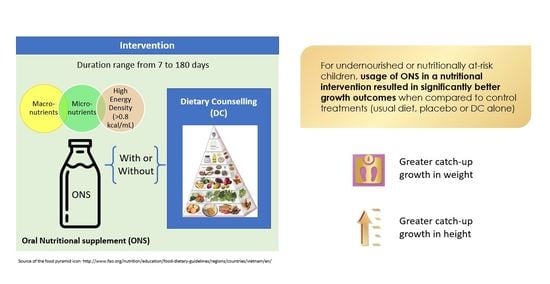
Effect of Oral Nutritional Supplementation on Growth in Children with Undernutrition: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Conduct of Review
2.2. Search Strategy
2.3. Inclusion Criteria
2.4. Anthropometric Measures
2.5. Nutritional Intake
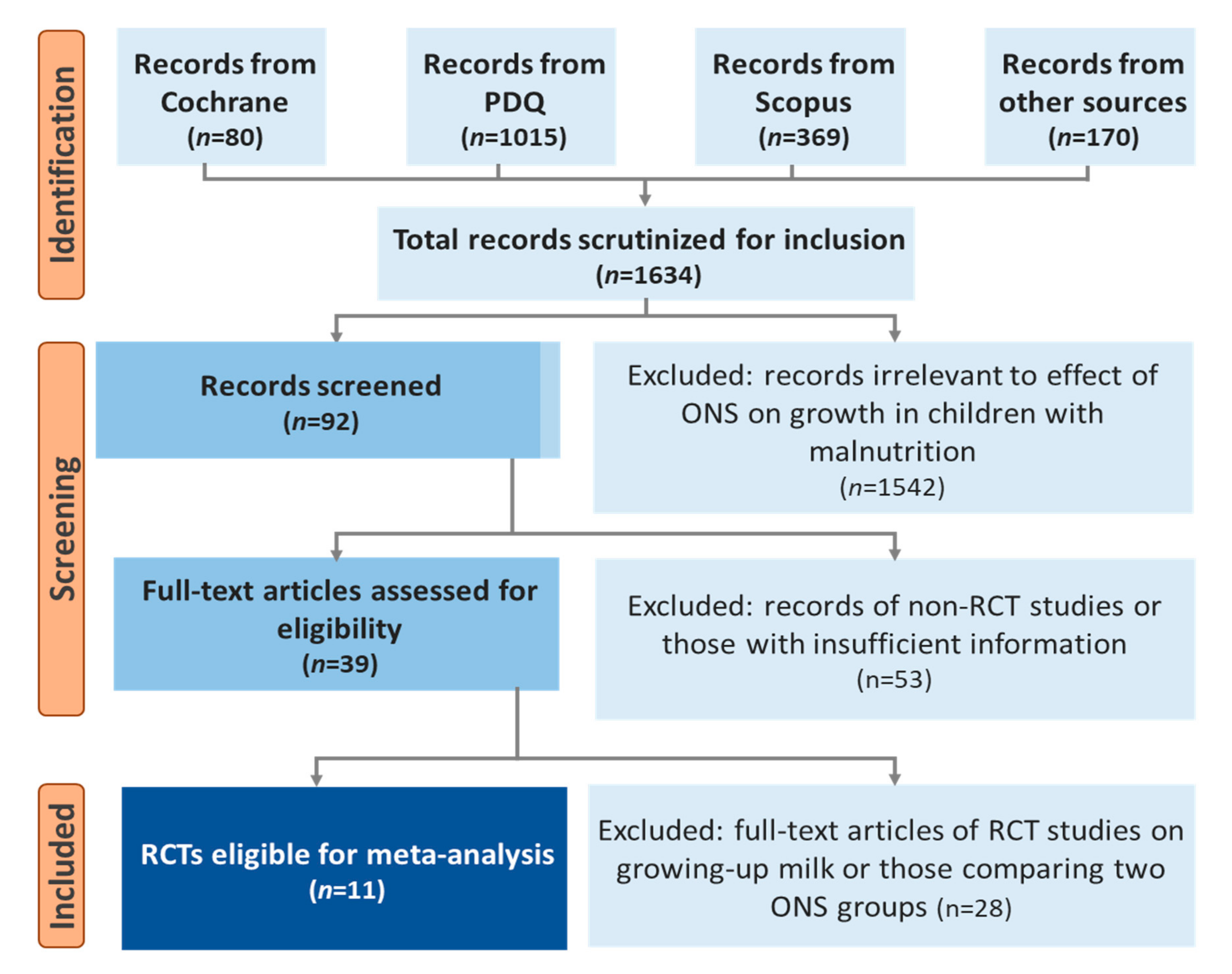
2.6. Study Selection and Data Extraction
2.7. Study Quality Assessment
