Impact of Maternal Daily Oral Low-Dose Vitamin A Supplementation on the Mother-Infant Pair: A Randomised Placebo-Controlled Trial in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
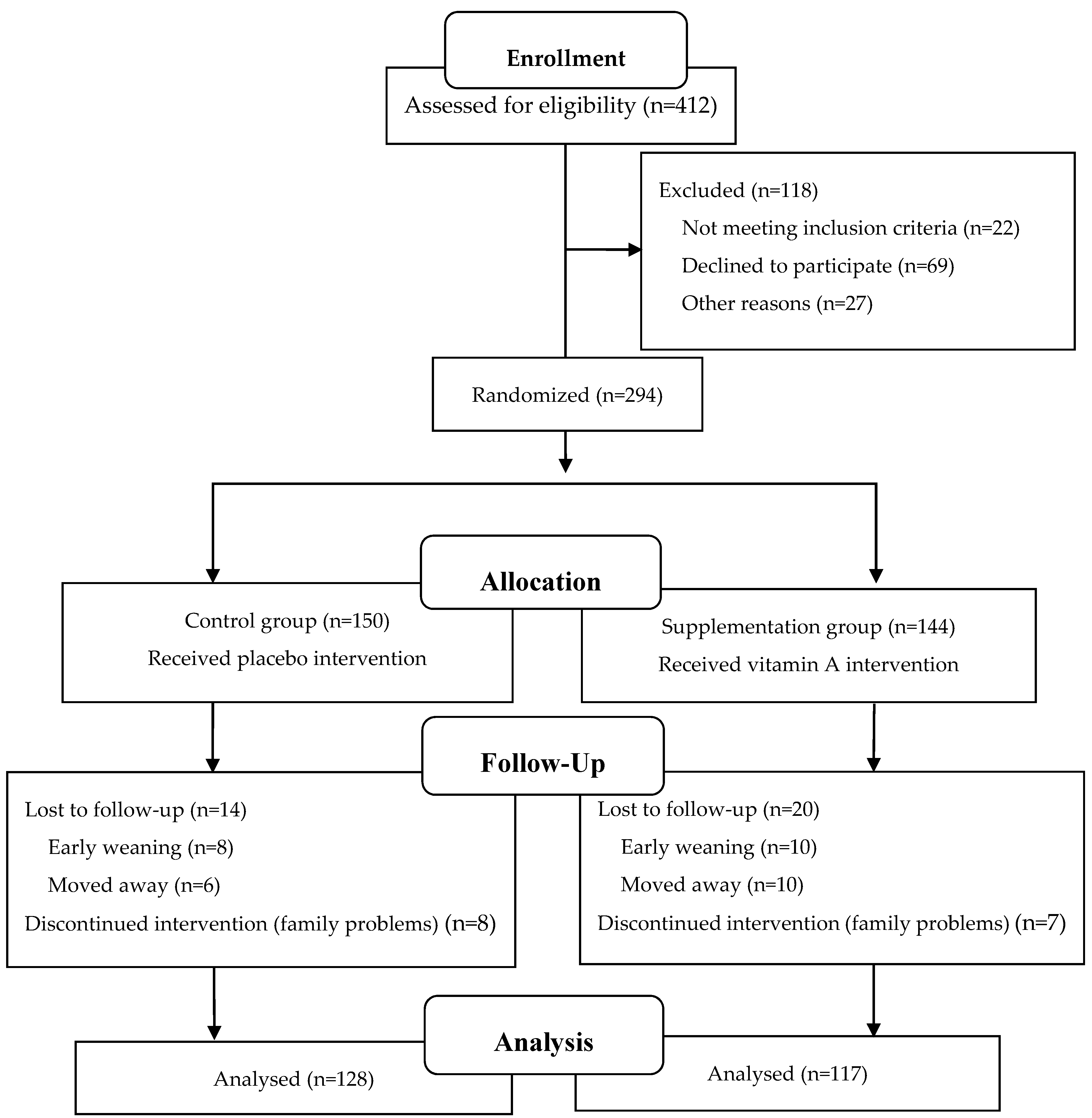
2.2. Study Design
2.3. Dietary Data Collection, Calculation, and Evaluation
2.4. Sample Collection and Retinol Concentration Detection
2.5. Quality Control
2.6. Sample Size and Statistical Analysis
3. Results
3.1. Participants and Baseline Characteristics
3.2. Maternal Daily Dietary Energy and Nutrient Intakes between Supplementation and Control Groups at Enrolment and the End of the Trial
3.3. Effect of Vitamin A Supplementation on Maternal Serum Retinol Concentration at the End of the Trial
3.4. Effect of Vitamin A Supplementation on Maternal Breast Milk Retinol Concentration at the End of the Trial
3.5. Effect of Maternal Vitamin A Supplementation on the Health Status of Infants during the Trial
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Debelo, H.; Novotny, J.A.; Ferruzzi, M.G. Vitamin A. Adv. Nutr. 2017, 8, 992–994. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Jin, C. Vitamin A and E Deficiency and Respiratory Tract Infection in Chinese Children; People’s Medical Publishing House: Beijing, China, 2019. [Google Scholar]
- Wang, R.; Chen, J.; Li, W.; Hu, Y.; Yang, X.; Yang, L. Evaluation of the vitamin A status in women of childbearing age from China city during 2010–2012. Acta Nutr. Sin. 2016, 38, 537–545. [Google Scholar]
- Chinese Nutrition Society. Chinese Dietary Guidelines (2016); People’s Medical Publishing House: Beijing, China, 2016. [Google Scholar]
- Ding, Y.; Indayati, W.; Basnet, T.B.; Li, F.; Luo, H.; Pan, H.; Wang, Z. Dietary intake in lactating mothers in China 2018: Report of a survey. Nutr. J. 2020, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005: WHO Global Database on Vitamin A Deficiency; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Yang, T.; Zhang, Y.; Ma, D.; Li, W.; Yang, X.; Wang, P. Survey on the nutrient intakes of lactating women in three cities of China. Acta Nutr. Sin. 2014, 36, 84–86. [Google Scholar]
- Zhou, Y.; Liu, W.; Li, Y.; Qin, Y.; LI, R.; Chen, Y.; Xu, Y. Prental multiple micronutrient supplementation among Chinese pregnant women. Food Nutr. China 2020, 26, 73–77. [Google Scholar]
- Huang, Z. Study on Dietary Nutritional Status and Milk Mineral Content of 269 Lactating Mothers [D]; Central South University of China: Changsha, China, 2014. [Google Scholar]
