The Dietary Approach to the Treatment of the Rare Genetic Tubulopathies Gitelman’s and Bartter’s Syndromes
Abstract
1. Introduction
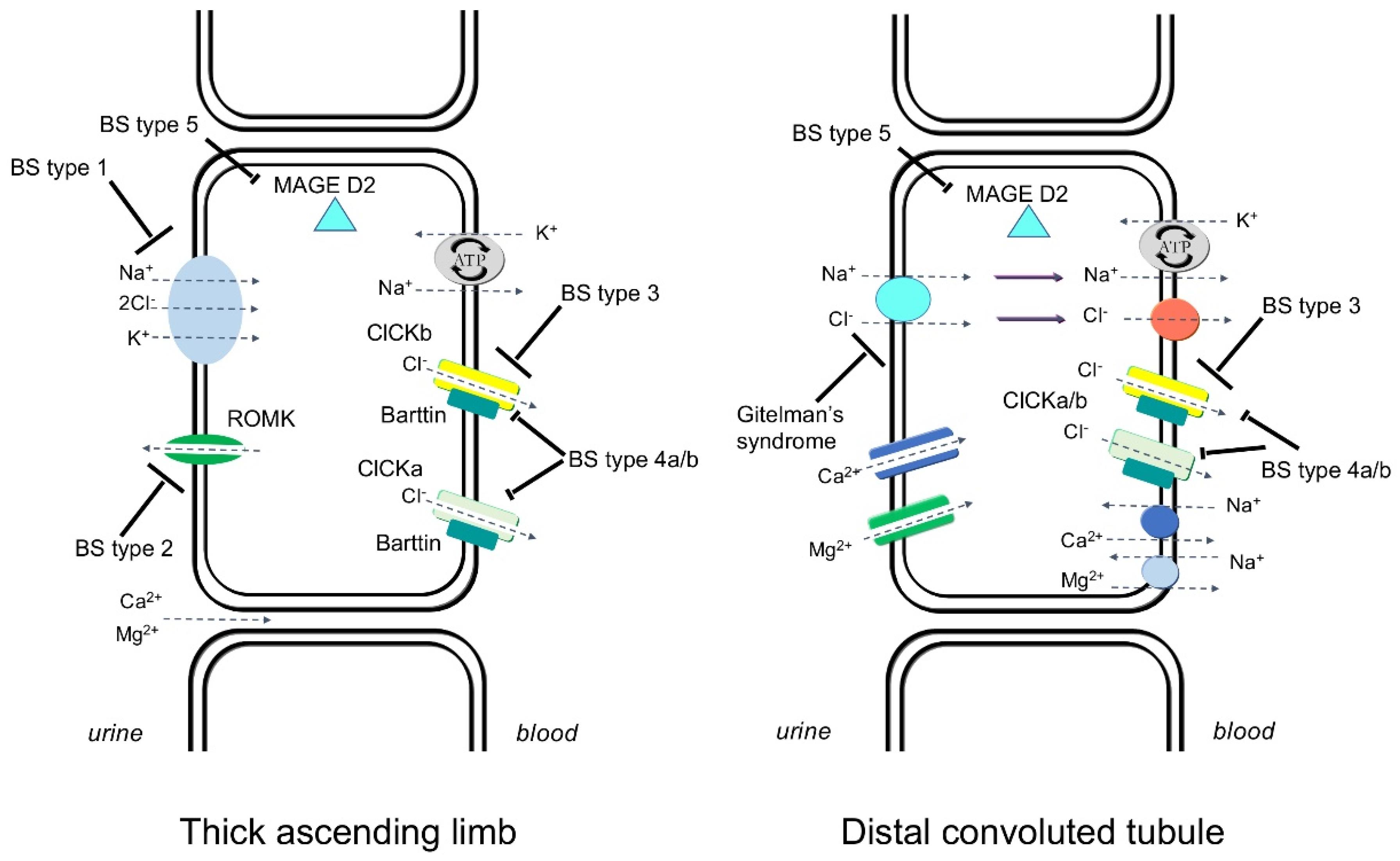
Gitelman’s Syndrome and Bartter’s Syndrome
2. Dietary Approach to Gitelman’s Syndrome
2.1. Sodium and Chloride
2.2. Potassium
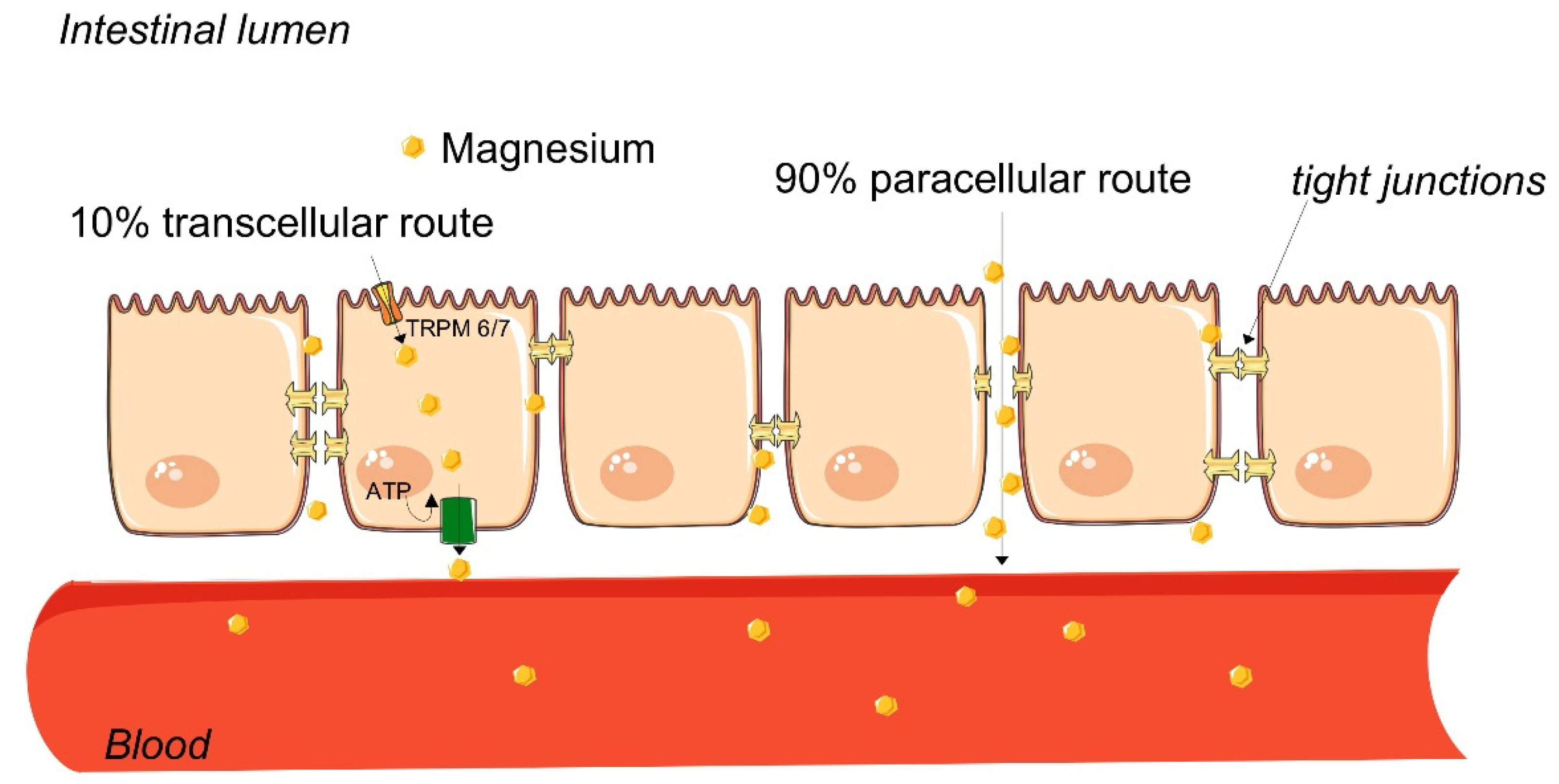
2.3. Magnesium
2.4. Food Causing Potassium and Magnesium Loss
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gitelman, H.J.; Graham, J.B.; Welt, L.G. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans. Assoc. Am. Physicians 1966, 79, 221–235. [Google Scholar]
- Bartter, F.C.; Pronove, P.; Gill, J.R.; Maccardle, R.C. Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. Am. J. Med. 1962, 33, 811–828. [Google Scholar] [CrossRef]
- Blanchard, A.; Bockenhauer, D.; Bolignano, D.; Calò, L.A.; Cosyns, E.; Devuyst, O.; Ellison, D.H.; Frankl, F.E.K.; Knoers, N.V.; Konrad, M.; et al. Gitelman syndrome: Consensus and guidance from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2017, 91, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Konrad, M.; Nijenhuis, T.; Ariceta, G.; Bertholet-Thomas, A.; Calò, L.A.; Capasso, G.; Emma, F.; Schlingmann, K.P.; Singh, M.; Trepiccione, F.; et al. Diagnosis and management of Bartter syndrome: Executive summary of the consensus and recommendations from the European Rare Kidney Disease Reference Network Working Group for Tubular Disorders. Kidney Int. 2021, 99, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Urwin, S.; Willows, J.; Sayer, J.A. The challenges of diagnosis and management of Gitelman syndrome. Clin. Endocrinol. 2020, 92, 3–10. [Google Scholar] [CrossRef]
- Kleta, R.; Bockenhauer, D. Bartter syndromes and other salt-losing tubulopathies. Nephron Physiol. 2006, 104, 73–80. [Google Scholar] [CrossRef]
- Costa, C.S.; Del-Ponte, B.; Assunção, M.C.F.; Santos, I.S. Consumption of ultra-processed foods and body fat during childhood and adolescence: A systematic review. Public Health Nutr. 2018, 21, 148–159. [Google Scholar] [CrossRef]
- Bockenhauer, D.; Bichet, D.G. Inherited secondary nephrogenic diabetes insipidus: Concentrating on humans. Am. J. Physiol. Ren. Physiol. 2013, 304, F1037–F1042. [Google Scholar] [CrossRef][Green Version]
