Gastrointestinal Tolerance and Protein Absorption Markers with a New Peptide Enteral Formula Compared to a Standard Intact Protein Enteral Formula in Critically Ill Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Intervention
2.3. Data Collection
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Study Product Intake and Gastrointestinal Tolerance
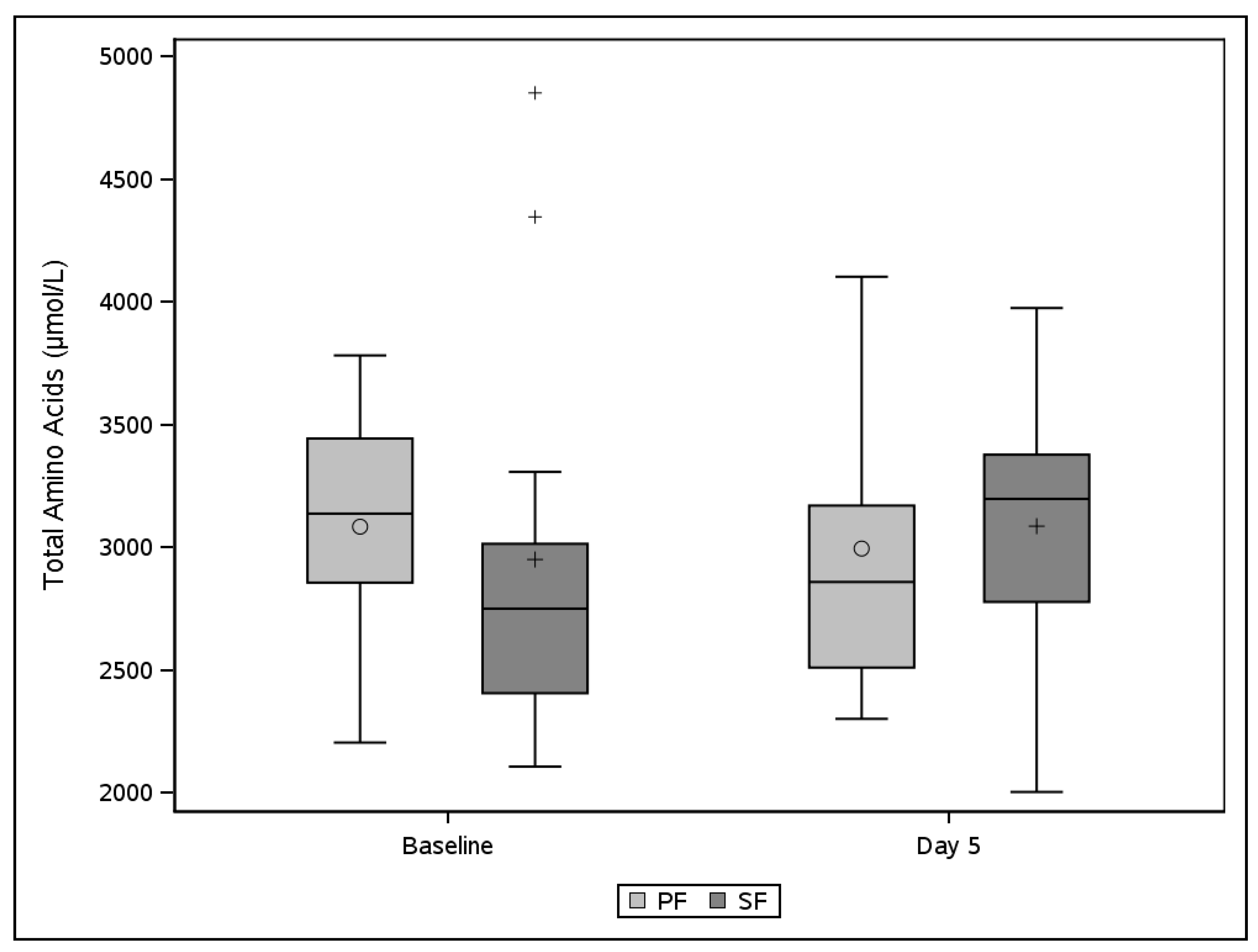
3.3. Plasma Amino Acids and Urine Parameters of Protein Metabolism
3.4. Clinical Outcome Parameters
3.5. Safety Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dhaliwal, R.; Cahill, N.; Lemieux, M.; Heyland, D.K. The Canadian critical care nutrition guidelines in 2013: An update on current recommendations and implementation strategies. Nutr. Clin. Pract. 2014, 29, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Kreymann, K.G.; Berger, M.M.; Deutz, N.E.; Hiesmayr, M.; Jolliet, P.; Kazandjiev, G.; Nitenberg, G.; Van den Berghe, G.; Wernerman, J.D.G.E.M.; Ebner, C.; et al. ESPEN Guidelines on Enteral Nutrition: Intensive care. Clin. Nutr. 2006, 25, 210–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lochs, H.; Dejong, C.; Hammarqvist, F.; Hébuterne, X.; Leon-Sanz, M.; Schütz, T.; van Gemert, W.; Van Gossum, A.; Valentini, L.; Lübke, H.; et al. ESPEN Guidelines on Enteral Nutrition: Gastroenterology. Clin. Nutr. 2006, 25, 260–274. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). J. Parenter. Enteral Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Sioson, M.S.; Martindale, R.; Abayadeera, A.; Abouchaleh, N.; Aditianingsih, D.; Bhurayanontachai, R.; Chiou, W.C.; Higashibeppu, N.; Nor, M.B.M.; Osland, E.; et al. Nutrition therapy for critically ill patients across the Asia-Pacific and Middle East regions: A consensus statement. Clin. Nutr. 2018, 24, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Weimann, A.; Braga, M.; Harsanyi, L.; Laviano, A.; Ljungqvist, O.; Soeters, P.; Jauch, K.W.; Kemen, M.; Hiesmayr, J.M.; Horbach, T.; et al. ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin. Nutr. 2006, 25, 224–244. [Google Scholar] [CrossRef]
- Chapman, M.J.; Deane, A.M. Gastrointestinal dysfunction relating to the provision of nutrition in the critically ill. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 207–212. [Google Scholar] [CrossRef]
- Clark, R.; Johnson, R. Malabsorption Syndromes. Nurs. Clin. N. Am. 2018, 53, 361–374. [Google Scholar] [CrossRef]
- Wierdsma, N.J.; Peters, J.H.; Weijs, P.J.; Keur, M.B.; Girbes, A.R.; van Bodegraven, A.A.; Beishuizen, A. Malabsorption and nutritional balance in the ICU: Fecal weight as a biomarker: A prospective observational pilot study. Crit. Care 2011, 15, R264. [Google Scholar] [CrossRef] [Green Version]