- Labrique, A.B.; Christian, P.; Klemm, R.D.; Rashid, M.; Shamim, A.A.; Massie, A.; Schulze, K.; Hackman, A.; West, K.P., Jr. A cluster-randomized, placebo-controlled, maternal vitamin A or beta-carotene supplementation trial in Bangladesh: Design and methods. Trials 2011, 12, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomiya, M.T.; de Arruda, I.K.; da Silva Diniz, A.; Santana, R.A.; da Silveira, K.C.; Andreto, L.M. The effect of vitamin A supplementation with 400,000 IU vs. 200,000 IU on retinol concentrations in the breast milk: A randomized clinical trial. Clin. Nutr. 2017, 36, 100–106. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A. Assessing vitamin A status: Past, present and future. J. Nutr. 2004, 134, 290S–293S. [Google Scholar] [CrossRef] [Green Version]
- Machado, M.R.; Kamp, F.; Nunes, J.C.; El-Bacha, T.; Torres, A.G. Breast milk content of vitamin A and E from early- to mid-lactation is affected by inadequate dietary intake in Brazilian adult women. Nutrients 2019, 11, 2025. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.M.; Saunders, C.; Ramalho, A.; Accioly, E. Serum vitamin A in mothers and newborns in the city of Rio de Janeiro. Int. J. Food Sci. Nutr. 2009, 60, 282–292. [Google Scholar] [CrossRef]
- WHO. Guideline: Vitamin A Supplementation in Postpartum Women; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Stoltzfus, R.J.; Underwood, B.A. Breast-milk vitamin A as an indicator of the vitamin A status of women and infants. Bull. World Health Organ. 1995, 73, 703–711. [Google Scholar]
- Roy, S.K.; Islam, A.; Molla, A.; Akramuzzaman, S.M.; Jahan, F.; Fuchs, G. Impact of a single megadose of vitamin A at delivery on breastmilk of mothers and morbidity of their infants. Eur. J. Clin. Nutr. 1997, 51, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, M.; Wu, J.; Sun, L.; Jiang, T.; Song, C. Study on establishment and evaluation of a novel method for dietary assessment with instant photography. Acta Nutr. Sin. 2014, 36, 288–295. [Google Scholar]
- Wang, Z. Food Atlas of Dietary Assessment by Instant Photography; School of Public Health Nanjing Medical University: Nanjing, China, 2011. [Google Scholar]
- Yang, Y.; Wang, G.; Pan, X. China Food Composition Tables, 6th ed.; Peking University Medical Press: Beijing, China, 2019. [Google Scholar]
- Chinese Nutrition Society. Chinese Dietary Reference Intakes (2013); Science Press: Beijing, China, 2014. [Google Scholar]
- Li, J.; Zheng, C.; Ni, J.; Li, W.; Lu, D.; Li, H.; Ma, W. Trend of vitamin A and vitamin E among pregnancy of Beijing in 2013–2016. J. Hyg. Res. 2019, 48, 56–60. [Google Scholar]
- de-Oliveira-Fonseca-Sally, E.; Antonio-dos-Anjos, L.; Gonçalves-Ramos, E.; de-Matos-Fonseca, V.; de-Andrade-Messias-da-Silva, B.; Wahrlich, V. Dietary intake of pregnant adolescents cared for in primary health care units of a Brazilian urban municipality. Nutr. Hosp. 2018, 35, 596–605. [Google Scholar]