- Scialla, J.J.; Anderson, C.A. Dietary acid load: A novel nutritional target in chronic kidney disease? Adv. Chronic Kidney Dis. 2013, 20, 141–149. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Fluid overload as a therapeutic target for the preservative management of chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2020, 29, 22–28. [Google Scholar] [CrossRef]
- Kimura, M.; Itokawa, Y. Cooking losses of minerals in foods and its nutritional significance. J. Nutr. Sci. Vitam. 1990, 36, S25–S32. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Felig, P.; Ferrannini, E.; Wahren, J. Effect of graded doses of insulin on splanchnic and peripheral potassium metabolism in man. Am. J. Physiol. Endocrinol. Metab. 1980, 238, E421–E427. [Google Scholar] [CrossRef]
- Nijenhuis, T.; Vallon, V.; van der Kemp, A.W.; Loffing, J.; Hoenderop, J.G.; Bindels, R.J. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J. Clin. Investig. 2005, 115, 1651–1658. [Google Scholar] [CrossRef]
- Kamel, K.S.; Harvey, E.; Douek, K.; Parmar, M.S.; Halperin, M.L. Studies on the pathogenesis of hypokalemia in Gitelman’s syndrome: Role of bicarbonaturia and hypomagnesemia. Am. J. Nephrol. 1998, 18, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Kuo, E. Mechanism of hypokalemia in magnesium deficiency. J. Am. Soc. Nephrol. 2007, 18, 2649–2652. [Google Scholar] [CrossRef] [PubMed]
- van Angelen, A.A.; van der Kemp, A.W.; Hoenderop, J.G.; Bindels, R.J. Increased expression of renal TRPM6 compensates for Mg2+ wasting during furosemide treatment. Nephrol. Dial. Transplant. Plus 2012, 5, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Agus, Z.S. Hypomagnesemia. J. Am. Soc. Nephrol. 1999, 10, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Punzi, L.; Calo, L.; Schiavon, F.; Pianon, M.; Rosada, M.; Todesco, S. Chondrocalcinosis is a feature of Gitelman’s variant of Bartter’s syndrome: A new look at the hypomagnesemia associated with calcium pyrophosphate dihydrate crystal deposition disease. Rev. Rhum. Engl. Ed. 1998, 65, 571–574. [Google Scholar]
- Calo, L.; Punzi, L.; Semplicini, A. Hypomagnesemia and chondrocalcinosis in Bartter’s and Gitelman’s syndrome: Review of the pathogenetic mechanisms. Am. J. Nephrol. 2000, 20, 347–350. [Google Scholar] [CrossRef]
- Knoers, N.V. Gitelman syndrome. Adv. Chronic Kidney Dis. 2006, 13, 148–154. [Google Scholar] [CrossRef]
