R. Role of Tight Glycemic Control during Acute Coronary Syndrome on CV Outcome in Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 3106056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caturano, A.; Galiero, R.; Pafundi, P.C.; Cesaro, A.; Vetrano, E.; Palmiero, G.; Rinaldi, L.; Salvatore, T.; Marfella, R.; Sardu, C.; et al. Does a strict glycemic control during acute coronary syndrome play a cardioprotective effect? Pathophysiology and clinical evidence. Diabetes Res. Clin. Pract. 2021, 178, 108959. [Google Scholar] [CrossRef]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Albanese, G.; Di Martino, A.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Sasso, F.C. The Diabetic Cardiomyopathy: The Contributing Pathophysiological Mechanisms. Front. Med. 2021, 8, 695792. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [Green Version]
- Laiteerapong, N.; Ham, S.A.; Gao, Y.; Moffet, H.H.; Liu, J.Y.; Huang, E.S.; Karter, A.J. The Legacy Effect in Type 2 Diabetes: Impact of Early Glycemic Control on Future Complications (The Diabetes & Aging Study). Diabetes Care 2019, 42, 416–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Robertson, R.P.; Harmon, J.; Tran, P.O.; Tanaka, Y.; Takahashi, H. Glucose toxicity in beta-cells: Type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 2003, 52, 581–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kietzmann, T.; Petry, A.; Shvetsova, A.; Gerhold, J.M.; Gorlach, A. The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1533–1554. [Google Scholar] [CrossRef]
- Januszyk, M.; Sorkin, M.; Glotzbach, J.P.; Vial, I.N.; Maan, Z.N.; Rennert, R.C.; Duscher, D.; Thangarajah, H.; Longaker, M.T.; Butte, A.J.; et al. Diabetes irreversibly depletes bone marrow-derived mesenchymal progenitor cell subpopulations. Diabetes 2014, 63, 3047–3056. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes, A. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S111–S124. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Haffner, S.M.; Heise, M.A.; Herman, W.H.; Holman, R.R.; Jones, N.P.; Kravitz, B.G.; Lachin, J.M.; O’Neill, M.C.; Zinman, B.; et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 2006, 355, 2427–2443. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Paldánius, P.M.; Proot, P.; Chiang, Y.; Stumvoll, M.; Del Prato, S. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): A 5-year, multicentre, randomised, double-blind trial. Lancet 2019, 394, 1519–1529. [Google Scholar] [CrossRef]
- Yeung, R.O.; Zhang, Y.; Luk, A.; Yang, W.; Sobrepena, L.; Yoon, K.H.; Aravind, S.R.; Sheu, W.; Nguyen, T.K.; Ozaki, R.; et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): A cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol. 2014, 2, 935–943. [Google Scholar] [CrossRef]
- Gopalan, A.; Mishra, P.; Alexeeff, S.E.; Blatchins, M.A.; Kim, E.; Man, A.; Karter, A.J.; Grant, R.W. Initial Glycemic Control and Care Among Younger Adults Diagnosed with Type 2 Diabetes. Diabetes Care 2020, 43, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.B.; Conner, C.; Nichols, G.A. Secondary failure of metformin monotherapy in clinical practice. Diabetes Care 2010, 33, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Murphree, D.H.; Arabmakki, E.; Ngufor, C.; Storlie, C.B.; McCoy, R.G. Stacked classifiers for individualized prediction of glycemic control following initiation of metformin therapy in type 2 diabetes. Comput. Biol. Med. 2018, 103, 109–115. [Google Scholar] [CrossRef]