- Nikniaz, L.; Mahdavi, R.; Ostadrahimi, A.; Hejazi, M.A.; Vatankhah, A.M. Effects of synbiotic supplementation on total antioxidant capacity of human breastmilk. Breastfeed. Med. 2013, 8, 217–222. [Google Scholar] [CrossRef]
- Martins, T.M.; Ferraz, I.S.; Daneluzzi, J.C.; Martinelli, C.E., Jr.; Del Ciampo, L.A.; Ricco, R.G.; Jordao, A.A., Jr.; Patta, M.C.; Vannucchi, H. Impact of maternal vitamin A supplementation on the mother-infant pair in Brazil. Eur. J. Clin. Nutr. 2010, 64, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.C.; West, C.E.; de Pee, S.; Bosch, D.; Phuong, H.D.; Hulshof, P.J.; Khoi, H.H.; Verhoef, H.; Hautvast, J.G. The contribution of plant foods to the vitamin A supply of lactating women in Vietnam: A randomized controlled trial. Am. J. Clin. Nutr. 2007, 85, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.; Burri, B.J.; Jamil, K.M.; Jamil, M. The effects of daily consumption of beta-cryptoxanthin-rich tangerines and beta-carotene-rich sweet potatoes on vitamin A and carotenoid concentrations in plasma and breast milk of Bangladeshi women with low vitamin A status in a randomized controlled trial. Am. J. Clin. Nutr. 2013, 98, 1200–1208. [Google Scholar] [PubMed] [Green Version]
- Rice, A.L.; Stoltzfus, R.J.; de Francisco, A.; Chakraborty, J.; Kjolhede, C.L.; Wahed, M.A. Maternal vitamin A or beta-carotene supplementation in lactating bangladeshi women benefits mothers and infants but does not prevent subclinical deficiency. J. Nutr. 1999, 129, 356–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dror, D.K.; Allen, L.H. Retinol-to-fat ratio and retinol concentration in human milk show similar time trends and associations with maternal factors at the population level: A systematic review and meta-analysis. Adv. Nutr. 2018, 9 (Suppl. 1), 332S–346S. [Google Scholar] [CrossRef]
- Muslimatun, S.; Schmidt, M.K.; West, C.E.; Schultink, W.; Hautvast, J.G.; Karyadi, D. Weekly vitamin A and iron supplementation during pregnancy increases vitamin A concentration of breast milk but not iron status in Indonesian lactating women. J. Nutr. 2001, 131, 2664–2669. [Google Scholar] [CrossRef] [Green Version]
- Klevor, M.K.; Haskell, M.J.; Lartey, A.; Adu-Afarwuah, S.; Zeilani, M.; Dewey, K.G. Lipid-based nutrient supplements providing approximately the recommended daily intake of vitamin A do not increase breast milk retinol concentrations among Ghanaian women. J. Nutr. 2016, 146, 335–342. [Google Scholar] [CrossRef]
- Gurgel, C.S.; de Araújo Pereira, L.A.; de Assis Costa, A.; da Silva Souza, M.A.; de Brito, P.A.; de Melo, L.R.; Dimenstein, R. Effect of routine prenatal supplementation on vitamin concentrations in maternal serum and breast milk. Nutrition 2017, 33, 261–265. [Google Scholar] [CrossRef]
- dos Santos Fernandes, T.F.; Figueiroa, J.N.; de Arruda, I.K.; da Silva Diniz, A. Effect on infant illness of maternal supplementation with 400 000 IU vs. 200 000 IU of vitamin A. Pediatrics 2012, 129, e960–e966. [Google Scholar] [CrossRef] [Green Version]
- Goenka, A.; Kollmann, T.R. Development of immunity in early life. J. Infect. 2015, 71 (Suppl. 1), S112–S120. [Google Scholar] [CrossRef]
- Boix-Amorós, A.; Collado, M.C.; Van’t Land, B.; Calvert, A.; Le Doare, K.; Garssen, J.; Hanna, H.; Khaleva, E.; Peroni, D.G.; Geddes, D.T.; et al. Reviewing the evidence on breast milk composition and immunological outcomes. Nutr. Rev. 2019, 77, 541–556. [Google Scholar] [CrossRef]
- Turfkruyer, M.; Verhasselt, V. Breast milk and its impact on maturation of the neonatal immune system. Curr. Opin. Infect. Dis. 2015, 28, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Keikha, M.; Bahreynian, M.; Saleki, M.; Kelishadi, R. Macro- and micronutrients of human milk composition: Are they related to maternal diet? A comprehensive systematic review. Breastfeed. Med. 2017, 12, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed Mohamed, A.; Salah Ahmed, E.M.; Farag, Y.M.; Bedair, N.I.; Nassar, N.A.; Ghanem, A.I. Dose-response association between vitamin D deficiency and atopic dermatitis in children, and effect modification by gender: A case-control study. J. Dermatol. Treat. 2021, 32, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Bishara, R.; Dunn, M.S.; Merko, S.E.; Darling, P. Nutrient composition of hindmilk produced by mothers of very low birth weight infants born at less than 28 weeks’ gestation. J. Hum. Lact. 2008, 24, 159–167. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Supplementation (n = 117) | Control (n = 128) | p-Value |
|---|---|---|---|
| Age, years | 30.1 ± 4.1 | 29.5 ± 3.4 | 0.16 |
| BMI at enrolment, kg/m2 | 23.48 ± 2.90 | 23.28 ± 2.49 | 0.34 |
| BMI at the end of trial, kg/m2 | 24.03 ± 2.7 | 23.04 ± 2.4 | 0.03 |
| Education, years | 15.44 ± 2.99 | 15.22 ± 3.63 | 0.58 |
| Gestational age, weeks | 39.30 ± 1.27 | 39.49 ± 1.25 | 0.24 |
| Primiparous, n (%) | 73 (62.4) | 88 (68.8) | 0.30 |
| Spontaneous labor, n (%) | 62 (53.0) | 84 (65.6) | 0.05 |
| Energy and Nutrients | Supplementation (n = 117) | Control (n = 128) | p-Value |
|---|---|---|---|
| At Enrolment | |||
| Energy (kcal/day) | 2189.9 (1878.1, 2620.8) | 2210.2 (1943.9, 2586.8) | 0.74 |
| Carbohydrate (g/day) | 264.8 (224.8, 325.9) | 258.8 (224.8, 314.5) | 0.94 |
