Lifestyle Factors and Genetic Variants Associated to Health Disparities in the Hispanic Population
Abstract
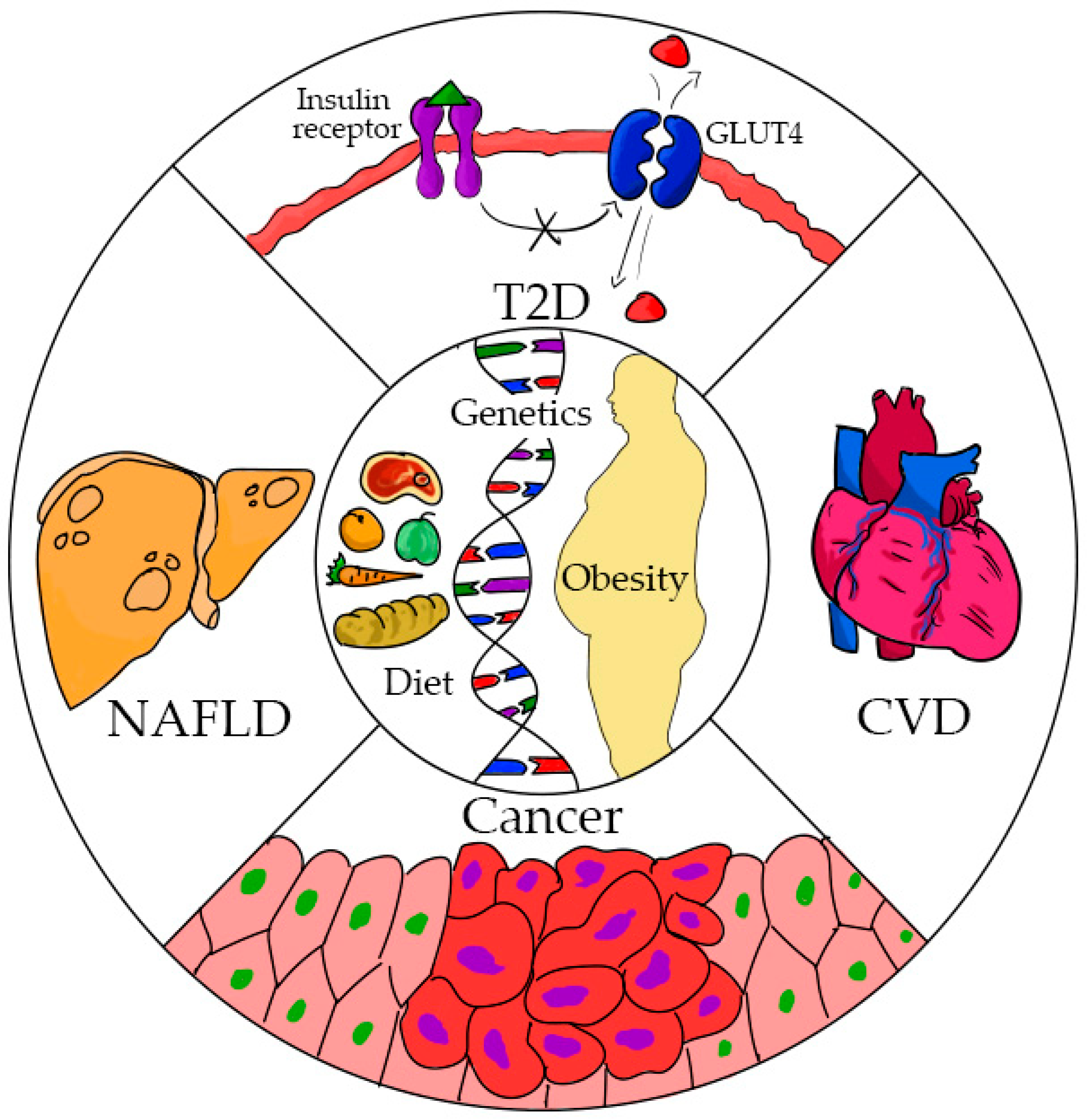
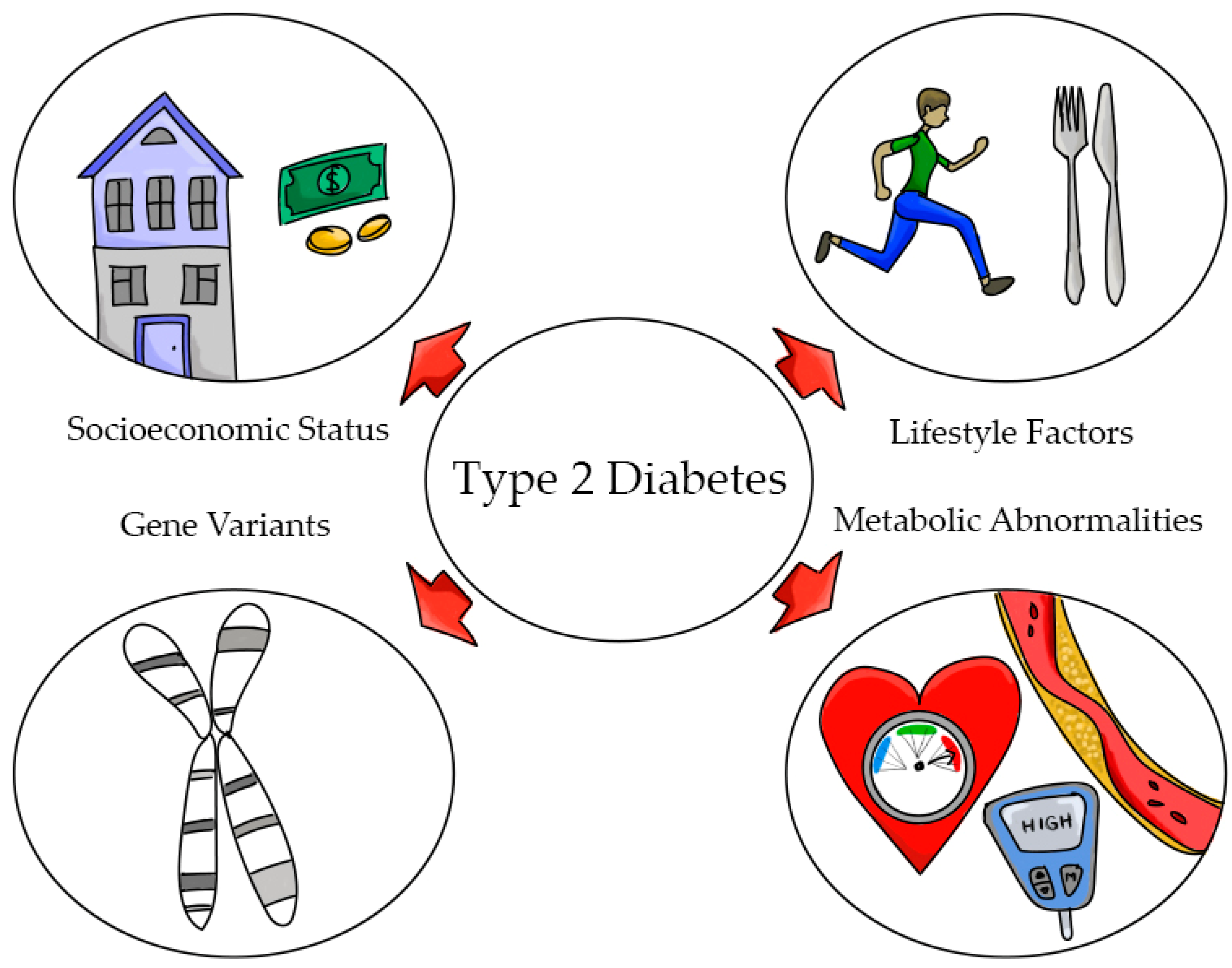
:1. Introduction
2. Obesity
3. Type 2 Diabetes (T2D)
4. Heart Disease
5. Non-Alcoholic Fatty Liver Disease (NAFLD)
6. Cancer
7. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Gakidou, E.E.; Frenk, J. Health inequalities and social group differences: What should we measure? Bull. World Health Organ. 1999, 77, 537–543. [Google Scholar]
- Agurs-Collins, T.; Persky, S.; Paskett, E.D.; Barkin, S.L.; Meissner, H.I.; Nansel, T.R.; Arteaga, S.S.; Zhang, X.; Das, R.; Farhat, T. Designing and Assessing Multilevel Interventions to Improve Minority Health and Reduce Health Disparities. Am. J. Public Health 2019, 109, S86–S93. [Google Scholar] [CrossRef]
- Davidson, J.A.; Kannel, W.; Lopez-Candales, A.; Morales, L.; Moreno, P.R.; Ovalle, F.; Rodriguez, C.J.; Rodbard, H.W.; Rosenson, R.S.; Stern, M. Avoiding the looming Latino/Hispanic cardiovascular health crisis: A call to action. J. Cardiometab. Syndr. 2007, 2, 238–243. [Google Scholar] [CrossRef]
- Szanto, K.B.; Li, J.; Cordero, P.; Oben, J.A. Ethnic differences and heterogeneity in genetic and metabolic makeup contributing to nonalcoholic fatty liver disease. Diabetes Metab. Syndr. Obesity Targets Ther. 2019, 12, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.J.; Daviglys, M.L.; Swett, K.; González, H.M.; Gallo, L.C.; Wassertheil-Smoller, S.; Giachel-lo, A.L.; Teng, Y.; Schneiderman, N.; Talavera, G.A.; et al. Dyslipidemia Patterns among Hispan-ics/Latinos in the United States of Diverse Background. Am. J. Med. 2014, 127, 1186–1194.e1. [Google Scholar] [CrossRef] [Green Version]
- Graves, K.D.; Huerta, E.; Cullen, J.; Kaufman, E.; Sheppard, V.; Luta, G.; Isaacs, C.; Schwartz, M.D.; Mandelblatt, J. Perceived risk of breast cancer among Latinas attending community clinics: Risk comprehension and relationship with mammography adherence. Cancer Causes Control. 2008, 19, 1373–1382. [Google Scholar] [CrossRef] [Green Version]