- Silk, D.B. Proteins, peptides and amino acids: Which and when? Nestle Nutr. Workshop Ser. Clin. Perform Programme 2000, 3, 257–271, discussion 271–274. [Google Scholar]
- Heyland, D.K.; Murch, L.; Cahill, N.; McCall, M.; Muscedere, J.; Stelfox, H.T.; Day, A.G. Enhanced protein-energy provision via the enteral route feeding protocol in critically ill patients: Results of a cluster randomized trial. Crit. Care Med. 2013, 41, 2743–2753. [Google Scholar] [CrossRef]
- Alexander, D.D.; Bylsma, L.C.; Elkayam, L.; Nguyen, D.L. Nutritional and health benefits of semi-elemental diets: A comprehensive summary of the literature. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 306–319. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, F.; Nitenberg, G.; Coudray-Lucas, C.; Lasser, P.; Giboudeau, J.; Cynober, L. Pharmacokinetic assessment of an oligopeptide-based enteral formula in abdominal surgery patients. Am. J. Clin. Nutr. 1998, 67, 124–128. [Google Scholar] [CrossRef]
- Wang, S.; Ma, L.; Zhuang, Y.; Jiang, B.; Zhang, X. Screening and risk factors of exocrine pancreatic insufficiency in critically ill adult patients receiving enteral nutrition. Crit. Care 2013, 17, R171. [Google Scholar] [CrossRef] [Green Version]
- Mowatt-Larssen, C.A.; Brown, R.O.; Wojtysiak, S.L.; Kudsk, K.A. Comparison of tolerance and nutritional outcome between a peptide and a standard enteral formula in critically ill, hypoalbuminemic patients. J. Parenter. Enteral Nutr. 1992, 16, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Petros, S.; Engelmann, L. Enteral nutrition delivery and energy expenditure in medical intensive care patients. Clin. Nutr. 2006, 25, 51–59. [Google Scholar] [CrossRef]
- Jakobsen, L.H.; Wirth, R.; Smoliner, C.; Klebach, M.; Hofman, Z.; Kondrup, J. Gastrointestinal tolerance and plasma status of carotenoids, EPA and DHA with a fiber-enriched tube feed in hospitalized patients initiated on tube nutrition: Randomized controlled trial. Clin. Nutr. 2017, 36, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Claus, D.; Geypens, B.; Hiele, M.; Geboes, K.; Rutgeerts, P.; Ghoos, Y. Amount and fate of egg protein escaping assimilation in the small intestine of humans. Am. J. Physiol. 