2.8. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Quality of Included Studies and Risk of Bias
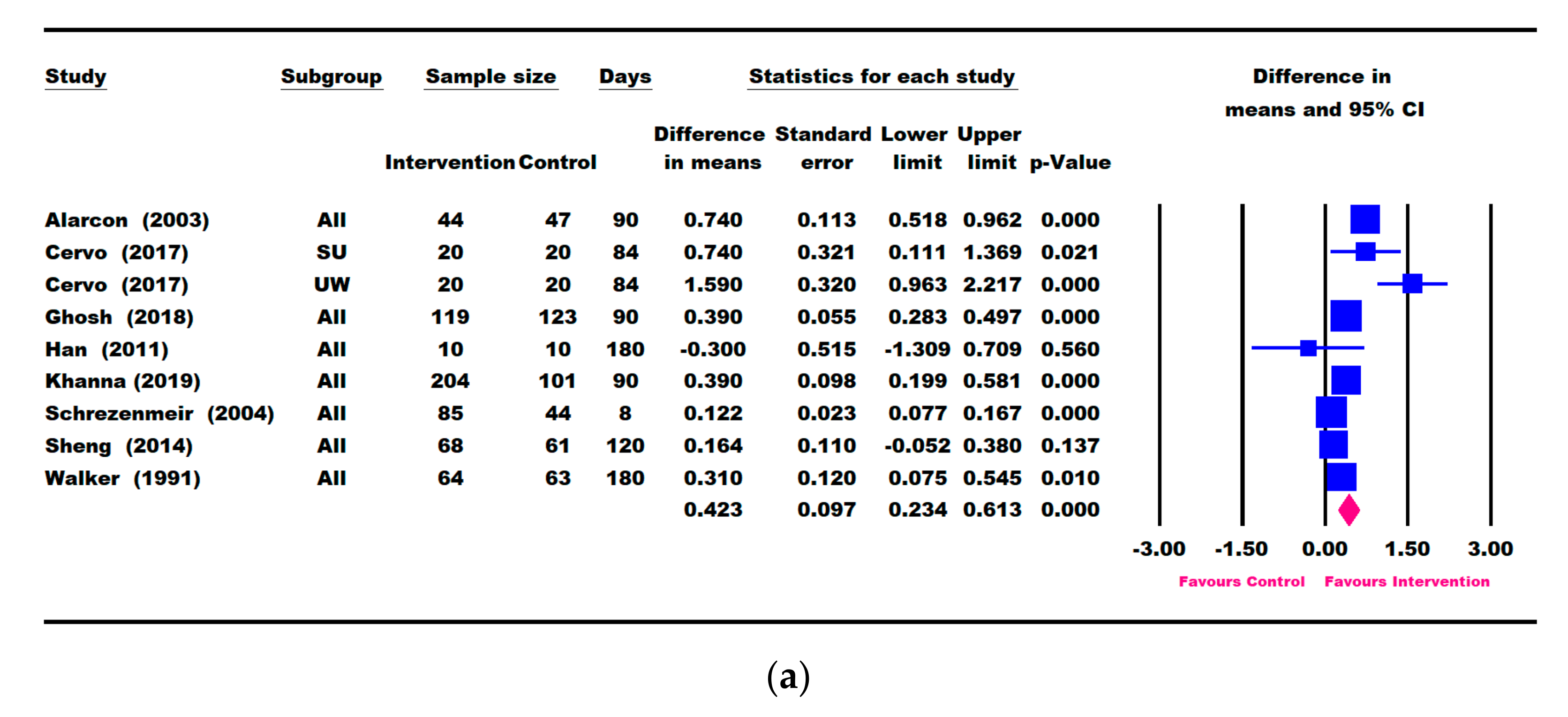
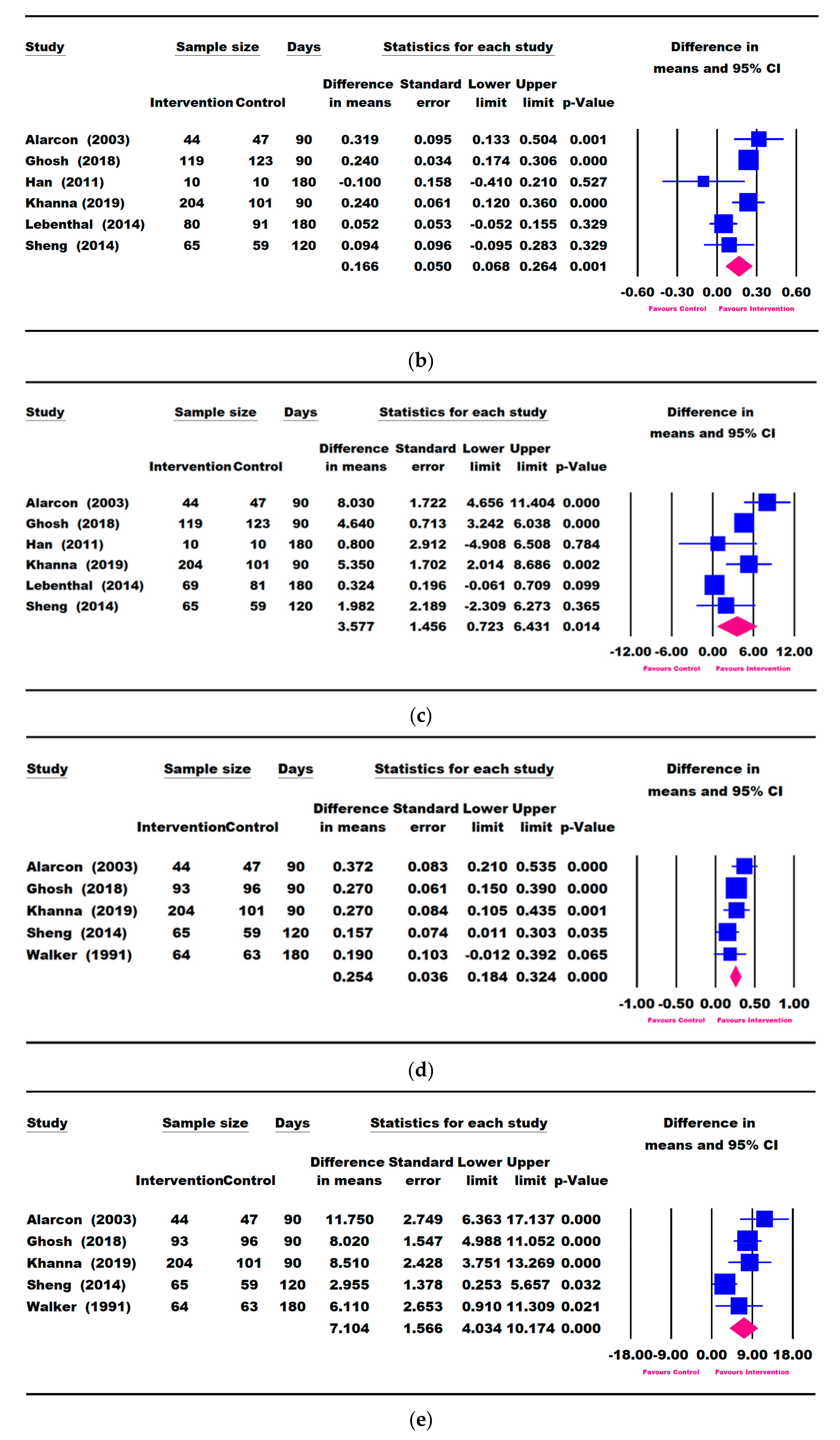
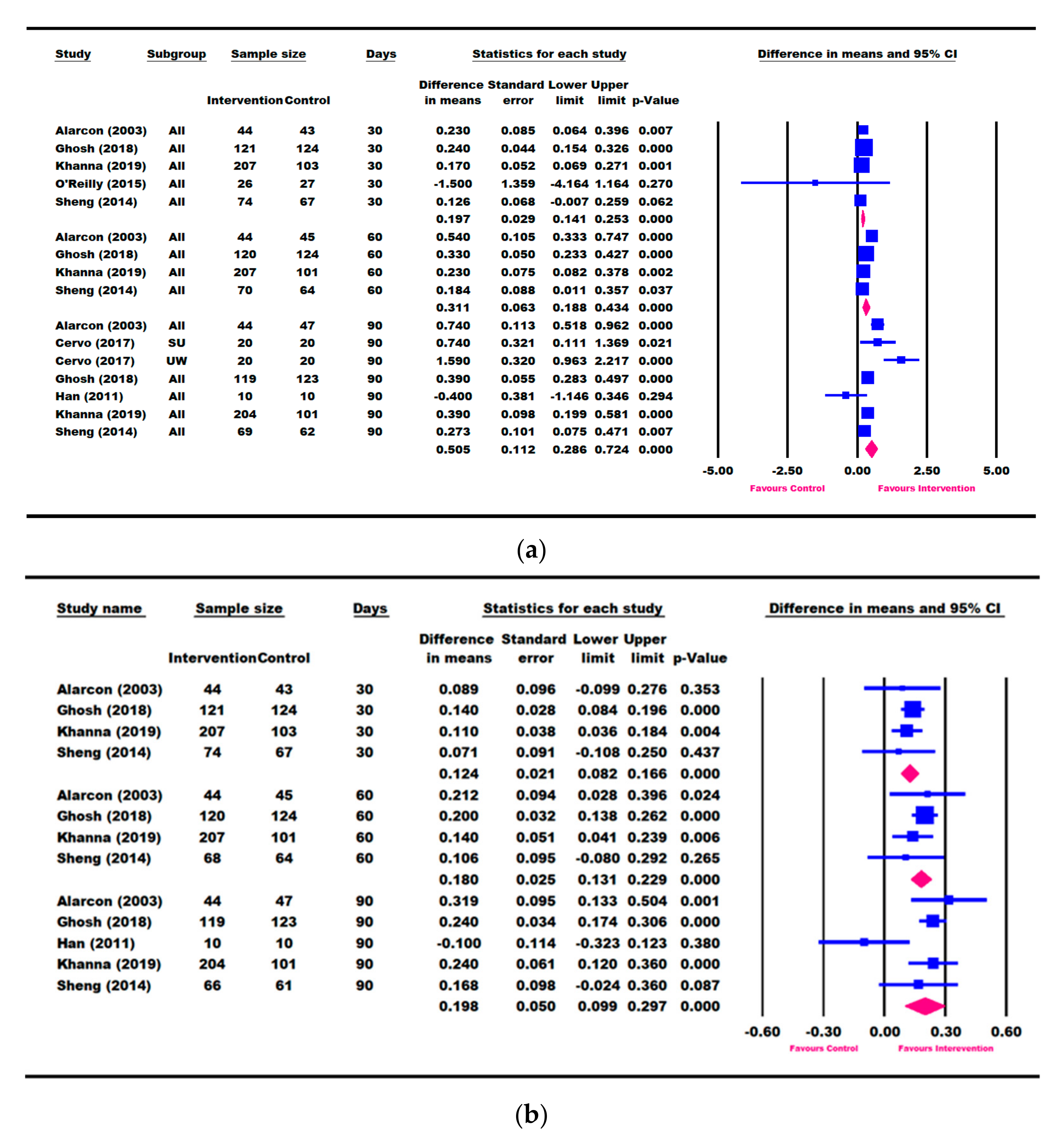
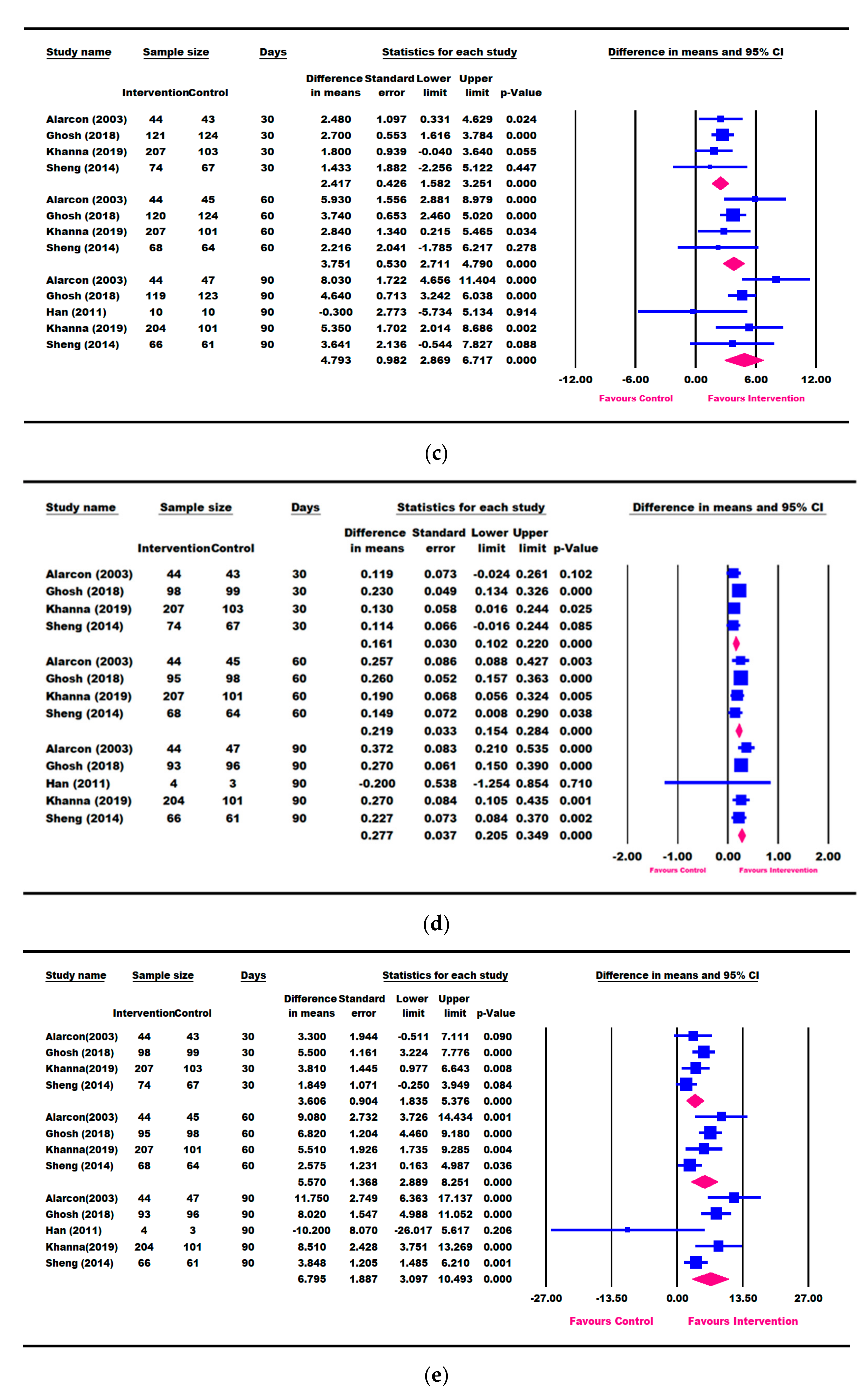
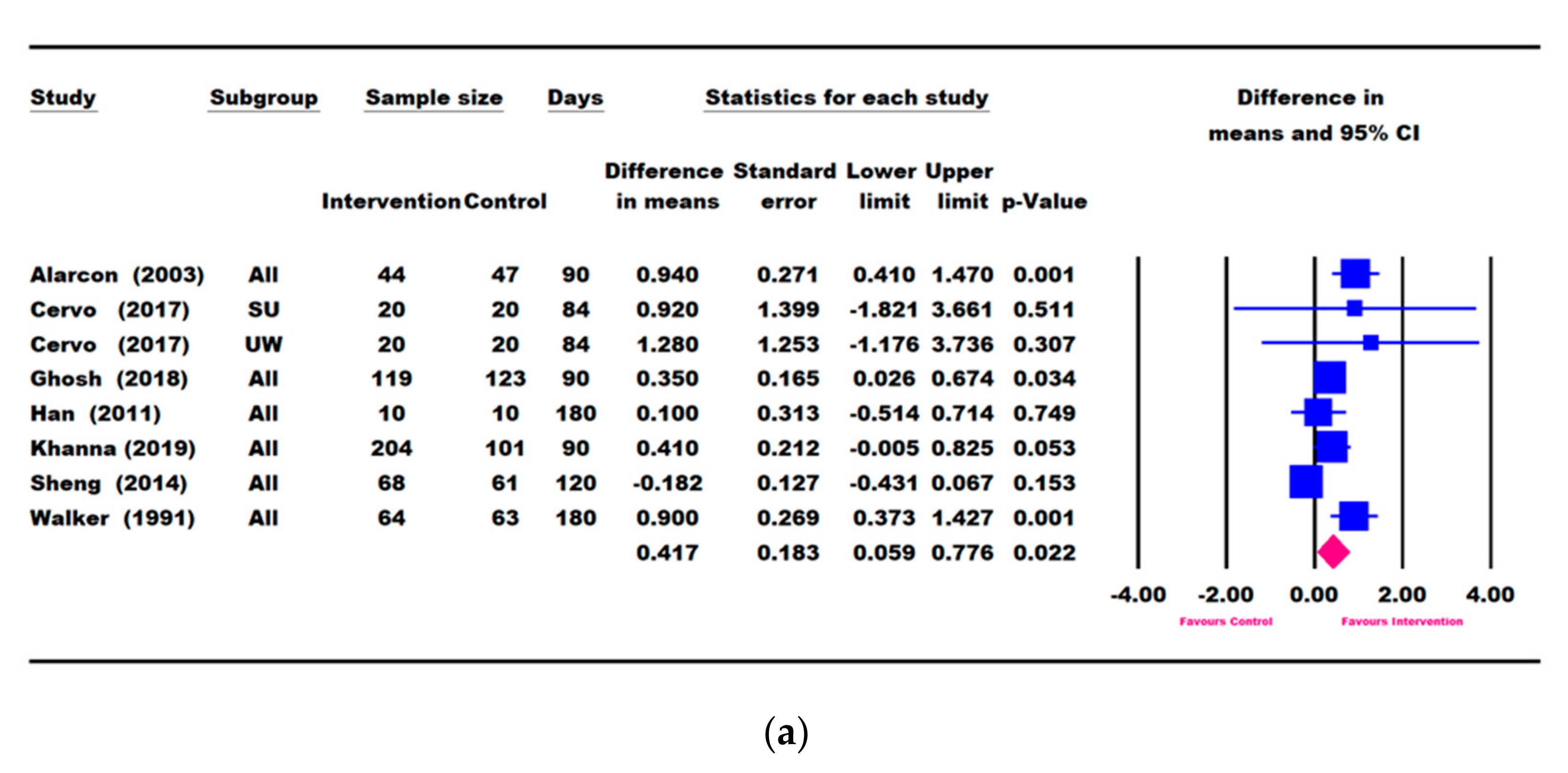
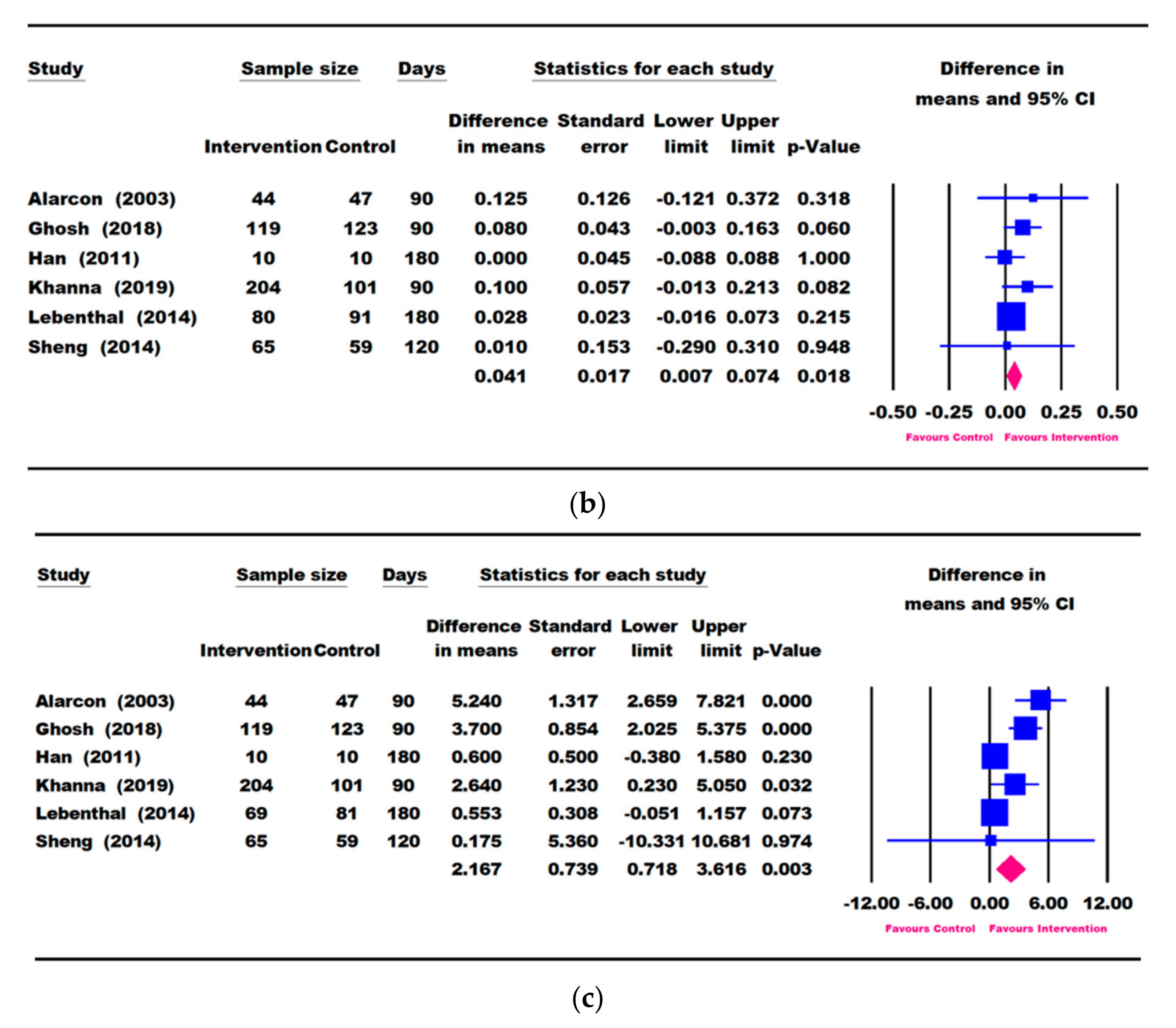
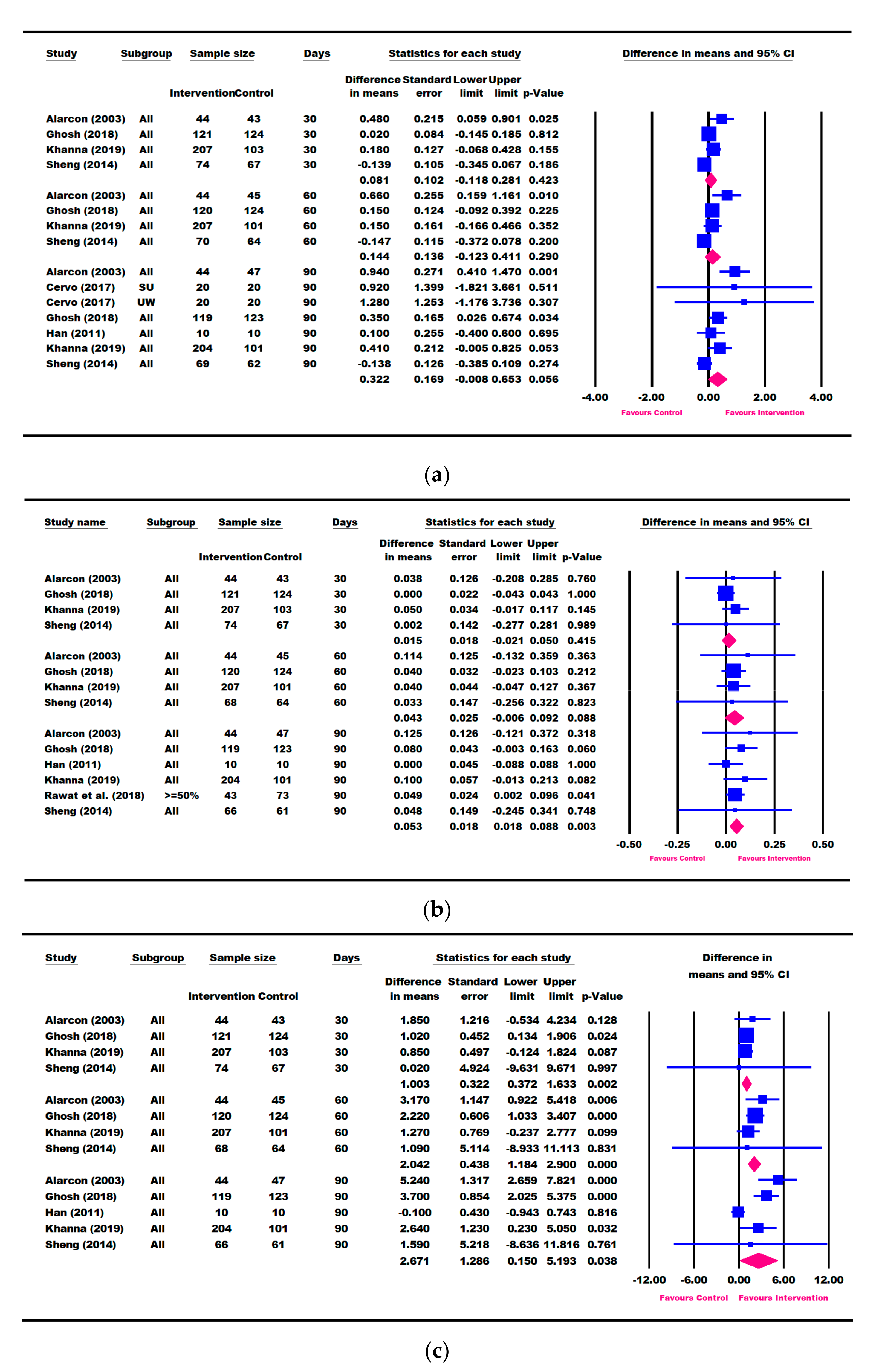
3.3. Results for Weight Parameters: Change in Weight, Weight-for-Age, and Weight-for-Height