- Gartenberg Livney, M.; Clark, C.M.; Karlawish, J.H.; Cartmell, S.; Negrón, M.; NuñezLopez, J.; Xie, S.X.; Entenza-Cabrera, F.; Vega, I.E.; Arnold, S.E. Ethnoracial Differences in the Clinical Characteristics of Alzheimer Disease at Initial Presentation at an Urban Alzheimer’s Disease Center. Am. J. Geriatr. Psychiatry 2011, 19, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Green, L.D.; Derr, J.N.; Knight, A. mtDNA Affinities of the Peoples of North-Central Mexico. Am. J. Hum. Genet. 2000, 66, 989–998. [Google Scholar] [CrossRef] [Green Version]
- Marrero, A.R.; Bravi, C.; Stuart, S.; Long, J.C.; Pereira das Neves Leite, F.; Kommers, T.; Carvalho, C.M.; Pena, S.D.; Ruiz-Linares, A.; Salzano, F.M.; et al. Preand post-Columbian gene and cultural continuity: The case of the Gaucho from southern Brazil. Hum. Hered. 2007, 64, 160–171. [Google Scholar] [CrossRef]
- Bryc, K.; Durand, E.Y.; Macpherson, J.M.; Reich, D.; Mountain, J.L. The Genetic Ancestry of African Americans, Latinos, and European Americans across the United States. Am. J. Hum. Genet. 2015, 96, 37–53. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Ray, N.; Rojas, W.; Parra, M.V.; Bedoya, G.; Gallo, C.; Poletti, G.; Mazzotti, G.; Hill, K.; Hurtado, A.M.; et al. Geographic Patterns of Genome Admixture in Latin American Mestizos. PLoS Genet. 2008, 4, e1000037. [Google Scholar] [CrossRef] [Green Version]
- Flegal, K.M.; Carroll, D.; Kit, B.K.; Ogden, C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. J. Am Med Assoc. 2012, 307, 491–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isasi, C.R.; Ayala, G.X.; Sotres-Alvarez, D.; Madanat, H.; Penedo, F.; Loria, C.M.; Elder, J.P.; Daviglus, M.L.; Barnhart, J.; Siega-Riz, A.M.; et al. Acculturation Related to Obesity in Hispanic/Latino Adults? Results from the Hispanic Community Health Study/Study of Latinos. J. Obes. 2015, 2015, 86276. [Google Scholar]
- Schneiderman, N.; Llabre, M.; Cowie, C.C.; Barnhart, J.; Carnethon, M.; Gallo, L.C.; Giachello, A.L.; Heiss, G.; Kaplan, R.C.; LaVange, L.M.; et al. Prevalence of Diabetes Among Hispanics/Latinos From Diverse Backgrounds: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care 2014, 37, 2233–2239. [Google Scholar] [CrossRef] [Green Version]