- Fine, K.D.; Santa Ana, C.A.; Fordtran, J.S. Diagnosis of magnesium-induced diarrhea. N. Engl. J. Med. 1991, 324, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Ranade, V.V.; Somberg, J.C. Bioavailability and pharmacokinetics of magnesium after administration of magnesium salts to humans. Am. J. Ther. 2001, 8, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Coudray, C.; Rambeau, M.; Feillet-Coudray, C.; Gueux, E.; Tressol, J.C.; Mazur, A.; Rayssiguier, Y. Study of magnesium bioavailability from ten organic and inorganic mg salts in mg-depleted rats using a stable isotope approach. Magnes. Res. 2005, 18, 215–223. [Google Scholar] [PubMed]
- Ahmed, F.; Mohammed, A. Magnesium: The Forgotten Electrolyte-A Review on Hypomagnesemia. Med. Sci. 2019, 7, 56. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975. [Google Scholar] [CrossRef]
- Gómez-Ramírez, S.; Brilli, E.; Tarantino, G.; Muñoz, M. Sucrosomial® Iron: A New Generation Iron for Improving Oral Supplementation. Pharmaceuticals 2018, 11, 97. [Google Scholar] [CrossRef]
- Brilli, E.; Khadge, S.; Fabiano, A.; Zambito, Y.; Williams, T.; Tarantino, G. Magnesium bioavailability after administration of sucrosomial® magnesium: Results of an ex-vivo study and a comparative, double-blinded, cross-over study in healthy subjects. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1843–1851. [Google Scholar] [CrossRef]
- Riccio, E.; Sabbatini, M.; Capuano, I.; Pellegrino, A.M.; Petruzzelli, L.A.; Pisani, A. Oral Sucrosomial® iron versus intravenous iron for recovering iron deficiency anaemia in ND-CKD patients: A cost- minimization analysis. BMC Nephrol. 2020, 21, 57. [Google Scholar] [CrossRef]
- Giordano, G.; Napolitano, M.; Di Battista, V.; Lucchesi, A. Oral high-dose sucrosomial iron vs intravenous iron in sideropenic anemia patients intolerant/refractory to iron sulfate: A multicentric randomized study. Ann. Hematol. 2020, 100, 2173–2179. [Google Scholar] [CrossRef]
- Bertani, L.; Tricò, D.; Zanzi, F.; Baiano Svizzero, G.; Coppini, F.; de Bortoli, N.; Bellini, M.; Antonioli, L.; Blandizzi, C.; Marchi, S. Oral Sucrosomial Iron Is as Effective as Intravenous Ferric Carboxy-Maltose in Treating Anemia in Patients with Ulcerative Colitis. Nutrients 2021, 13, 608. [Google Scholar] [CrossRef]
