Dietary Phytochemicals in Zinc Homeostasis: A Strategy for Prostate Cancer Management
Abstract
:1. Introduction
2. Biochemistry of Zn in the Healthy Prostate
3. Zn and Metabolic Reprogramming in PCa
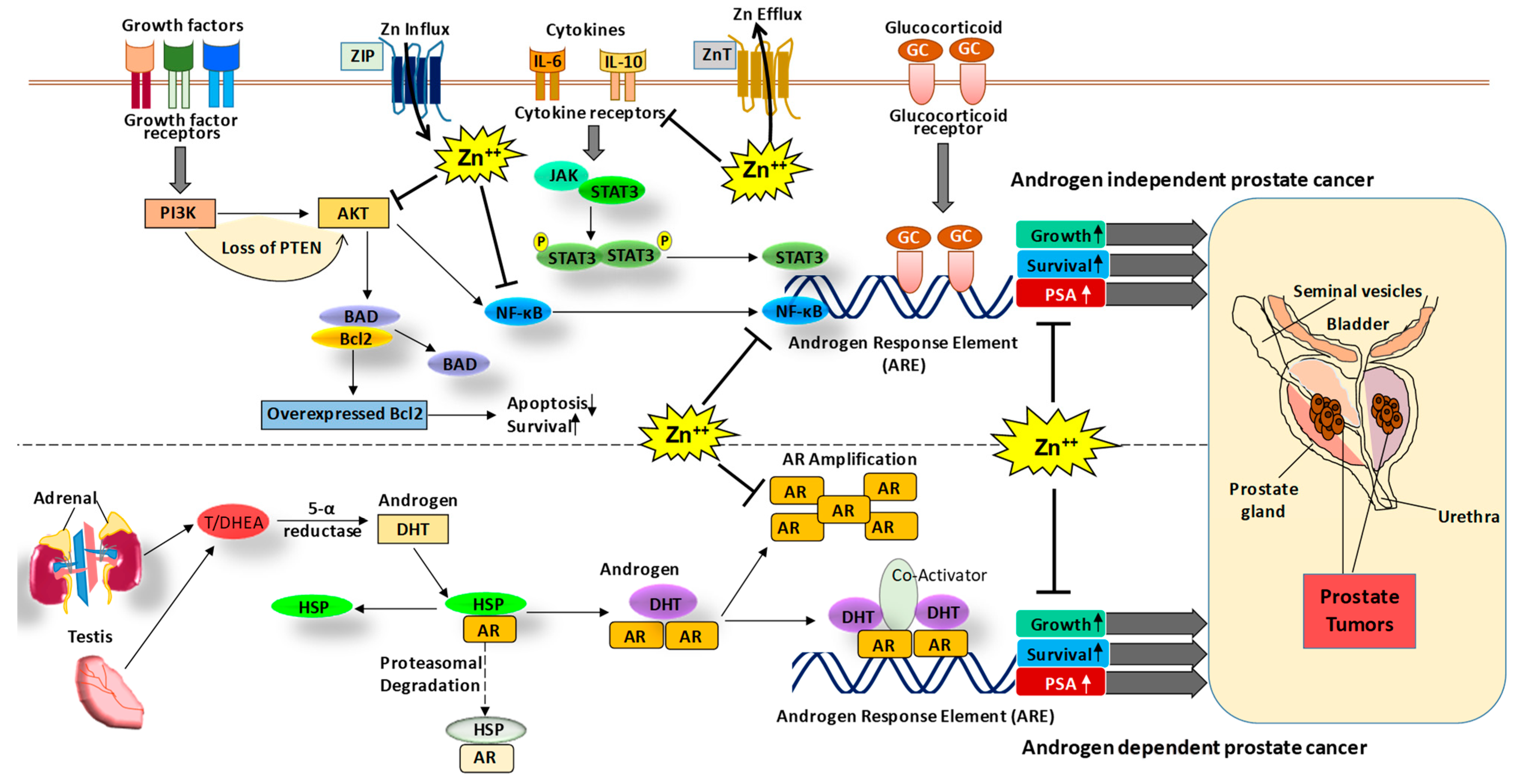
4. Role of Zn in the Healthy Prostate and PCa
5. Lower Levels of Zn Support Prostate Malignancy
6. Important Regulators of Zn Homeostasis in the Prostate
7. Challenges with Zn Supplementation
8. Zn Deficiency and PCa Epidemiology
9. Naturally Occurring Dietary Phytochemicals Known to Enhance the Absorption/Bioaccumulation of Zn
9.1. Resveratrol
9.2. Quercetin
9.3. Epigallocatechin-3-Gallate
9.4. Curcumin
10. Agents Known to Inhibit the Absorption of Zn
11. Role of Zn in other Prostatic Diseases
12. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Leitzmann, M.F.; Stampfer, M.J.; Wu, K.; Colditz, G.A.; Willett, W.C.; Giovannucci, E.L. Zinc supplement use and risk of prostate cancer. J. Natl. Cancer Inst. 2003, 95, 1004–1007. [Google Scholar] [CrossRef] [Green Version]
- Costello, L.C.; Franklin, R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, L.C.; Feng, P.; Milon, B.; Tan, M.; Franklin, R.B. Role of zinc in the pathogenesis and treatment of prostate cancer: Critical issues to resolve. Prostate Cancer Prostatic Dis. 2004, 7, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.K.; Pitschmann, A.; Ahmad, N. Resveratrol-zinc combination for prostate cancer management. Cell Cycle 2014, 13, 1867–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santillo, V.M.; Lowe, F.C. Role of vitamins, minerals and supplements in the prevention and management of prostate cancer. Int. Braz. J. Urol. 2006, 32, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Henry, G.H.; Malewska, A.; Joseph, D.B.; Malladi, V.S.; Lee, J.; Torrealba, J.; Mauck, R.J.; Gahan, J.C.; Raj, G.V.; Roehrborn, C.G.; et al. A Cellular Anatomy of the Normal Adult Human Prostate and Prostatic Urethra. Cell Rep. 2018, 25, 3530–3542.