- Kalia, H.S.; Gaglio, P.J. The Prevalence and Pathobiology of Nonalcoholic Fatty Liver Disease in Patients of Different Races or Ethnicities. Clin. Liver Dis. 2016, 20, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Siega-Riz, A.M.; Sotres-Alvarez, D.; Ayala, G.X.; Ginsberg, M.; Himes, J.H.; Liu, K.; Loria, C.M.; Mossavar-Rahmani, Y.; Rock, C.L.; Rodriguez, B.; et al. Food-group and nutrient-density intakes by Hispanic and Latino backgrounds in the Hispanic Community Health Study/Study of Latinos. Am. J. Clin. Nutr. 2014, 99, 1487–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siega-Riz, A.M.; Pace, N.D.; Butera, N.M.; Van Horn, L.; Daviglus, M.L.; Harnack, L.; Mossavar-Rahmani, Y.; Rock, C.L.; Pereira, R.I.; Sotres-Alvarez, D. How Well Do U.S. Hispanics Adhere to the Dietary Guidelines for Americans? Results from the Hispanic Community Health Study/Study of Latinos. Health Equity 2019, 3, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.A.; Fulton, J.E.; Schoenborn, C.A.; Loustalot, F. Trend and prevalence estimates based on the 2008 Physical Activity Guidelines for Americans. Am. J. Prev. Med. 2010, 39, 305–313. [Google Scholar] [CrossRef]
- Levin, B.E. Factors promoting and ameliorating the development of obesity. Physiol. Behav. 2005, 86, 633–639. [Google Scholar] [CrossRef]
- Rodriguez, C.J.; Cai, J.; Swett, K.; González, H.M.; Talavera, G.A.; Wruck, L.M.; Wassertheil-Smoller, S.; Lloyd-Jones, D.; Kaplan, R.C.; Daviglus, M.L. High Cholesterol Awareness, Treatment, and Control Among Hispanic/Latinos: Results From the Hispanic Community Health Study/Study of Latinos. J. Am. Heart Assoc. 2015, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- National Heart Lung and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. The Evidence Report; National Institutes of Health: Rockville, MD, USA, 1998.
- Sturm, R. The Effects Of Obesity, Smoking, And Drinking On Medical Problems And Costs. Health Aff. 2002, 21, 245–253. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebron, K.; Andersen, C.J.; Aguilar, D.; Blesso, C.N.; Barona, J.; Dugan, C.; Jones, J.L.; Al-Sarraj, T.; Fernandez, M.L. A larger body mass index is associated with increased atherogenic dyslipidemia, insulin resistance, and low-grade inflammation in individuals with metabolic syndrome. Metab. Syndr. Relat. Disord. 