- William, J.H.; Danziger, J. Proton-pump inhibitor-induced hypomagnesemia: Current research and proposed mechanisms. World J. Nephrol. 2016, 5, 152–157. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; Kittanamongkolchai, W.; Srivali, N.; Edmonds, P.J.; Ungprasert, P.; O’Corragain, O.A.; Korpaisarn, S.; Erickson, S.B. Proton pump inhibitors linked to hypomagnesemia: A systematic review and meta-analysis of observational studies. Ren. Fail. 2015, 37, 1237–1241. [Google Scholar] [CrossRef]
- Zittermann, A. Magnesium deficit-overlooked cause of low vitamin D status? BMC Med. 2013, 11, 229. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopath Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Rameshrad, M.; Hosseinzadeh, H. Toxicological Effects of Glycyrrhiza glabra (Licorice): A Review. Phytother. Res. 2017, 31, 1635–1650. [Google Scholar] [CrossRef] [PubMed]
- Francini-Pesenti, F.; Puato, M.; Piccoli, A.; Brocadello, F. Liquorice-induced hypokalaemia and water retention in the absence of hypertension. Phytother. Res. 2008, 22, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Knochel, J.P.; Schlein, E.M. On the mechanism of rhabdomyolysis in potassium depletion. J. Clin. Investig. 1972, 51, 1750–1758. [Google Scholar] [CrossRef]
- Yang, L.Y.; Yin, J.H.; Yang, J.; Ren, Y.; Xiang, C.Y.; Wang, C.Y. Liquorice-induced severe hypokalemic rhabdomyolysis with Gitelman syndrome and diabetes: A case report. World J. Clin. Cases 2019, 7, 1200–1205. [Google Scholar] [CrossRef]
- Rodrigo, R.; Thielemann, L.; Olea, M.; Muñoz, P.; Cereceda, M.; Orellana, M. Effect of ethanol ingestion on renal regulation of water and electrolytes. Arch. Med. Res. 1998, 29, 209–218. [Google Scholar]
- De Marchi, S.; Cecchin, E.; Basile, A.; Bertotti, A.; Nardini, R.; Bartoli, E. Renal tubular dysfunction in chronic alcohol abuse—Effects of abstinence. N. Engl. J. Med. 1993, 329, 1927–1934. [Google Scholar] [CrossRef]
- Mahendran, M.; Agarwal, S.; Ray, A.; Vikram, N.K. Binge alcohol consumption leading to hypokalemic rhabdomyolysis. BMJ Case Rep. 2019, 12, e229307. [Google Scholar] [CrossRef]
- Knobel, U.; Modarres, G.; Schneemann, M.; Schmid, C. Gitelman’s syndrome with persistent hypokalemia-don’t forget licorice, alcohol, lemon juice, iced tea and salt depletion: A case report. J. Med. Case Rep. 2011, 5, 312. [Google Scholar] [CrossRef]