1999, 277, G935–G943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geypens, B.A.; Claus, D.; Evenepoel, P.; Hiele, M.; Maes, B.; Peeters, M.; Ghoos, Y. Influence of dietary protein supplements on the formation of bacterial metabolites in the colon. Gut 1997, 41, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Elia, M.; Carter, A.; Bacon, S.; Winearls, C.G.; Smith, R. Clinical usefulness of urinary 3-methylhistidine excretion in indicating muscle protein breakdown. Br. Med. J. 1981, 282, 351–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaser, A.R.; Malbrain, M.L.; Starkopf, J.; Fruhwald, S.; Jakob, S.M.; De Waele, J.; Spies, C. Gastrointestinal function in intensive care patients: Terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012, 38, 384–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compher, C.; Chittams, J.; Sammarco, T.; Nicolo, M.; Heyland, D.K. Greater Protein and Energy Intake May Be Associated With Improved Mortality in Higher Risk Critically Ill Patients: A Multicenter, Multinational Observational Study. Crit. Care Med. 2017, 45, 156–163. [Google Scholar] [CrossRef]
- Elke, G.; Wang, M.; Weiler, N.; Day, A.G.; Heyland, D.K. Close to recommended caloric and protein intake by enteral nutrition is associated with better clinical outcome of critically ill septic patients: Secondary analysis of a large international nutrition database. Crit. Care 2014, 18, R29. [Google Scholar] [CrossRef] [Green Version]
- Lee, Z.Y.; Ibrahim, N.A.; Mohd-Yusof, B.N. Prevalence and duration of reasons for enteral nutrition feeding interruption in a tertiary intensive care unit. Nutrition 2018, 53, 26–33. [Google Scholar] [CrossRef]
- Jeppesen, P.B. Spectrum of short bowel syndrome in adults: Intestinal insufficiency to intestinal failure. J. Parenter. Enteral Nutr. 2014, 38 (Suppl. 1), 8s–13s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikfarjam, M.; Wilson, J.S.; Smith, R.C. Diagnosis and management of pancreatic exocrine insufficiency. Med. J. Aust. 2017, 207, 161–165. [Google Scholar] [CrossRef]
- Brinson, R.R.; Kolts, B.E. Diarrhea associated with severe hypoalbuminemia: A comparison of a peptide-based chemically defined diet and standard enteral alimentation. Crit. Care Med. 1988, 16, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Meredith, J.W.; Ditesheim, J.A.; Zaloga, G.P. Visceral protein levels in trauma patients are greater with peptide diet than with intact protein diet. J. Trauma 1990, 30, 825–828, discussion 828–829. [Google Scholar] [CrossRef] [PubMed]
- Jakob, S.M.; Bütikofer, L.; Berger, D.; Coslovsky, M.; Takala, J. A randomized controlled pilot study to evaluate the effect of an enteral formulation designed to improve gastrointestinal tolerance in the critically ill patient-the SPIRIT trial. Crit. Care 2017, 21, 140. [Google Scholar] [CrossRef] [Green Version]