- Brown, J.B.; Nichols, G.A.; Perry, A. The burden of treatment failure in type 2 diabetes. Diabetes Care 2004, 27, 1535–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maple-Brown, L.J.; Ye, C.; Retnakaran, R. Area-under-the-HbA1c-curve above the normal range and the prediction of microvascular outcomes: An analysis of data from the Diabetes Control and Complications Trial. Diabetes Med. 2013, 30, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Van Wijngaarden, R.P.T.; Overbeek, J.A.; Heintjes, E.M.; Schubert, A.; Diels, J.; Straatman, H.; Steyerberg, E.W.; Herings, R.M.C. Relation Between Different Measures of Glycemic Exposure and Microvascular and Macrovascular Complications in Patients with Type 2 Diabetes Mellitus: An Observational Cohort Study. Diabetes Ther. 2017, 8, 1097–1109. [Google Scholar] [CrossRef]
- Nichols, G.A.; Rosales, A.G.; Perrin, N.A.; Fortmann, S.P. The association between different A1C-based measures of glycemia and risk of cardiovascular disease hospitalization. Diabetes Care 2014, 37, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Kornelius, E.; Chiou, J.Y.; Yang, Y.S.; Lu, Y.L.; Peng, C.H.; Huang, C.N. The Diabetes Shared Care Program and Risks of Cardiovascular Events in Type 2 Diabetes. Am. J. Med. 2015, 128, 977–985.e3. [Google Scholar] [CrossRef]
- Glycemic Targets: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S73–S84. [CrossRef]
- Khunti, K.; Chen, H.; Cid-Ruzafa, J.; Fenici, P.; Gomes, M.B.; Hammar, N.; Ji, L.; Kosiborod, M.; Pocock, S.; Shestakova, M.V.; et al. Glycaemic control in patients with type 2 diabetes initiating second-line therapy: Results from the global DISCOVER study programme. Diabetes Obes. Metab. 2020, 22, 66–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Elbasha, E.H.; Chhatwal, J. Theoretical Foundations and Practical Applications of Within-Cycle Correction Methods. Med. Decis. Mak. 2016, 36, 115–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Int. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.M.; Shields, B.M.; Henley, W.E.; Jones, A.G.; Hattersley, A.T. Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: An analysis using clinical trial data. Lancet Diabetes Endocrinol. 2019, 7, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Zeitler, P.; Hirst, K.; Copeland, K.C.; El Ghormli, L.; Levitt Katz, L.; Levitsky, L.L.; Linder, B.; McGuigan, P.; White, N.H.; Wilfley, D.; et al. HbA1c After a Short Period of Monotherapy with Metformin Identifies Durable Glycemic Control Among Adolescents with Type 2 Diabetes. Diabetes Care 2015, 38, 2285–2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, F.; Chen, H.; Cooper, A.; Gomes, M.B.; Ji, L.; Leigh, P.; Ramirez, L.; Shestakova, M.V.; Shimomura, I.; Siddiqui, A.; et al. What are the factors associated with long-term glycaemic control in patients with type 2 diabetes and elevated glycated haemoglobin (≥ 7.0%) at initiation of second-line therapy? Results from the DISCOVER study. Diabetes Obes. Metab. 2021, 23, 2336–2343. [Google Scholar] [CrossRef]
- Brasacchio, D.; Okabe, J.; Tikellis, C.; Balcerczyk, A.; George, P.; Baker, E.K.; Calkin, A.C.; Brownlee, M.; Cooper, M.E.; El-Osta, A. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes 2009, 58, 1229–1236. [Google Scholar] [CrossRef] [Green Version]
- El-Osta, A.; Brasacchio, D.; Yao, D.; Pocai, A.; Jones, P.L.; Roeder, R.G.; Cooper, M.E.; Brownlee, M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 2008, 205, 2409–2417. [Google Scholar] [CrossRef]
- Kahn, S.E.; Lachin, J.M.; Zinman, B.; Haffner, S.M.; Aftring, R.P.; Paul, G.; Kravitz, B.G.; Herman, W.H.; Viberti, G.; Holman, R.R.; et al. Effects of rosiglitazone, glyburide, and metformin on beta-cell function and insulin sensitivity in ADOPT. Diabetes 2011, 60, 1552–1560. [Google Scholar] [CrossRef] [Green Version]
- Caturano, A.; Galiero, R.; Pafundi, P.C. Metformin for Type 2 Diabetes. JAMA 2019, 322, 1312. [Google Scholar] [CrossRef]
- Al-Rasheedi, A.A. The Role of Educational Level in Glycemic Control among Patients with Type II Diabetes Mellitus. Int. J. Health Sci. 2014, 8, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Choi, J.H.; Kim, K.J.; An, J.H.; Kim, H.Y.; Kim, S.G.; Kim, N.H. Determinants of Long-Term Durable Glycemic Control in New-Onset Type 2 Diabetes Mellitus. Diabetes Metab. J. 2017, 41, 284–295. [Google Scholar] [CrossRef]
- Chaturvedi, N.; Stephenson, J.M.; Fuller, J.H. The relationship between socioeconomic status and diabetes control and complications in the EURODIAB IDDM Complications Study. Diabetes Care 1996, 19, 423–430. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Abbasi, F.; Cheal, K.; Chu, J.; Lamendola, C.; Reaven, G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann. Int. Med. 2003, 139, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Sasso, F.C.; Pafundi, P.C.; Simeon, V.; De Nicola, L.; Chiodini, P.; Galiero, R.; Rinaldi, L.; Nevola, R.; Salvatore, T.; Sardu, C.; et al. Efficacy and durability of multifactorial intervention on mortality and MACEs: A randomized clinical trial in type-2 diabetic kidney disease. Cardiovasc. Diabetol. 2021, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Consortium, R. Lack of Durable Improvements in beta-Cell Function Following Withdrawal of Pharmacological Interventions in Adults with Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes. Diabetes Care 2019, 42, 1742–1751. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Without Treatment Failure (n = 757) | With Treatment Failure (n = 291) | p |
|---|---|---|---|
| Age (year) | 56.8 ± 12.6 | 53.2 ± 12.1 | <0.001 |
| Male gender (%) | 54.2 | 57.7 | 0.298 |
| Education history | 0.058 | ||
| Elementary school or uneducated (%) | 25.9 | 28.3 | |
| High school (%) | 47.7 | 52.4 | |
| University or higher (%) | 26.4 | 19.3 | |
| SBP (mmHg) | 134.3 ± 18.1 | 132.0 ± 20.7 | 0.107 |
| DBP (mmHg) | 79.9 ± 11.9 | 80.2 ± 12.5 | 0.699 |
| BMI (kg/m2) | 26.2 ± 4.6 | 26.6 ± 4.8 | 0.198 |
| Glycemic burden AUC’ (%/90 days) | 3.4 ± 8.9 | 7.3 ± 12.7 | <0.001 |
| Glycemic burden AUC’’(%/90 days) | 1.9 ± 3.0 | 3.5 ± 3.0 | <0.001 |
| Laboratory parameters | |||
| Baseline HbA1c (%) | 9.3 ± 2.6 | 10.7 ± 2.8 | <0.001 |
| Triglyceride (mg/dL) | 151.5 ± 103.3 | 181.9 ± 144.8 | 0.001 |
| Total cholesterol (mg/dL) | 188.8 ± 44.5 | 196.5 ± 48.5 | 0.015 |
| HDL-cholesterol (mg/dL) | 44.0 ± 12.3 | 42.9 ± 12.0 | 0.171 |
| LDL-cholesterol (mg/dL) | 115.1 ± 36.6 | 119.1 ± 41.8 | 0.153 |
| eGFR (mL/min/1.73 m2) | 95.4 ± 30.7 | 100.9 ± 43.6 | 0.024 |
| ALT (u/L) | 40.4 ± 35.1 | 42.7 ± 41.5 | 0.385 |
| Initial insulin pump (%) | 7.7 | 16.8 | <0.001 |
| Initially prescribed OAD | |||
| Biguanides (%) | 87.2 | 85.9 | 0.584 |
| TZD (%) | 4.9 | 5.8 | 0.531 |
| DPP4i (%) | 46.2 | 44.3 | 0.579 |
| GLP1RA (%) | 0.1 | 0.0 | 0.535 |
| SU (%) | 35.4 | 53.6 | <0.001 |
| Glinides (%) | 3.3 | 3.4 | 0.914 |
| AGI (%) | 1.2 | 2.7 | 0.073 |
| SGLT2i (%) | 0.3 | 0.0 | 0.380 |
| Initially prescribed OAD (category number) | <0.001 | ||