2015, 13, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Neeland, I.J.; Turer, A.T.; Vega, G.L. Ethnic and gender susceptibility to metabolic risk. Metab. Syndr. Relat. Disord. 2014, 12, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.W.; Wong, G.L.; Choi, P.C.; Chan, A.W.; Li, M.K.; Chan, H.Y.; Chim, A.M.; Yu, J.; Sung, J.J.; Chan, H.L. Disease progression of non-alcoholic fatty liver disease: A prospective study with paired liver biopsies at 3 years. Gut 2010, 59, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S.; Tiribelli, C. The spectrum of liver disease in the general population: Lesson from the Dionysos study. J. Hepatol. 2001, 35, 531–537. [Google Scholar] [CrossRef]
- Machado, M.; Marques-Vidal, P.; Cortez-Pinto, H. Hepatic histology in obese patients undergoing bariatric surgery. J. Hepatol. 2006, 45, 600–606. [Google Scholar] [CrossRef]
- American Society of Clinical Oncology. The state of cancer care in America, 2014: A report by the American Society of Clinical Oncology. J. Oncol. Pract. 2014, 10, 119–142. [Google Scholar] [CrossRef] [Green Version]
- Arnold, M.; Pandeya, N.; Byrnes, G.; Renehan, A.; A Stevens, G.; Ezzati, M.; Ferlay, J.; Miranda, J.J.; Romieu, I.; Dikshit, R.; et al. Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol. 2015, 16, 36–46. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes, A. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.E. Diabetes in the hispanic or latino population: Genes, environment, culture, and more. Curr. Diabetes Rep. 2005, 5, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Kollannoor-Samuel, G.; Segura Perez, S.; Shebi, F.M.; Hawley, N.L.; Damio, G.; Chhabra, J.; Vega-Lopez, S.; Fernandez, M.L.; Perez-Escamilla, R. Nutrition facts panel use is associated with diety quality and Dietary patterns among Latinos with Type 2 diabetes. Public Health Nutr. 2017, 20, 2909–2919. [Google Scholar] [CrossRef] [Green Version]
- Semega, L.J.F.; Fontenot, K.R.; Kollar, M.A. Income and poverty in the United States 2016. 2017. Available online: https://www.census.gov/content/dam/Census/library/publications/2017/demo/P60-259.pdf. (accessed on 20 April 2021).