3.4. Results for Height Parameters: Change in Height and Height-for-Age
3.5. Other Growth Outcomes
3.6. Nutritional Intake
3.7. Publication Bias and Heterogeneity
4. Discussion
Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Onis, M.; Branca, F. Childhood stunting: A global perspective. Matern. Child Nutr. 2016, 12 (Suppl. 1), 12–26. [Google Scholar] [CrossRef] [PubMed]
- Mwangome, M.; Prentice, A.M. Tackling the triple threats of childhood malnutrition. BMC Med. 2019, 17, 210. [Google Scholar] [CrossRef]
- UNICEF-WHO-World Bank. Levels and Trends in Child Malnutrition: Key Findings of the 2020 Edition of the Joint Child. Malnutrition Estimates; United Nations Children’s Fund (UNICEF), World Health Organization, International Bank for Reconstruction and Development/The World Bank: Geneva, Switzerland, 2020. [Google Scholar]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Briend, A.; Khara, T.; Dolan, C. Wasting and stunting--similarities and differences: Policy and programmatic implications. Food Nutr. Bull. 2015, 36, S15–S23. [Google Scholar] [CrossRef]
- UNICEF. Progress for Children: A Report Card on Nutrition. Available online: https://www.unicef.org/progressforchildren/2006n4/index_undernutrition.html (accessed on 14 November 2020).