- Fried, M.D.; Khoshoo, V.; Seeke, D.J.; Gilday, D.L.; Ash, J.M.; Peneharz, P.B. Decrease in gastric emptying time and episodes of regurgitation in children with spastic quadriplegia fed a whey-based formula. J. Pediatr. 1992, 120 Pt 1, 569–572. [Google Scholar] [CrossRef]
- Viall, C.; Porcelli, K.; Teran, J.C.; Varma, R.N.; Steffee, W. A double-blind clinical trial comparing the gastrointestinal side effects of two enteral feeding formulas. J. Parenter. Enter. Nutr. 1990, 14, 265–269. [Google Scholar] [CrossRef] [PubMed]
- van den Braak, C.C.; Klebach, M.; Abrahamse, E.; Minor, M.; Hofman, Z.; Knol, J.; Ludwig, T. A novel protein mixture containing vegetable proteins renders enteral nutrition products non-coagulating after in vitro gastric digestion. Clin. Nutr. 2013, 32, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Beaufrère, B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E340–E348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebau, F.; Király, E.; Olsson, D.; Wernerman, J.; Rooyackers, O. Uptake of dietary amino acids into arterial blood during continuous enteral feeding in critically ill patients and healthy subjects. Clin. Nutr. 2021, 40, 912–918. [Google Scholar] [CrossRef]
- Steinhardt, H.J.; Wolf, A.; Jakober, B.; Schmuelling, R.M.; Langer, K.; Brandl, M.; Adibi, S.A. Nitrogen absorption in pancreatectomized patients: Protein versus protein hydrolysate as substrate. J. Lab. Clin. Med. 1989, 113, 162–167. [Google Scholar]
- Mundi, M.S. Reduction in health care utilization with transition to peptide based diet in intolerant home enteral nutrition patients. Nutr. Clin. Pract. 2020, 35, 487–494. [Google Scholar] [CrossRef]
| SF (N = 13) | PF (N = 13) | p-Value 1 | ||
|---|---|---|---|---|
| Sex (male) | n (%) | 6 (46.2%) | 8 (61.5%) | 0.695 |
| Age (years) | Mean (SD) | 54.5 (16.8) | 56.5 (18.8) | 0.786 |
| BMI (kg/m2) | Mean (SD) | 27.40 (4.98) | 29.25 (5.63) | 0.383 |
| Admission diagnosis | 1.000 | |||
| Medical | n (%) | 8 (61.5%) | 9 (69.2%) | |
| Surgical non-trauma | n (%) | 4 (30.8%) | 4 (30.8%) | |
| Trauma non-surgical | n (%) | 1 (7.7%) | 0 (0.0%) | |
| SOFA score | Median [IQR] | 10 (8−11) | 7 (6−11) | 0.286 |
| APACHE II score | Median [IQR] | 21 (16−24) | 26 (18−28) | 0.353 |
| Predicted mortality (%) | Mean (SD) | 37.55 (19.97) | 46.58 (22.86) | 0.294 |
| Adjusted predicted mortality (%) | Mean (SD) | 23.4 (17.5) | 35.8 (26.0) | 0.164 |
| Total (N = 26) | SF (N = 13) | PF (N = 13) | |
|---|---|---|---|
| Total no. days | 123 | 62 | 61 |
| Total % of days target not reached | 86.2% | 82.3% | 90.2% |
| Reported reasons 1 | |||
| Start-up period, n (%) | 23.6% | 22.6% | 24.6% |
| Symptoms of intolerance, n (%) | 7.3% | 4.8% | 9.8% |
| Medical investigation, n (%) | 12.2% | 9.7% | 14.8% |
| Energy intake other routes, n (%) | 10.6% | 4.8% | 16.4% |
| Other 2, n (%) | 42.3% | 45.2% | 39.3% |
| SF (N = 13) | PF (N = 13) | p Value 4 | ||
|---|---|---|---|---|
| Diarrhea 2 | n (%) | 3 (23.1%) | 5 (38.5%) | 0.388 |
| Constipation 3 | n (%) | 7 (53.8%) | 3 (23.1%) | 0.115 |
| Vomiting | n (%) | 1 (7.7%) | 2 (15.4%) | 0.549 |
| GRV > 250 mL | n (%) | 5 (38.5%) | 4 (30.8%) | 0.691 |
| GRV > 500 mL | n (%) | 1 (7.7%) | 2 (15.4%) | 0.535 |
| SF (N = 13) | PF (N = 13) | p Value 1 | ||
|---|---|---|---|---|
| n = 8 | n = 9 | |||
| p-Cresol (mg/24 h) | Median (Q1−Q3) | 21 (10−54) | 48 (6−151) | 0.520 |
| Phenol (mg/24 h) | Median (Q1−Q3) | 1 (0−22) | 0 (0−2) | 0.317 |
| Total nitrogen (g/24 h) | Median (Q1−Q3) | 18 (9−20) | 15 (5−23) | 0.981 |
| Nitrogen balance (g) | Median (Q1−Q3) | −46 (−56−13) | −38 (−57−49) | 0.548 |
| Creatinine (mmol/24 h) | Median (Q1−Q3) | 5 (3−9) | 4 (2−8) | 0.924 |
| 3-Methylhistidine (µmol/24 h) | Median (Q1−Q3) | 268 (106−345) | 262 (32−390) | 0.775 |
| SF (N = 13) | PF (N = 13) | p Value | ||
|---|---|---|---|---|
| Mortality rates (28 days) | n (%) | 4 (30.8%) | 4 (30.8%) | 0.930 1 |
| Duration of ICU stay (days) | Mean (SD) | 12.7 (6.0) | 14.5 (8.2) | 0.539 2 |
| Duration of hospital stay (days) | Median (Q1−Q3) | 16 (10−29) | 29 (17−29) | 0.361 3 |
| Duration of first ventilation period (days) | Median (Q1−Q3) | 10 (8−14) | 11 (6−14) | 0.618 3 |
| SOFA score (at Day 5) | Median (Q1−Q3) | 7 (5−10) | 6 (5−10) | 0.713 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Brito-Ashurst, I.; Klebach, M.; Tsompanaki, E.; Kaul, S.; van Horssen, P.; Hofman, Z. Gastrointestinal Tolerance and Protein Absorption Markers with a New Peptide Enteral Formula Compared to a Standard Intact Protein Enteral Formula in Critically Ill Patients. Nutrients 2021, 13, 2362. https://doi.org/10.3390/nu13072362
de Brito-Ashurst I, Klebach M, Tsompanaki E, Kaul S, van Horssen P, Hofman Z. Gastrointestinal Tolerance and Protein Absorption Markers with a New Peptide Enteral Formula Compared to a Standard Intact Protein Enteral Formula in Critically Ill Patients. Nutrients. 2021; 13(7):2362. https://doi.org/10.3390/nu13072362
Chicago/Turabian Stylede Brito-Ashurst, Ione, Marianne Klebach, Eleni Tsompanaki, Sundeep Kaul, Peter van Horssen, and Zandrie Hofman. 2021. "Gastrointestinal Tolerance and Protein Absorption Markers with a New Peptide Enteral Formula Compared to a Standard Intact Protein Enteral Formula in Critically Ill Patients" Nutrients 13, no. 7: 2362. https://doi.org/10.3390/nu13072362