- Schiller, J.S.; Lucas, J.W.; Ward, B.W.; Peregoy, J.A. Summary health statistics for U.S. Adults: National Health Interview Survey, 2010. Vital Health Stat. 2012. Available online: http://www.cdc.gov/nchs/data/series/sr_10/sr10_252.pdf (accessed on 20 April 2021).
- Lorenzo, C.; Lee, R.; Haffner, S.M. Impaired Glucose Tolerance and Obesity as Effect Modifiers of Ethnic Disparities of the Progression to Diabetes: The San Antonio Heart Study. Diabetes Care 2012, 35, 2548–2552. [Google Scholar] [CrossRef] [Green Version]
- Laaksonen, D.E.; Lakka, H.M.; Salonen, J.T.; Niskanen, L.K.; Rauramaa, R.; Lakka, T.A. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care 2002, 25, 1612–1618. [Google Scholar] [CrossRef] [Green Version]
- Fung, T.T.; McCullough, M.; van Dam, R.M.; Hu, F.B. A prospective study of overall diet quality and risk of type 2 diabetes in women. Diabetes Care 2007, 30, 1753–1757. [Google Scholar] [CrossRef] [Green Version]
- Sofinanou, A.; Fung, T.T.; Tucker, K.L. Differences in diet pattern adherence by nativity and duration of US residence in the Mexican-American population. J. Am. Diet. Assoc. 2011, 111, 1563–1569. [Google Scholar] [CrossRef]
- Grant, S.F.; Thorleifsson, G.; Reynisdottir, I.; Benediktsson, R.; Manolescu, A.; Sainz, J.; Helgason, A.; Stefansson, H.; Emilsson, V.; Helgadottir, A.; et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 2006, 38, 320–323. [Google Scholar] [CrossRef]
- Yi, F.; Brubaker, P.L.; Jin, T. TCF-4 Mediates Cell Type-specific Regulation of Proglucagon Gene Expression by β-Catenin and Glycogen Synthase Kinase-3β. J. Biol. Chem. 2005, 280, 1457–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, N.D.; Lethtinen, A.B.; Langefeld, C.D.; Campbell, J.K.; Haffner, S.M.; Norris, J.M.; Bergman, R.N. Association of TCF7L2 Gene Polymorphisms with Reduced Acute Insulin Response in Hispanic Americans. J. Clin. Endocrin. Metab. 2008, 93, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Harris, M.I.; Flegal, K.M.; Cowie, C.C.; Eberhardt, M.S.; E Goldstein, D.; Little, R.R.; Wiedmeyer, H.-M.; Byrd-Holt, D.D. Prevalence of Diabetes, Impaired Fasting Glucose, and Impaired Glucose Tolerance in U.S. Adults: The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 1998, 21, 518–524. [Google Scholar] [CrossRef]
- Neel, J.V. Diabetes mellitus: A “thrifty” genotype rendered detrimental by “progress”? Am. J. Hum. Genet. 1962, 14, 353–362. [Google Scholar]
- Consortium, S.T.D. Sequence variants in SLC16A11 are a common risk factor for type 2 diabetes in Mexico. Nature 2014, 506, 97–101. [Google Scholar] [CrossRef]
- Rusu, V.; Hoch, E.; Mercader, J.M.; Tenen, D.E.; Gymrek, M.; Hartigan, C.R.; DeRan, M.; Von Grotthuss, M.; Fontanillas, P.; Spooner, A.; et al. Type 2 Diabetes Variants Disrupt Function of SLC16A11 through Two Distinct Mechanisms. Cell 2017, 170, 199–212.e20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Feng, Z.; Zhang, Y.; Sun, Y.; Chen, Y.; Liu, X.; Li, S.; Zhou, T.; Chen, L.; Wei, Y.; et al. Gain-of-Function Mutations of SLC16A11 Contribute to the Pathogenesis of Type 2 Diabetes. Cell Rep. 2019, 26, 884–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, T.M.; Bittner, V. Biomarkers of atherosclerosis: Clinical applications. Curr. Cardiol. Rep. 2008, 10, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, M.N.; Cabrera, R.M.; Saucedo, M.S.; Fernandez, M.L. Dietary cholesterol does not increase biomarkers for chronic disease in a pediatric population at risk from Northern Mexico. Am. J. Clin. Nutr. 2004, 80, 855–861. [Google Scholar] [CrossRef] [Green Version]
- Posadas-Romero, C.; Tapia-Conyer, R.; Lerman-Garber, I. Cholesterol levels and prevalence of hypercholesterolemia in a Mexican adult population. Atherosclerosis 1995, 118, 275–284. [Google Scholar] [CrossRef]
- Crespo, C.; Loria, C.; Burt, V. Hypertension and other cardiovascular disease risk factors among Mexican Americans, Cuban Americans, and Puerto Ricans from the Hispanic Health and Nutrition Examination Survey. Public Health Rep. 1996, 111, 7–10. [Google Scholar]
- Daviglus, M.L.; Talavera, G.A.; Aviles-Santa, M.L.; Allison, M.; Cai, J.; Criqui, M.H.; Gellman, M.; Giachello, A.L.; Gouskova, N.; Kaplan, R.C.; et al. Prevalence of Major Cardiovascular Risk Factors and Cardiovascular Diseases Among Hispanic/Latino Individuals of Diverse Backgrounds in the United States. JAMA 2012, 308, 1775–1784. [Google Scholar] [CrossRef]
- Heiss, G.; Snyder, M.L.; Teng, Y.; Schneiderman, N.; Llabre, M.M.; Cowie, C.; Carnethon, M.; Kaplan, R.; Giachello, A.; Gallo, L.; et al. Prevalence of Metabolic Syndrome Among Hispanics/Latinos of Diverse Background: The Hispanic Community Health Study/Study of Latinos. Diabetes Care 2014, 37, 2391–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raatz, S.K.; Conrad, Z.; Johnson, L.K.; Picklo, M.J.; Jahns, L. Relationship of the Reported Intakes of Fat and Fatty Acids to Body Weight in US Adults. Nutrition 2017, 9, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannu, G.S.; Zaman, M.J.; Gupta, A.; Rehman, H.U.; Myint, P.K. Evidence of Lifestyle Modification in the Management of Hypercholesterolemia. Curr. Cardiol. Rev. 2013, 9, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Toth, P.P.; Fazio, S.; Wong, N.D.; Hull, M.; Nichols, G.A. Risk of cardiovascular events in patients with hypertriglyceridaemia: A review of real-world evidence. Diabetes Obes. Metab. 2020, 22, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Goran, M.I.; Bosy-Westphal, A.; King, J.C.; Schmidt, L.A.; Schwarz, J.-M.; Stice, E.; Sylvetsky, A.C.; Turnbaugh, P.J.; Bray, G.A.; et al. Pathways and mechanisms linking dietary components to cardiometabolic disease: Thinking beyond calories. Obes. Rev. 2018, 19, 1205–1235. [Google Scholar] [CrossRef]
- Bowden, N.W.; An, S.S.; Palmer, N.D.; Brown, W.M.; Norris, J.M.; Haffner, S.M.; Hawkins, G.A.; Guo, X.; Rotter, J.I.; Chen, Y.-D.I.; et al. Molecular basis of a linkage peak: Exome sequencing and family-based analysis identify a rare genetic variant in the ADIPOQ gene in the IRAS Family Study. Hum. Mol. Genet. 2010, 19, 4112–4120. [Google Scholar] [CrossRef] [Green Version]
- Attar, M.J.H.; Mohammadi, S.; Karimi, M.; Hossein-Nezhad, A.; Hosseini, S.H.; Eshraghian, M.R.; Jafari, N.; Rahmani, M.; Karimi, F.; Nezhad, M.K. Association of adiponectin with dietary factors and cardiovascular risk factors in type 2 diabetes mellitus patients. Diabetes Metab. Syndr. Clin. Res. Rev. 2013, 7, 3–7. [Google Scholar] [CrossRef]
- Romero-Hidalgo, S.; Villarreal-Molina, T.; González-Barrios, J.A.; Canizales-Quinteros, S.; Arellano, M.E.R.; Yañez-Velazco, L.B.; Bernal-Alcantara, D.A.; Villa, A.R.; Antuna-Puente, B.; Acuña-Alonzo, V.; et al. Carbohydrate Intake Modulates the Effect of the ABCA1-R230C Variant on HDL Cholesterol Concentrations in Premenopausal Women. J. Nutrients 2011, 142, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Vidal-Quintanar, R.L.; Mendivil, R.L.; Peña, M.; Fernandez, M.L. Lime-treated maize husks lower plasma LDL-cholesterol in normal and hypercholesterolemic adult men from northern Mexico. Brit. J. Nutrients 1999, 81, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Bellentani, S.; Marino, M. Epidemiology and natural history of non-alcoholic fatty liver disease (NAFLD). Ann. Hepatol. 2009, 8, S4–S8. [Google Scholar] [CrossRef]