| Fat (g/day) | 85.0 (64.5, 97.4) | 83.0 (67.2, 101.4) | 0.27 |
| Fat to carbohydrate ratio | 0.30 (0.24, 0.39) | 0.34 (0.23, 0.40) | 0.34 |
| Protein (g/day) | 102.8 (87.4, 128.9) | 102.3 (81.3, 114.6) | 0.94 |
| Vitamin A (µg RAE/day) | 711.5 (521.6, 1143.3) | 732 (502.5, 1078.7) | 0.56 |
| At the end of trial | |||
| Energy (kcal/day) | 2019.5 (1754.3, 2312.4) | 1978.8 (1701.6, 2229.7) | 0.50 |
| Carbohydrate (g/day) | 245.9 (191.1, 295.9) | 248.1 (200.0, 285.3) | 0.65 |
| Fat (g/day) | 69.8 (53.4, 88.1) | 73.4 (54.2, 83.4) | 0.27 |
| Fat to carbohydrate ratio | 0.29 (0.24, 0.39) | 0.28 (0.22, 0.35) | 0.95 |
| Protein (g/day) | 83.67 (73.96, 108.2) | 81.94 (68.1, 101.5) | 0.94 |
| Vitamin A (µg RAE/day) | 706.6 (567.4, 1097.6) | 783.9 (477.6, 1066.9) | 0.77 |
| Dependent Variables and Models | R2 | β | β (95% CI) | p-Value |
|---|---|---|---|---|
| Serum retinol concentration, µmol/L | ||||
| Model 1 | 0.038 | 0.170 | 0.060–0.274 | <0.01 |
| Model 2 | 0.507 | 0.168 | 0.087–0.249 | <0.01 |
| Model 3 | 0.508 | 0.171 | 0.089–0.252 | <0.01 |
| Breast milk retinol concentration, µmol/L | ||||
| Model 1 | 0.053 | 0.146 | 0.077–0.254 | <0.01 |
| Model 2 | 0.319 | 0.144 | 0.064–0.223 | <0.01 |
| Model 3 | 0.323 | 0.147 | 0.068–0.277 | <0.01 |
| Disease Patterns | Supplementation (n = 117) | Control (n = 128) | RR (95% CI) | p-Value 1 | Adjusted RR (95%CI) | p-Value 2 |
|---|---|---|---|---|---|---|
| Febrile illness | 10 (8.5) | 7 (5.5) | 1.62 (0.59–4.39) | 0.35 | 1.63 (0.59–4.52) | 0.35 |
| Respiratory tract infection | 9 (7.7) | 18 (14.1) | 0.61 (0.31–1.23) | 0.16 | 0.60 (0.29–1.22) | 0.16 |
| Diarrhea | 8 (6.8) | 14 (10.9) | 0.60 (0.24–1.48) | 0.27 | 0.59 (0.29–1.22) | 0.26 |
| Eczema | 14 (12.0) | 12 (9.3) | 0.90 (0.41–1.99) | 0.80 | 0.87 (0.39–1.95) | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Hu, P.; Yang, Y.; Xu, F.; Li, F.; Lu, X.; Xie, Z.; Wang, Z. Impact of Maternal Daily Oral Low-Dose Vitamin A Supplementation on the Mother-Infant Pair: A Randomised Placebo-Controlled Trial in China. Nutrients 2021, 13, 2370. https://doi.org/10.3390/nu13072370
Ding Y, Hu P, Yang Y, Xu F, Li F, Lu X, Xie Z, Wang Z. Impact of Maternal Daily Oral Low-Dose Vitamin A Supplementation on the Mother-Infant Pair: A Randomised Placebo-Controlled Trial in China. Nutrients. 2021; 13(7):2370. https://doi.org/10.3390/nu13072370
Chicago/Turabian StyleDing, Ye, Ping Hu, Yue Yang, Fangping Xu, Fang Li, Xiaolong Lu, Zhencheng Xie, and Zhixu Wang. 2021. "Impact of Maternal Daily Oral Low-Dose Vitamin A Supplementation on the Mother-Infant Pair: A Randomised Placebo-Controlled Trial in China" Nutrients 13, no. 7: 2370. https://doi.org/10.3390/nu13072370