| No OAD (%) | |||
| Monotherapy (%) | 5.9 | 9.6 | |
| Two combination therapy (%) | 33.2 | 20.3 | |
| Three or more combination therapy (%) | 38.6 | 38.5 | |
| Initially prescribed insulin use | 22.3 | 31.6 | 0.017 |
| No insulin (%) | 86.5 | 79.7 | |
| Basal insulin (%) | 11.8 | 16.8 | |
| Basal and pre-prandial insulin (%) | 1.7 | 3.4 | |
| Stable glycemic period (months) | 45.1 ± 31.8 | 26.8 ± 21.1 | <0.001 |
| Variables | Multivariable (Forward) | |
|---|---|---|
| HR (95% CI) | p | |
| Age (per 1 year) | 0.959 (0.947, 0.971) | <0.001 |
| Education history | 0.005 | |
| Elementary school or uneducated | Reference | |
| High school | 0.681 (0.492, 0.942) | 0.020 |
| University or higher | 0.495 (0.324, 0.754) | 0.001 |
| Glycemic burden AUC’ (per 1 %/90 days) | 1.027 (1.019, 1.035) | <0.001 |
| Laboratory parameters | ||
| Baseline HbA1c (%) | 1.118 (1. 065, 1. 174) | <0.001 |
| Initially prescribed OAD | ||
| SU (vs. no use SU) | 1.459 (1.026, 2.076) | 0.036 |
| AGI (vs. no use AGI) | 2.496 (1.150, 5.422) | 0.021 |
| Initially prescribed OAD (categories) | 0.004 | |
| No OAD | Reference | |
| Monotherapy | 0.474(0.294, 0.763) | 0.002 |
| Two combination therapy | 0.457(0.281, 0.743) | 0.002 |
| Three or more combination therapy | 0.370(0.211, 0.650) | 0.001 |
| Variables | Multivariable (Forward) | |
|---|---|---|
| HR (95% CI) | p | |
| Age (per 1 year) | 0.965 (0.952, 0.978) | <0.001 |
| Education history | 0.026 | |
| Elementary school or uneducated | Reference | |
| High school | 0.721 (0.504, 1.030) | 0.073 |
| University or higher | 0.531 (0.335, 0.841) | 0.007 |
| Glycemic burden AUC’ (per 1 %/90 days) | 1.026 (1.018, 1.034) | <0.001 |
| Laboratory parameters | ||
| Baseline HbA1c (%) | 1.130 (1.058, 1.171) | <0.001 |
| Initially prescribed OAD | ||
| AGIs (vs. no use AGIs) | 2.161 (1.007, 4.638) | 0.048 |
| Variables | Multivariate (Forward) | |
|---|---|---|
| Unstandardized Coefficient β (95% CI) | p | |
| Age (per 1 year) | 0.938 (0.903, 0.975) | 0.001 |
| SBP (per 1 mmHg) | 0.976 (0.953, 0.999) | 0.041 |
| Glycemic burden AUC’’ (per 1 %/90 days) | 1.128 (1.016, 1.253) | 0.024 |
| Laboratory parameters | ||
| Triglyceride (per 1 mg/dL) | 1.004 (1.002, 1.006) | 0.001 |
| HDL-cholesterol (per 1 mg/dL) | 0.960 (0.924, 0.997) | 0.033 |
| ALT (per 1 u/L) | 0.977 (0.955, 0.999) | 0.044 |
| Initially prescribed OAD (categories) | <0.001 | |
| No OAD (%) | Reference | |
| Monotherapy (%) | 0.164 (0.072, 0.371) | <0.001 |
| Two combination therapy (%) | 0.123 (0.042, 0.365) | <0.001 |
| Three or more combination therapy (%) | 1.256 (0.090, 17.486) | 0.865 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, W.-L.; Huang, H.-C.; Lin, H.-C.; Lo, W.-C.; Chen, S.-C.; Lee, M.-Y. Greater Glycemic Burden Is Associated with Further Poorer Glycemic Control in Newly-Diagnosed Type 2 Diabetes Mellitus Patients. Nutrients 2022, 14, 320. https://doi.org/10.3390/nu14020320
Wen W-L, Huang H-C, Lin H-C, Lo W-C, Chen S-C, Lee M-Y. Greater Glycemic Burden Is Associated with Further Poorer Glycemic Control in Newly-Diagnosed Type 2 Diabetes Mellitus Patients. Nutrients. 2022; 14(2):320. https://doi.org/10.3390/nu14020320
Chicago/Turabian StyleWen, Wei-Lun, Hui-Chun Huang, Hsiu-Chu Lin, Wan-Ching Lo, Szu-Chia Chen, and Mei-Yueh Lee. 2022. "Greater Glycemic Burden Is Associated with Further Poorer Glycemic Control in Newly-Diagnosed Type 2 Diabetes Mellitus Patients" Nutrients 14, no. 2: 320. https://doi.org/10.3390/nu14020320