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, L.C.; Franklin, R.B.; Feng, P. Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer. Mitochondrion 2005, 5, 143–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, R.B.; Milon, B.; Feng, P.; Costello, L.C. Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer. Front. Biosci. 2005, 10, 2230–2239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddad, A.; Mohiuddin, S.S. Biochemistry, Citric Acid Cycle; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Costello, L.C.; Franklin, R.B. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: Connecting the dots. Mol. Cancer 2006, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Eidelman, E.; Twum-Ampofo, J.; Ansari, J.; Siddiqui, M.M. The Metabolic Phenotype of Prostate Cancer. Front. Oncol. 2017, 7, 131. [Google Scholar] [CrossRef]
- Zaichick, V.; Sviridova, T.V.; Zaichick, S.V. Zinc in the human prostate gland: Normal, hyperplastic and cancerous. Int. Urol. Nephrol. 1997, 29, 565–574. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. Zinc is decreased in prostate cancer: An established relationship of prostate cancer! J. Biol. Inorg. Chem. 2011, 16, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Zadra, G.; Photopoulos, C.; Loda, M. The fat side of prostate cancer. Biochim. Biophys. Acta 2013, 1831, 1518–1532. [Google Scholar] [CrossRef] [Green Version]
- Dang, Q.; Chen, Y.A.; Hsieh, J.T. The dysfunctional lipids in prostate cancer. Am. J. Clin. Exp. Urol. 2019, 7, 273–280. [Google Scholar] [PubMed]
- Chhabra, G.; Singh, C.K.; Ndiaye, M.A.; Fedorowicz, S.; Molot, A.; Ahmad, N. Prostate cancer chemoprevention by natural agents: Clinical evidence and potential implications. Cancer Lett. 2018, 422, 9–18. [Google Scholar] [CrossRef] [PubMed]
- To, P.K.; Do, M.H.; Cho, Y.S.; Kwon, S.Y.; Kim, M.S.; Jung, C. Zinc Inhibits Expression of Androgen Receptor to Suppress Growth of Prostate Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, E.D.; