Effects of Heat-Killed Levilactobacillus brevis KB290 in Combination with β-Carotene on Influenza Virus Infection in Healthy Adults: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Experimental Beverages
2.3. Subjects
2.4. Endpoints
2.5. Background Survey, Preliminary Survey, and Daily Questionnaire
2.6. Safety Analysis
2.7. Statistical Analyses
3. Results
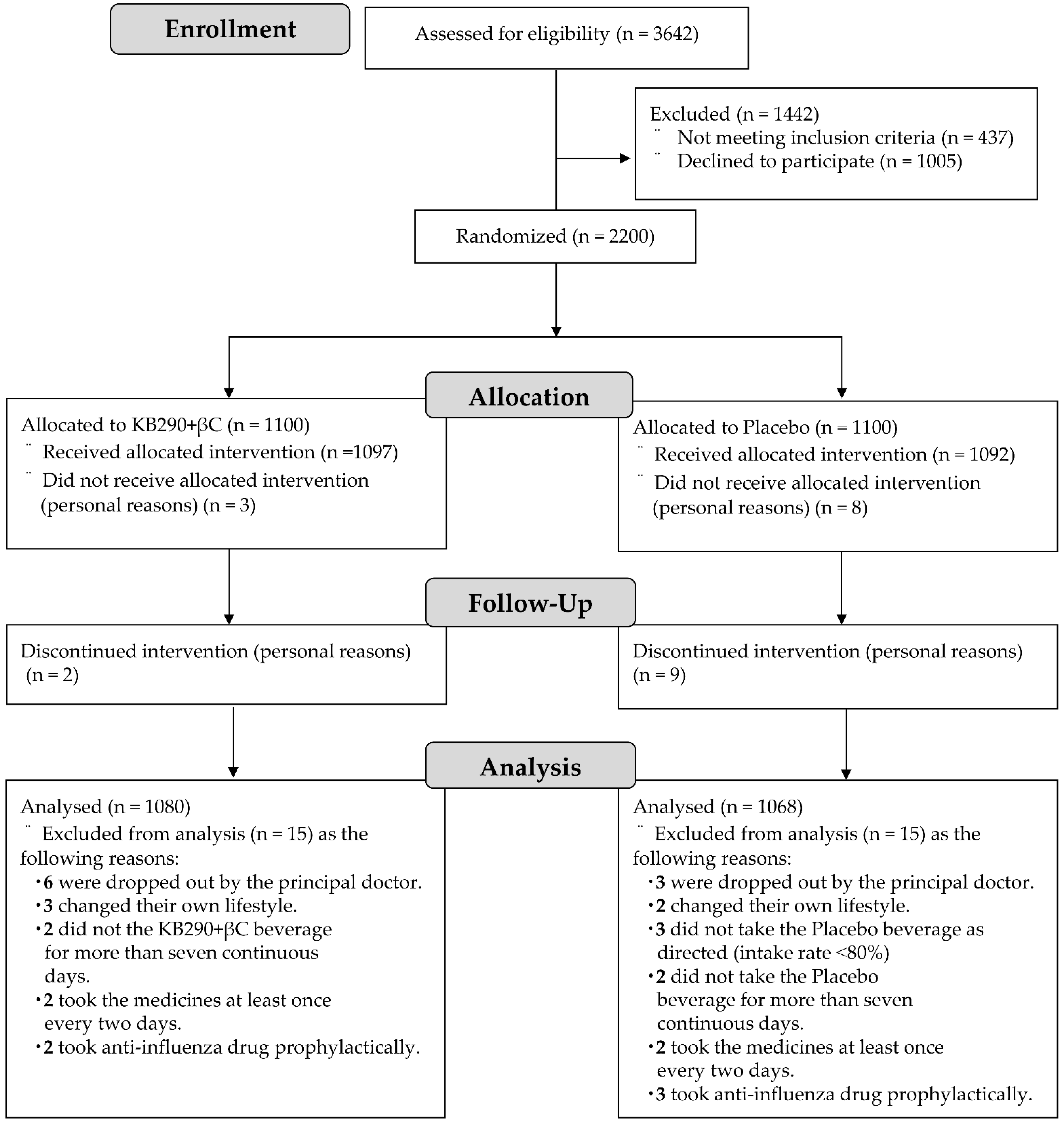
3.1. Flowchart and Characteristics of the Trial Subjects
3.2. Primary and Secondary Endpoints
3.3. Subgroup Analysis
3.4. Safety Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Influenza (Seasonal), 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 19 September 2020).
- Lam, P.-P.; Coleman, B.L.; Green, K.; Powis, J.; Richardson, D.; Katz, K.; Borgundvaag, B.; Smith-Gorvie, T.; Kwong, J.C.; Bondy, S.J.; et al. Predictors of influenza among older adults in the emergency department. BMC Infect. Dis. 2016, 16, 616–625. [Google Scholar] [CrossRef] [Green Version]
- Monto, A.S.; Gravenstein, S.; Elliott, M.; Colopy, M.; Schweinle, J. Clinical signs and symptoms predicting influenza infection. Arch. Intern. Med. 2000, 160, 3243–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Evaluation of Influenza Vaccine Effectiveness: A Guide to the Design and Interpretation of Observational Studies, 2017. Available online: https://apps.who.int/iris/handle/10665/255203 (accessed on 19 September 2020).
- Ujike, M.; Shimabukuro, K.; Mochizuki, K.; Obuchi, M.; Kageyama, T.; Shirakura, M.; Kishida, N.; Yamashita, K.; Horikawa, H.; Kato, Y.; et al. Oseltamivir-resistant influenza viruses A (H1N1) during 2007-2009 influenza seasons, Japan. Emerg. Infect. Dis. 2010, 16, 926–935. [Google Scholar] [CrossRef]
- Shin, K.; Wakabayashi, H.; Yamauchi, K.; Teraguchi, S.; Tamura, Y.; Kurokawa, M.; Shiraki, K. Effects of orally administered bovine lactoferrin and lactoperoxidase on influenza virus infection in mice. J. Med. Microbiol. 2005, 54, 717–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [Green Version]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Pino-Lagos, K.; Guo, Y.; Noelle, R.J. Retinoic acid: A key player in immunity. Biofactors 2010, 36, 430–436. [Google Scholar] [CrossRef]
- Takeda, S.; Takeshita, M.; Kikuchi, Y.; Dashnyam, B.; Kawahara, S.; Yoshida, H.; Watanabe, W.; Muguruma, M.; Kurokawa, M. Efficacy of oral administration of heat-killed probiotics from Mongolian dairy products against influenza infection in mice: Alleviation of influenza infection by its immunomodulatory activity through intestinal immunity. Int. Immunopharmacol. 2011, 11, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.-N.; Lee, D.-H.; Lee, Y.-N.; Park, J.-K.; Yuk, S.-S.; Yang, S.-Y.; Lee, H.-J.; Woo, S.-H.; Kim, H.-M.; Lee, J.-B.; et al. Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice. Antiviral Res. 2012, 93, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Satomi, S.; Khanum, S.; Miller, P.; Suzuki, S.; Suganuma, H.; Heiser, A.; Gupta, S.K. Short Communication: Oral administration of heat-killed Lactobacillus brevis KB290 in combination with retinoic acid provides protection against influenza virus infection in mice. Nutrients 2020, 12, 2925. [Google Scholar] [CrossRef]
- Hathcock, J.N.; Hattan, D.G.; Jenkins, M.Y.; McDonald, J.T.; Sundaresan, P.R.; Wilkening, V.L. Evaluation of vitamin A toxicity. Am. J. Clin. Nutr. 1990, 52, 183–202. [Google Scholar] [CrossRef]
- Lobo, G.P.; Hessel, S.; Eichinger, A.; Noy, N.; Moise, A.R.; Wyss, A.; Palczewski, K.; Lintig, J.V. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta, beta-carotene absorption and vitamin A production. FASEB J. 2010, 24, 1656–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Meyskens, F.L., Jr.; Omenn, G.S.; Valanis, B.; Williams, J.H., Jr. The beta-carotene and retinol efficacy trial: Incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J. Natl. Cancer Inst. 2004, 96, 1743–1750. [Google Scholar] [CrossRef] [Green Version]
- The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; Goodman, G.; Thornquist, M.; Grizzle, J.; Rosenstock, L.; Barnhart, S.; Balmes, J.; Cherniack, M.G.; Cullen, M.R.; Glass, A.; et al. The beta-carotene and retinol efficacy trial (CARET) for chemoprevention of lung cancer in high risk populations: Smokers and asbestos-exposed workers. Cancer Res. 1994, 54, 2038s–2043s. [Google Scholar]
- Kuster, S.P.; Shah, P.S.; Coleman, B.L.; Lam, P.-P.; Tong, A.; Wormsbecker, A.; McGeer, A. Incidence of influenza in healthy adults and healthcare workers: A systematic review and meta-analysis. PLoS ONE 2011, 6, e26239. [Google Scholar] [CrossRef] [Green Version]
- Sugimura, T.; Takahashi, H.; Jounai, K.; Ohshio, K.; Kanayama, M.; Tazumi, K.; Tanihata, Y.; Miura, Y.; Fujiwara, D.; Yamamoto, N. Effects of oral intake of plasmacytoid dendritic cells-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus. Br. J. Nutr. 2015, 114, 727–733. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, H.; Ishikane, M.; Ueda, P. Seasonal influenza activity during the SARS-CoV-2 outbreak in Japan. JAMA 2020, 323, 1969–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Minister of Health, Labour and Welfare. Outbreak of Patients with Pneumonia Associated with Coronavirus [1st case], 2020. Available online: https://www.mhlw.go.jp/stf/newpage_08906.html (accessed on 18 November 2020).
- World Health Organization. 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4 (accessed on 18 November 2020).
- Minister of Health, Labour and Welfare. 2020. Available online: https://www.mhlw.go.jp/content/10900000/000597148.pdf (accessed on 18 November 2020).
- Minister of Health, Labour and Welfare. 2020. Available online: https://www.mhlw.go.jp/content/10900000/000597149.pdf (accessed on 18 November 2020).
- Minister of Health, Labour and Welfare. 2020. Available online: https://www.mhlw.go.jp/content/10900000/000597150.pdf (accessed on 18 November 2020).
- Deyle, E.R.; Maher, M.C.; Hernandez, R.D.; Basu, S.; Sugihara, G. Global environmental drivers of influenza. Proc. Natl. Acad. Sci. USA 2016, 113, 13081–13086. [Google Scholar] [CrossRef] [Green Version]
- Muto, K.; Yamamoto, I.; Nagasu, M.; Tanaka, M.; Wada, K. Japanese citizens’ behavioral changes and preparedness against COVID-19: An online survey during the early phase of the pandemic. PLoS ONE 2020, 15, e0234292. [Google Scholar] [CrossRef]
- World Health Organization. 2017. Available online: https://www.who.int/influenza/preparedness/pandemic/guidance_pandemic_influenza_surveillance_2017/en/ (accessed on 21 November 2020).
- Matsumoto, M.; Waki, N.; Suganuma, H.; Takahashi, I.; Kurauchi, S.; Sawada, K.; Tokuda, I.; Misawa, M.; Ando, M.; Itoh, K.; et al. Association between biomarkers of cardiovascular diseases and the blood concentration of carotenoids among the general population without apparent illness. Nutrients 2020, 12, 2310. [Google Scholar] [CrossRef] [PubMed]
- Minister of Health, Labour and Welfare. 2020. Available online: https://www.mhlw.go.jp/content/10900000/000687163.pdf (accessed on 18 June 2021).
- Minister of Health, Labour and Welfare. 2020. Available online: https://www.mhlw.go.jp/content/10904750/000586561.pdf (accessed on 29 June 2021).
- Waki, N.; Matsumoto, M.; Fukui, Y.; Suganuma, H. Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among schoolchildren: An open-label pilot study. Lett. Appl. Microbiol. 2014, 59, 565–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoshita, T.; Maruyama, K.; Suyama, K.; Nishijima, M.; Akamatsu, K.; Jogamoto, A.; Katakami, K.; Saito, I. The effects of OLL1073R-1 yogurt intake on influenza incidence and immunological markers among women healthcare workers: A randomized controlled trial. Food Funct. 2019, 10, 8129–8136. [Google Scholar] [CrossRef] [PubMed]
- Namba, K.; Hatano, M.; Tomoko Yaeshima, T.; Takase, M.; Suzuki, K. Effects of Bifidobacterium longum BB536 Administration on influenza infection, influenza vaccine antibody titer, and cell-mediated immunity in the elderly. Biosci. Biotechnol. Biochem. 2010, 74, 939–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waki, N.; Yajima, N.; Suganuma, H.; Buddle, B.M.; Luo, D.; Heiser, A.; Zheng, T. Oral administration of Lactobacillus brevis KB290 to mice alleviates clinical symptoms following influenza virus infection. Lett. Appl. Microbiol. 2014, 58, 87–93. [Google Scholar] [CrossRef]
- Sasaki, E.; Suzuki, S.; Fukui, Y.; Yajima, N. Cell-bound exopolysaccharides of Lactobacillus brevis KB290 enhance cytotoxic activity of mouse splenocytes. J. Appl. Microbiol. 2015, 118, 506–514. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar] [CrossRef]
- Matsumiya, T.; Stafforini, D.M. Function and regulation of retinoic acid-inducible gene-I. Crit. Rev. Immunol. 2010, 30, 489–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Total (n = 2148) | KB290 + βC (n = 1080) | Placebo (n = 1068) | p-Value | |
|---|---|---|---|---|---|