- Martinez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, Y. Citrate metabolism in blood transfusions and its relationship due to metabolic alkalosis and respiratory acidosis. Int. J. Clin. Exp. Med. 2015, 8, 6578–6584. [Google Scholar]
- Hamm, L.; Hering-Smith, K.S. Pathophysiology of hypocitraturic nephrolithiasis. Endocrinol. Metab. Clin. N. Am. 2002, 31, 885–893. [Google Scholar] [CrossRef]
- Siener, R.; Jahnen, A.; Hesse, A. Influence of a mineral water rich in calcium, magnesium and bicarbonate on urine composition and the risk of calcium oxalate crystallization. Eur. J. Clin. Nutr. 2004, 58, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Vitoria, I. The nutritional limitations of plant-based beverages in infancy and childhood. Nutr. Hosp. 2017, 34, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
| Food | Potassium (mg) | Carbohydrates (g) | Energy (Kilocalories) |
|---|---|---|---|
| Vegetables | |||
| Broccoli, raw | 303 | 6.3 (sugars 1.4) | 39 |
| Kale, raw | 348 | 4.4 (sugars 0.8) | 43 |
| Lettuce, raw | 253 | 3 (sugars 1.2) | 20 |
| Mushrooms (white), raw | 318 | 3.3 (sugars 2.0) | 22 |
| Spinach, raw | 460 | 3.6 (sugars 0.4) | 23 |
| Tomatoes, canned | 198 | 7.3 (sugars 4.4) | 32 |
| Potatoes, boiled | 372 | 20.1 (sugars 0.9) | 87 |
| Swiss chard, raw | 379 | 3.7 (sugars 1.1) | 19 |
| Legumes | |||
| Beans (white), dry | 1540 | 60.3 (sugars 2.1) | 333 |
| Green peas, raw | 244 | 14.4 (sugars 5.7) | 81 |
| Soy milk | 158 | 14.4 (sugars 5.7) | 38 |
| Fruits | |||
| Apricot, raw | 259 | 11.1 (sugars 9.2) | 48 |
| Apricot, dried | 1162 | 62.6 (sugars 53.4) | 241 |
| Avocados, raw | 485 | 8.6 (sugars 0.3) | 167 |
| Banana, raw | 326 | 8.6 (sugars 0.3) | 89 |
| Kiwifruit, green, raw | 312 | 14.7 (sugars 9.0) | 61 |
| Orange, raw | 166 | 11.5 (sugars 9.1) | 46 |
| Watermelon | 173 | 7.5 (sugars 6.2) | 30 |
| Grapefruit juice | 141 | 7.7 (sugars 7.7) | 37 |
| Fruit juice blend, 100% fruit | 101 | 11.2 (sugars 10.4) | 46 |
| Nuts | |||
| Almonds | 733 | 21.6 (sugars 4.3) | 579 |
| Hazelnuts | 680 | 17.7 (sugars 4.3) | 628 |
| Walnuts | 441 | 13.7 (sugars 2.61) | 654 |
| Meat and fish | |||
| Halibut (Atlantic and Pacific), raw | 435 | 0 | 91 |
| Salmon (Atlantic, farmed), raw | 363 | 0 | 208 |
| Beef (short loin), raw | 266 | 0 | 145 |
| Pork (shoulder, fresh), raw | 302 | 0 | 236 |
| Other | |||
| Chocolate, dark 60–69% | 567 | 52.4 (sugars 36.7) | 579 |
| Cocoa powder | 1524 | 57.8 (sugars 1.75) | 228 |
| Food | Magnesium (mg) | Carbohydrates (g) | Energy (Kilocalories) |
|---|---|---|---|
| Nuts | |||
| Almonds | 270 | 21.6 (sugars 4.3) | 579 |
| Hazelnuts | 163 | 17.7 (sugars 4.3) | 628 |
| Walnuts | 158 | 13.7 (sugars 2.6) | 654 |
| Legumes | |||
| Beans (black), dry | 180 | 40.8 (sugars 2.4) | 270 |
| Chickpeas, dry | 45 | 25.5 (sugars 4.5) | 210 |
| Green peas, raw | 33 | 14.4 (sugars 5.7) | 81 |
| Lentils, raw | 33 | 63.4 (sugars 2.0) | 352 |
| Cereals and starchy foods | |||
| Bran flakes | 229 | 80.5 (sugars 18.6) | 328 |
| Quinoa, uncooked | 197 | 64.2 (sugars 2.7) | 368 |
| Quinoa, cooked | 64 | 21.3 (sugars 0.9) | 120 |
| Wheat germ, crude | 239 | 51.8 (sugars 16.8) | 360 |
| Whole bread | 69 | 55.9 (sugars 2.9) | 262 |
| Vegetables | |||
| Spinach, raw | 79 | 3.6 (sugars 0.4)) | 23 |
| Swiss card, raw | 81 | 3.7 (sugars 1.1) | 19 |
| Others | |||
| Avocado | 29 | 8.6 (sugars 0.3) | 167 |
| Chocolate, dark 60–69% | 176 | 52.4 (sugars 36.7) | 579 |
| Chocolate, dark 70–85% | 228 | 45.9 (sugars 0) | 598 |
| Mackerel, raw | 60 | 0 | 205 |
| Tofu | 30 | 2.35 (sugars 24) | 71 |
| Tofu, yogurt | 40 | 16 (sugars 1.2) | 94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francini, F.; Gobbi, L.; Ravarotto, V.; Toniazzo, S.; Nalesso, F.; Spinella, P.; Calò, L.A. The Dietary Approach to the Treatment of the Rare Genetic Tubulopathies Gitelman’s and Bartter’s Syndromes. Nutrients 2021, 13, 2960. https://doi.org/10.3390/nu13092960
Francini F, Gobbi L, Ravarotto V, Toniazzo S, Nalesso F, Spinella P, Calò LA. The Dietary Approach to the Treatment of the Rare Genetic Tubulopathies Gitelman’s and Bartter’s Syndromes. Nutrients. 2021; 13(9):2960. https://doi.org/10.3390/nu13092960
Chicago/Turabian StyleFrancini, Francesco, Laura Gobbi, Verdiana Ravarotto, Silvia Toniazzo, Federico Nalesso, Paolo Spinella, and Lorenzo A Calò. 2021. "The Dietary Approach to the Treatment of the Rare Genetic Tubulopathies Gitelman’s and Bartter’s Syndromes" Nutrients 13, no. 9: 2960. https://doi.org/10.3390/nu13092960
APA StyleFrancini, F., Gobbi, L., Ravarotto, V., Toniazzo, S., Nalesso, F., Spinella, P., & Calò, L. A. (2021). The Dietary Approach to the Treatment of the Rare Genetic Tubulopathies Gitelman’s and Bartter’s Syndromes. Nutrients, 13(9), 2960. https://doi.org/10.3390/nu13092960