- Harrison, S.A.; Neuschwander-Tetri, B.A. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin. Liver Dis. 2004, 8, 861–879. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Cortez-Pinto, H. Non-alcoholic fatty liver disease. What the clinician needs to know. World J. Gastroenterol. 2014, 20, 12956–12980. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L. The Metabolic Syndrome. Nutrients Rev. 2007, 64, S30–S34. [Google Scholar] [CrossRef]
- Athyros, V.G.; Alexandrides, T.K.; Bilianou, H.; Cholongitas, E.; Doumas, M.; Ganotakis, E.S.; Mantzoros, C. The use of statins alone, or in combination with pioglitazone and other drugs, for the treatment of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and related cardiovascular risk. An expert panel statement. Metabolism 2017, 71, 17–32. [Google Scholar] [CrossRef]
- Doycheva, I.; Loomba, R. Effect of Metformin on Ballooning Degeneration in Nonalcoholic Steatohepatitis (NASH): When to Use Metformin in Nonalcoholic Fatty Liver Disease (NAFLD). Adv. Ther. 2014, 31, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.R.; Troy, T.N.; Huo, D.; O’Brien, B.L.; Jensen, D.M.; Hart, J. Influence of ethnicity on histological differences in non-alcoholic fatty liver disease. J. Hepatol. 2009, 50, 797–804. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Marzuillo, P.; Del Giudice, E.M.; Santoro, N. Pediatric fatty liver disease: Role of ethnicity and genetics. World J. Gastroenterol. 2014, 20, 7347–7355. [Google Scholar] [CrossRef]
- Romeo, S.; Huang-Doran, I.; Baroni, M.G.; Kotronen, A. Unravelling the pathogenesis of fatty liver disease: Patatin-like phospholipase domain-containing 3 protein. Curr. Opin. Lipidol. 2010, 21, 247–252. [Google Scholar] [CrossRef]
- Speliotes, E.K.; Yerges-Armstrong, L.M.; Wu, J.; Hernaez, R.; Kim, L.J.; Palmer, C.D. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011, 7, e1001324. [Google Scholar] [CrossRef] [PubMed]
- Palmer, N.D.; Musani, S.K.; Yerges-Armstrong, L.; Feitosa, M.F.; Bielak, L.F.; Hernaez, R.; Kahali, B. Characterization of European-ancestry NAFLD-Associated Variants in Individuals of African and Hispanic Descent. Hepatology 2013, 58, 966–975. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef]
- Chilton, F.H.; Murphy, R.C.; Wilson, B.A.; Sergeant, S.; Ainsworth, H.; Seeds, M.C.; Mathias, R.A. Diet-gene interactions and PUFA metabolism. A potential contributor to health disparities and human diseases. Nutrients 2014, 6, 1993–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maki, K.C.; Eron, F.; Cassans, M.E.; Dicklin, M.R.; Davidson, M. W-6 polyunsaturated fatty acids and cardiometabolic health:Current evidence, controversies and research gaps. Adv. Nutr. 2018, 9, 688–700. [Google Scholar]
- Markland, M.; Wu, J.H.Y.; Imamura, F.; Del Gobbo, L.C.; Fretts, A.; de Goede, J.; Shi, P. Biomarkers of dietary faty acids and incident cardiovascular disease and mortality: An individual level pooled analysis of 30 cohort studies. Circulation 2019, 139, 2422–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chilton, F.H.; Dutta, R.; Reynolds, L.M.; Sergeant, S.; Mathias, R.A.; Seeds, M.C. Precision nutrition and Omega-3 Polyunsaturated fatty acids: A case of personalized supplementation approaches for the prevention and management of human diseases. Nutrients 2017, 9, 1165. [Google Scholar] [CrossRef]
- Allott, E.H.; Hursting, S.D. Obesity and cancer: Mechanistic insights from transdisciplinary studies. Endocr. Relat. Cancer 2015, 22, R365–R386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavala, V.A.; Bracci, P.M.; Carethers, J.M.; Carvajal-Carmona, L.; Coggins, N.B.; Cruz-Correa, M.R.; Davis, M.; de Smith, A.J.; Dutil, J.; Figueiredo, J.C.; et al. Cancer health disparities in racial/ethnic minorities in the United States. Br. J. Cancer 2021, 124, 315–332. [Google Scholar] [CrossRef]
- Nahleh, Z.; Otoukesh, S.; Mirshahidi, H.R.; Nguyen, A.L.; Nagaraj, G.; Botrus, G.; Badri, N.; Diab, N.; Alvarado, A.; Sanchez, L.A.; et al. Disparities in breast cancer: A multi-institutional comparative analysis focusing on American Hispanics. Cancer Med. 2018, 7, 2710–2717. [Google Scholar] [CrossRef]
- Wallace, T.A.; Martin, D.N.; Ambs, S. Interactions among genes, tumor biology and the environment in cancer health disparities: Examining the evidence on a national and global scale. Carcinog 2011, 32, 1107–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, K.; Cokkinides, V.; Jemal, A.; Cardinez, C.J.; Murray, T.; Samuels, A.; Ward, E.; Thun, M.J. Cancer statistics for Hispanics, 2003. CA Cancer J. Clin. 2003, 53, 208–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, M.C.; Fejerman, L.; Das, R.; Setiawan, V.W.; Cruz-Correa, M.R.; Perez-Stable, E.J.; Figueiredo, J.C. Variability in Cancer Risk and Outcomes Within US Latinos by National Origin and Genetic Ancestry. Curr. Epidemiol. Rep. 2016, 3, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Ginsburg, O.; Rochon, P.A.; Sun, P.; Narod, S.A. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 2015, 313, 165–173. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Cancer Facts & Figures for Hispanics/Latinos 2012–2014. 2012. Available online: http://www.cancer.org/research/cancerfactsfigures/cancerfactsfiguresforhispanicslatinos/cancer-facts-figureshispanics-2012-2014 (accessed on 21 April 2021).
- Guerrero, S.; López-Cortés, A.; Indacochea, A.; García-Cárdenas, J.M.; Zambrano, A.K.; Cabrera-Andrade, A.; Guevara-Ramírez, P.; González, D.A.; Leone, P.E.; Paz-Y-Miño, C. Analysis of Racial/Ethnic Representation in Select Basic and Applied Cancer Research Studies. Sci. Rep. 2018, 8, 13978. [Google Scholar] [CrossRef] [PubMed]
- Penedo, F.J.; Yanez, B.; Castañeda, S.F.; Gallo, L.C.; Wortman, K.; Gouskova, N.; Simon, M.; Arguelles, W.; Llabre, M.; Sanchez-Johnsen, L.; et al. Self-Reported Cancer Prevalence among Hispanics in the US: Results from the Hispanic Community Health Study/Study of Latinos. PLoS ONE 2016, 11, e0146268. [Google Scholar] [CrossRef]
| Disease | Genes | Allele | Protein | Function |
|---|---|---|---|---|
| T2D | ||||
| TCT7L2 | rs7903146-T, rs112255372 | Transcription factor | Involved in the production of wnt genes. Decreased production of glucagon-like peptide-1 | |
| SLC16A11 | rs77086571 | Monocarboxylate transporter | Transport monocarboxylic acids via a proton coupled mechanism | |
| NAFLD | ||||
| PNPLA3 | rs738409 | Enzyme with lipase activity | Hydrolyzes triglycerides and retinyl esters | |
| CVD | ||||
| ADIPOQ | rs200573126 | Adiponectin | Low adiponectin levels leads to inflammation and insulin resistance. | |
| R230C | Rs9282541 | ABCIA1 | Involved in reverse cholesterol transport and in HDL cholesterol levels | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez, M.L. Lifestyle Factors and Genetic Variants Associated to Health Disparities in the Hispanic Population. Nutrients 2021, 13, 2189. https://doi.org/10.3390/nu13072189
Fernandez ML. Lifestyle Factors and Genetic Variants Associated to Health Disparities in the Hispanic Population. Nutrients. 2021; 13(7):2189. https://doi.org/10.3390/nu13072189
Chicago/Turabian StyleFernandez, Maria Luz. 2021. "Lifestyle Factors and Genetic Variants Associated to Health Disparities in the Hispanic Population" Nutrients 13, no. 7: 2189. https://doi.org/10.3390/nu13072189