- Nassar, M.F.; Shaaban, S.Y.; Nassar, J.F.; Younis, N.T.; Abdel-Mobdy, A.E. Language skills and intelligence quotient of protein energy malnutrition survivors. J. Trop. Pediatr. 2012, 58, 226–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandjaja; Poh, B.K.; Rojroonwasinkul, N.; Le Nyugen, B.K.; Budiman, B.; Ng, L.O.; Soonthorndhada, K.; Xuyen, H.T.; Deurenberg, P.; Parikh, P.; et al. Relationship between anthropometric indicators and cognitive performance in Southeast Asian school-aged children. Br. J. Nutr. 2013, 110 (Suppl. 3), S57–S64. [Google Scholar] [CrossRef] [PubMed]
- Perignon, M.; Fiorentino, M.; Kuong, K.; Burja, K.; Parker, M.; Sisokhom, S.; Chamnan, C.; Berger, J.; Wieringa, F.T. Stunting, poor iron status and parasite infection are significant risk factors for lower cognitive performance in Cambodian school-aged children. PLoS ONE 2014, 9, e112605. [Google Scholar] [CrossRef] [Green Version]
- Prentice, A.M.; Ward, K.A.; Goldberg, G.R.; Jarjou, L.M.; Moore, S.E.; Fulford, A.J.; Prentice, A. Critical windows for nutritional interventions against stunting. Am. J. Clin. Nutr. 2013, 97, 911–918. [Google Scholar] [CrossRef] [Green Version]
- Sudfeld, C.R.; McCoy, D.C.; Fink, G.; Muhihi, A.; Bellinger, D.C.; Masanja, H.; Smith, E.R.; Danaei, G.; Ezzati, M.; Fawzi, W.W. Malnutrition and its determinants are associated with suboptimal cognitive, communication, and motor development in Tanzanian children. J. Nutr. 2015, 145, 2705–2714. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Jensen, S.K.G.; Wade, M.; Kumar, S.; Westerlund, A.; Kakon, S.H.; Haque, R.; Petri, W.A.; Nelson, C.A. Growth faltering is associated with altered brain functional connectivity and cognitive outcomes in urban Bangladeshi children exposed to early adversity. BMC Med. 2019, 17, 199. [Google Scholar] [CrossRef] [Green Version]
- IEG. What Can We Learn from Nutrition Impact Evaluations? Lessons from a Review of Interventions to Reduce Childhood Malnutrition in Developing Countries; The International Bank for Reconstruction and Development; The World Bank: Washington, DC, USA, 2010. [Google Scholar]
- Gat-Yablonski, G.; Phillip, M. Nutritionally-induced catch-up growth. Nutrients 2015, 7, 517–551. [Google Scholar] [CrossRef] [Green Version]
- Black, M.M.; Perez-Escamilla, R.; Rao, S.F. Integrating nutrition and child development interventions: Scientific basis, evidence of impact, and implementation considerations. Adv. Nutr. 2015, 6, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Leroy, J.L.; Ruel, M.; Habicht, J.P.; Frongillo, E.A. Linear growth deficit continues to accumulate beyond the first 1000 days in low- and middle-income countries: Global evidence from 51 national surveys. J. Nutr. 2014, 144, 1460–1466. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.P.; Iannotti, L.; Dewey, K.G.; Michaelsen, K.F.; Onyango, A.W. Contextualising complementary feeding in a broader framework for stunting prevention. Matern. Child. Nutr. 2013, 9 (Suppl. 2), 27–45. [Google Scholar] [CrossRef] [PubMed]
- Jee, Y.H.; Baron, J.; Phillip, M.; Bhutta, Z.A. Malnutrition and catch-up growth during childhood and puberty. World Rev. Nutr. Diet. 2014, 109, 89–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martorell, R.; Khan, L.K.; Schroeder, D.G. Reversibility of stunting: Epidemiological findings in children from developing countries. Eur. J. Clin. Nutr. 1994, 48 (Suppl. 1), S45–S57. [Google Scholar] [PubMed]
- Sudfeld, C.R.; McCoy, D.C.; Danaei, G.; Fink, G.; Ezzati, M.; Andrews, K.G.; Fawzi, W.W. Linear growth and child development in low- and middle-income countries: A meta-analysis. Pediatrics 2015, 135, e1266–e1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiadis, A.; Penny, M.E. Child undernutrition: Opportunities beyond the first 1000 days. Lancet Public Health 2017, 2, e399. [Google Scholar] [CrossRef] [Green Version]
- Huynh, D.T.; Estorninos, E.; Capeding, R.Z.; Oliver, J.S.; Low, Y.L.; Rosales, F.J. Longitudinal growth and health outcomes in nutritionally at-risk children who received long-term nutritional intervention. J. Hum. Nutr. Diet. 2015, 28, 623–635. [Google Scholar] [CrossRef]
- Alarcon, P.A.; Lin, L.H.; Noche, M., Jr.; Hernandez, V.C.; Cimafranca, L.; Lam, W.; Comer, G.M. Effect of oral supplementation on catch-up growth in picky eaters. Clin. Pediatr. 2003, 42, 209–217. [Google Scholar] [CrossRef]
- Fiore, P.; Castagnola, E.; Merolla, R. Effect of nutritional intervention on physical growth in children at risk of malnutrition. Int. Pediatr. 2002, 17, 179–183. [Google Scholar]
- WHO. The WHO Chilld Growth Standards. Available online: https://www.who.int/childgrowth/standards/en/ (accessed on 25 April 2021).
- WHO. The Z-Score or Standard Deviation Classification System. Available online: https://www.who.int/nutgrowthdb/about/introduction/en/index4.html (accessed on 23 April 2021).
- Mehta, N.M.; Corkins, M.R.; Lyman, B.; Malone, A.; Goday, P.S.; Carney, L.N.; Monczka, J.L.; Plogsted, S.W.; Schwenk, W.F.; American Society for Parenteral and Enteral Nutrition (ASPEN) Board of Directors. Defining pediatric malnutrition: A paradigm shift toward etiology-related definitions. JPEN J. Parenter. Enter. Nutr. 2013, 37, 460–481. [Google Scholar] [CrossRef] [Green Version]
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E.; Lancet Nutrition Interventions Review Group; et al. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- UNICEF. The State of the World’s Children 2019. Children, Food and Nutrition: Growing Well in a Changing World; United Nations Children’s Fund (UNICEF): New York, NY, USA, 2019. [Google Scholar]
- Luby, S.P.; Rahman, M.; Arnold, B.F.; Unicomb, L.; Ashraf, S.; Winch, P.J.; Stewart, C.P.; Begum, F.; Hussain, F.; Benjamin-Chung, J.; et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: A cluster randomised controlled trial. Lancet Glob. Health 2018, 6, e302–e315. [Google Scholar] [CrossRef] [Green Version]
- Tickell, K.D.; Atlas, H.E.; Walson, J.L. Environmental enteric dysfunction: A review of potential mechanisms, consequences and management strategies. BMC Med. 2019, 17, 181. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Salam, R.A.; Hadi, Y.B.; Sadiq Sheikh, S.; Bhutta, A.Z.; Weise Prinzo, Z.; Bhutta, Z.A. Preventive lipid-based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes. Cochrane Database Syst. Rev. 2019, 5, CD012611. [Google Scholar] [CrossRef] [PubMed]
- Goudet, S.M.; Bogin, B.A.; Madise, N.J.; Griffiths, P.L. Nutritional interventions for preventing stunting in children (birth to 59 months) living in urban slums in low- and middle-income countries (LMIC). Cochrane Database Syst. Rev. 2019, 6, CD011695. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.L.; Stein, A.D. The impact of nutritional interventions beyond the first 2 years of life on linear growth: A systematic review and meta-analysis. Adv. Nutr. 2017, 8, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Goldsmith, P.D.; Winter-Nelson, A. The importance of animal source foods for nutrient sufficiency in the developing world: The Zambia scenario. Food Nutr. Bull. 2016, 37, 303–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 4. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Francis, D.K.; Smith, J.; Saljuqi, T.; Watling, R.M. Oral protein calorie supplementation for children with chronic disease. Cochrane Database Syst. Rev. 2015, 5, CD001914. [Google Scholar] [CrossRef] [Green Version]
- Smyth, R.L.; Rayner, O. Oral calorie supplements for cystic fibrosis. Cochrane Database Syst. Rev. 2014, 5, CD000406. [Google Scholar] [CrossRef] [Green Version]
- Braegger, C.; Decsi, T.; Dias, J.A.; Hartman, C.; Kolacek, S.; Koletzko, B.; Koletzko, S.; Mihatsch, W.; Moreno, L.; Puntis, J.; et al. Practical approach to paediatric enteral nutrition: A comment by the ESPGHAN committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 110–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatima, S.; Ali Khan, S.; Fatima, F. Nutritional supplements and their use in the treatment of malnutrition in developing countries. J. Ayub Med. Coll. Abbottabad 2015, 27, 911–922. [Google Scholar]
- Gera, T.; Sachdev, H.S. Lipid-Based Nutrient Supplements for the Treatment of 6- to 59-Month-Old Children with Moderate Acute Malnutrition. Available online: https://www.crd.york.ac.uk/prospero/display_record.asp?ID=CRD42016036458 (accessed on 25 April 2021).