| Age | 20–29 | 572 | 283 | 289 | 0.44 |
| 30–39 | 505 | 256 | 249 | ||
| 40–49 | 642 | 337 | 305 | ||
| 50–59 | 429 | 204 | 225 | ||
| Average age | 38.7 ± 11.1 | 38.7 ± 11.0 | 38.7 ± 11.2 | ||
| Sex | Female/male | 1141/1007 (53.1%) | 570/510 (52.8%) | 571/497 (53.5%) | 0.75 |
| Region | Hokkaido | 720 | 360 | 360 | 1.0 |
| Kanto | 719 | 360 | 359 | ||
| Kyushu | 709 | 360 | 349 | ||
| Influenza vaccine (including plans) | Yes/No | 1011/1137 (47.1%) | 506/574 (46.9%) | 505/563 (47.3%) | 0.84 |
| Residing with children aged <18 years | Yes/No | 786/1362 (36.6%) | 395/685 (36.6%) | 391/677 (36.6%) | 0.99 |
| Daily use of public transportation | Yes/No | 852/1296 (39.7%) | 427/653 (39.5%) | 425/643 (39.8%) | 0.90 |
| KB290 + βC | Placebo | RR | 95% CI | p-Value | |
|---|---|---|---|---|---|
| Influenza incidence | 31/1080 (2.9%) | 36/1068 (3.4%) | 0.852 | 0.531; 1.366 | 0.51 |
| Fever incidence | 111/1080 (10.3%) | 109/1068 (10.2%) | 1.007 | 0.784; 1.294 | 0.96 |
| Fever duration | 1.7 ± 1.2 | 1.8 ± 1.0 | - | - | 0.33 |
| KB290 + βC | Placebo | RR | 95% CI | p-Value | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 16/570 (2.8%) | 20/571 (3.5%) | 0.796 | 0.408; 1.552 | 0.50 |
| Male | 15/510 (2.9%) | 16/497 (3.2%) | 0.911 | 0.445; 1.863 | 0.80 |
| Age | |||||
| ˂40 years | 10/539 (1.9%) | 21/538 (3.9%) | 0.465 | 0.217; 0.998 | 0.044 |
| ≥40 years | 21/541 (3.9%) | 15/530 (2.8%) | 1.387 | 0.707; 2.720 | 0.34 |
| Region | |||||
| Hokkaido | 9/360 (2.5%) | 16/360 (4.4%) | 0.551 | 0.240; 1.264 | 0.15 |
| Kanto | 13/360 (3.6%) | 12/359 (3.3%) | 1.083 | 0.487; 2.408 | 0.84 |
| Kyushu | 9/360 (2.5%) | 8/349 (2.3%) | 1.093 | 0.417; 2.866 | 0.86 |
| Influenza vaccination status | |||||
| Vaccinated | 15/434 (3.5%) | 12/459 (2.6%) | 1.334 | 0.617; 2.882 | 0.46 |
| Unvaccinated | 16/646 (2.5%) | 24/609 (3.9%) | 0.619 | 0.326; 1.177 | 0.15 |
| KB290 + βC | Placebo | RR | 95% CI | p-Value | |
|---|---|---|---|---|---|
| Fever incidence | |||||
| Aged ˂ 40 years | 51/539 (9.5%) | 74/538 (13.8%) | 0.655 | 0.449; 0.957 | 0.028 |
| Aged ≥ 40 years | 60/541 (11.1%) | 35/530 (6.6%) | 1.764 | 1.141; 2.727 | 0.010 |
| Fever duration | |||||
| Aged ˂ 40 years | 1.5 ± 1.0 | 1.7 ± 0.9 | - | - | 0.018 |
| Aged ≥ 40 years | 2.0 ± 1.2 | 1.8 ± 1.2 | - | - | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satomi, S.; Waki, N.; Arakawa, C.; Fujisawa, K.; Suzuki, S.; Suganuma, H. Effects of Heat-Killed Levilactobacillus brevis KB290 in Combination with β-Carotene on Influenza Virus Infection in Healthy Adults: A Randomized Controlled Trial. Nutrients 2021, 13, 3039. https://doi.org/10.3390/nu13093039
Satomi S, Waki N, Arakawa C, Fujisawa K, Suzuki S, Suganuma H. Effects of Heat-Killed Levilactobacillus brevis KB290 in Combination with β-Carotene on Influenza Virus Infection in Healthy Adults: A Randomized Controlled Trial. Nutrients. 2021; 13(9):3039. https://doi.org/10.3390/nu13093039
Chicago/Turabian StyleSatomi, Shohei, Naoko Waki, Chinatsu Arakawa, Kazuhiko Fujisawa, Shigenori Suzuki, and Hiroyuki Suganuma. 2021. "Effects of Heat-Killed Levilactobacillus brevis KB290 in Combination with β-Carotene on Influenza Virus Infection in Healthy Adults: A Randomized Controlled Trial" Nutrients 13, no. 9: 3039. https://doi.org/10.3390/nu13093039
APA StyleSatomi, S., Waki, N., Arakawa, C., Fujisawa, K., Suzuki, S., & Suganuma, H. (2021). Effects of Heat-Killed Levilactobacillus brevis KB290 in Combination with β-Carotene on Influenza Virus Infection in Healthy Adults: A Randomized Controlled Trial. Nutrients, 13(9), 3039. https://doi.org/10.3390/nu13093039