- Oluwaniyi, T.; Adegboye, A.; Impact of Lipid Based Nutrient Supplements for Prevention of Childhood Malnutrition: A Systematic Review. PROSPERO: International Prospective Register of Systematic Reviews 2015 CRD42015025019. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42015025019 (accessed on 25 April 2021).
- Ge, K.Y.; Chang, S.Y. Definition and measurement of child malnutrition. Biomed. Environ. Sci 2001, 14, 283–291. [Google Scholar]
- Agency for Healthcare Research and Quality. Systematic Review Data Repository. Available online: https://srdr.ahrq.gov/ (accessed on 30 July 2020).
- Schulz, K.F.; Chalmers, I.; Hayes, R.J.; Altman, D.G. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995, 273, 408–412. [Google Scholar] [CrossRef]
- Schrezenmeir, J.; Heller, K.; McCue, M.; Llamas, C.; Lam, W.; Burow, H.; Kindling-Rohracker, M.; Fischer, W.; Sengespeik, H.C.; Comer, G.M.; et al. Benefits of oral supplementation with and without synbiotics in young children with acute bacterial infections. Clin. Pediatr. 2004, 43, 239–249. [Google Scholar] [CrossRef]
- Han, J.C.; Damaso, L.; Welch, S.; Balagopal, P.; Hossain, J.; Mauras, N. Effects of growth hormone and nutritional therapy in boys with constitutional growth delay: A randomized controlled trial. J. Pediatr. 2011, 158, 427–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, X.; Tong, M.; Zhao, D.; Leung, T.F.; Zhang, F.; Hays, N.P.; Ge, J.; Ho, W.M.; Northington, R.; Terry, D.L.; et al. Randomized controlled trial to compare growth parameters and nutrient adequacy in children with picky eating behaviors who received nutritional counseling with or without an oral nutritional supplement. Nutr. Metab. Insights 2014, 7, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.K.; Kishore, B.; Shaikh, I.; Satyavrat, V.; Kumar, A.; Shah, T.; Pote, P.; Shinde, S.; Berde, Y.; Low, Y.L.; et al. Effect of oral nutritional supplementation on growth and recurrent upper respiratory tract infections in picky eating children at nutritional risk: A randomized, controlled trial. J. Int. Med. Res. 2018, 46, 2186–2201. [Google Scholar] [CrossRef] [PubMed]
- Lebenthal, Y.; Yackobovitch-Gavan, M.; Lazar, L.; Shalitin, S.; Tenenbaum, A.; Shamir, R.; Phillip, M. Effect of a nutritional supplement on growth in short and lean prepubertal children: A prospective, randomized, double-blind, placebo-controlled study. J. Pediatr. 2014, 165, 1190–1193. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Kishore, B.; Shaikh, I.; Satyavrat, V.; Kumar, A.; Shah, T.; Pote, P.; Shinde, S.; Berde, Y.; Low, Y.L.; et al. Continuation of oral nutritional supplementation supports continued growth in nutritionally at-risk children with picky eating behaviour: A post-intervention, observational follow-up study. J. Int. Med. Res. 2018, 46, 2615–2632. [Google Scholar] [CrossRef] [Green Version]
- Cervo, M.M.C.; Mendoza, D.S.; Barrios, E.B.; Panlasigui, L.N. Effects of nutrient-fortified milk-based formula on the nutritional status and psychomotor skills of preschool children. J. Nutr. Metab. 2017, 2017, 6456738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, S.P.; Powell, C.A.; Grantham-McGregor, S.M.; Himes, J.H.; Chang, S.M. Nutritional supplementation, psychosocial stimulation, and growth of stunted children: The Jamaican study. Am. J. Clin. Nutr. 1991, 54, 642–648. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.; Boland, S.; Slattery, D.; Ward, F. A prospective, randomised, open labelled trial to examine the clinical efficacy of an oral nutritional supplement with regards to improving the nutritional status of undernourished children (2–10 years) in the hospital and community setting over 6 weeks. Clin. Nutr. ESPEN 2015, 10, e202–e203. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, P.; Premakumari, S.; Haripriya, S. Supplementation of milk based health drink enriched with micro nutrients—Part-I impact on growth and haemoglobin status of 7–12 year old children. Ind. J. Nutr. Dietet. 2008, 45, 449–465. [Google Scholar]
- Khanna, D.; Yalawar, M.; Verma, G.; Baggs, G.; Devitt, A.; Bhavya, C.; Bhatnagar, S.; Ghosh, A.; Jog, P.; Khadilkar, A.; et al. Oral nutritional supplementation in picky eating children (P11-114-19). Curr. Dev. Nutr. 2019, 3, nzz048-P11. [Google Scholar] [CrossRef] [Green Version]
- Rawat, V.; Frappin, G.; Kalita, P.; Mittal, N.; Fendell, L.; Yackobovitch-Gavan, M.; Lebenthal, Y.; Tenenbaum, A.; Shamir, R.; Lazar, L.; et al. Effect of nutritional supplementation on growth in short and lean prepubertal children over 6 months: A Post-hoc analysis. In Proceedings of the 5th International Conference on Nutrition and Growth, Paris, France, 1–3 March 2018. [Google Scholar]
- Yackobovitch-Gavan, M.; Lebenthal, Y.; Lazar, L.; Shalitin, S.; Demol, S.; Tenenbaum, A.; Shamir, R.; Phillip, M. Effect of nutritional supplementation on growth in short and lean prepubertal children after 1 year of intervention. J. Pediatr. 2016, 179, 154–159. [Google Scholar] [CrossRef]
- Bouma, S. Diagnosing pediatric malnutrition. Nutr. Clin. Pract. 2017, 32, 52–67. [Google Scholar] [CrossRef] [Green Version]
- Guldan, G.S.; Fan, H.C.; Ma, X.; Ni, Z.Z.; Xiang, X.; Tang, M.Z. Culturally appropriate nutrition education improves infant feeding and growth in rural Sichuan, China. J. Nutr. 2000, 130, 1204–1211. [Google Scholar] [CrossRef] [Green Version]
- Santos, I.; Victora, C.G.; Martines, J.; Goncalves, H.; Gigante, D.P.; Valle, N.J.; Pelto, G. Nutrition counseling increases weight gain among Brazilian children. J. Nutr. 2001, 131, 2866–2873. [Google Scholar] [CrossRef] [Green Version]
- Michaelsen, K.F.; Hoppe, C.; Roos, N.; Kaestel, P.; Stougaard, M.; Lauritzen, L.; Molgaard, C.; Girma, T.; Friis, H. Choice of foods and ingredients for moderately malnourished children 6 months to 5 years of age. Food Nutr. Bull. 2009, 30, S343–S404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golden, M.H. Proposed recommended nutrient densities for moderately malnourished children. Food Nutr. Bull. 2009, 30, S267–S342. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.; Smith, T. Malnutrition: Causes and consequences. Clin. Med. 2010, 10, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Prader, A. Catch-up growth. Postgrad. Med. J. 1978, 54 (Suppl. 1), 133–146. [Google Scholar]
- Cuttler, L.; Misra, M.; Koontz, M. Chapter 22—Somatic growth and maturation. In Endocrinology: Adult and Pediatric, 7th ed.; Jameson, J.L., De Groot, L.J., de Kretser, D.M., Giudice, L.C., Grossman, A.B., Melmed, S., Potts, J.T., Weir, G.C., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2016; pp. 382–417. [Google Scholar] [CrossRef]
- Hirvonen, K. Measuring catch-up growth in malnourished populations. Ann. Hum. Biol. 2014, 41, 67–75. [Google Scholar] [CrossRef] [PubMed]

| Study | Country | Subjects * | n | Age Range | Mean Age, Years (SD) | % Boys | Intervention Type | Intervention Intervals, Days | GRADE Quality |
|---|---|---|---|---|---|---|---|---|---|
| Walker, 1991 | Jamaica | HAZ < −2 WHZ ≤ 0 | 127 | 9–24 m | 1.56 (0.34) | 56 | ONS vs. no ONS | 180, 365 | Moderate |
| Alarcon, 2003 | Philippines, Taiwan | WHP < 25th percentile, picky eater | 104 | 3–5 y | 4.04 (0.54) | 51 | ONS-DC vs. DC | 30, 60, 90 | High |
| Schrezenmeir, 2004 | Germany | Acute infection | 129 | 1–6 y | 4.16 (1.48) | 56 | ONS vs. fruit-flavored drink | 8 | High |
| Vijayalakshmi, 2008 | India | Height < NCHS standard | 842 | 7–12 y | 9.49 (1.72) | 50 | ONS vs. usual diet | 365 | Low |
| Han, 2011 | USA | CDGM | 20 | 7–11 y | 9.30 (1.33) | 100 | ONS vs. usual diet | 90, 180, 365 | High |
| Sheng, 2014 | China | WHP < 25th percentile, picky eater | 142 | 2.5–5 y | 3.71 (0.70) | 49 | ONS-DC vs. DC | 30, 60, 90, 120 | High |
| Lebenthal, 2014 Yackobovitch, 2016 Rawat, 2018 | Israel | HAP and WAP ≤ 10th percentile; WAP ≤ HAP | 200 | 3–9 y | 5.50 (1.50) | 75 | ONS vs. low-caloric, low-protein control | 90, 180 | High |
| O’Reilly, 2015 | Ireland | Undernourished | 67 | 2–10 y | 4.80 (2.00) | NR | ONS-DC vs. DC | 16, 42 | Low |
| Cervo, 2017 | Philippines | 3 ≤ WAZ < −2 or WAZ < −3 | 80 | 3–5 y | 4.19 (1.27) | 37 | ONS vs. usual diet | 84 ^ | Moderate |
| Ghosh (1), 2018 Ghosh (2), 2018 | India | −2 ≤ WAZ < −1 Picky eater, URTI | 255 | 2–6 y | 3.67 (1.19) | 63 | ONS-DC vs. DC | 10, 30, 60, 90 | High |
| Khanna, 2019 | India | 3% < WHZ < 15% Picky eater | 321 | 2–4 y | 2.94 (0.54) | 60 | ONS-DC vs. DC | 7, 30, 60, 90 | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, F.; Hannon, B.A.; Hustead, D.S.; Aw, M.M.; Liu, Z.; Chuah, K.A.; Low, Y.L.; Huynh, D.T.T. Effect of Oral Nutritional Supplementation on Growth in Children with Undernutrition: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3036. https://doi.org/10.3390/nu13093036
Zhang Z, Li F, Hannon BA, Hustead DS, Aw MM, Liu Z, Chuah KA, Low YL, Huynh DTT. Effect of Oral Nutritional Supplementation on Growth in Children with Undernutrition: A Systematic Review and Meta-Analysis. Nutrients. 2021; 13(9):3036. https://doi.org/10.3390/nu13093036
Chicago/Turabian StyleZhang, Zhiying, Fei Li, Bridget A. Hannon, Deborah S. Hustead, Marion M. Aw, Zhongyuan Liu, Khun Aik Chuah, Yen Ling Low, and Dieu T. T. Huynh. 2021. "Effect of Oral Nutritional Supplementation on Growth in Children with Undernutrition: A Systematic Review and Meta-Analysis" Nutrients 13, no. 9: 3036. https://doi.org/10.3390/nu13093036
APA StyleZhang, Z., Li, F., Hannon, B. A., Hustead, D. S., Aw, M. M., Liu, Z., Chuah, K. A., Low, Y. L., & Huynh, D. T. T. (2021). Effect of Oral Nutritional Supplementation on Growth in Children with Undernutrition: A Systematic Review and Meta-Analysis. Nutrients, 13(9), 3036. https://doi.org/10.3390/nu13093036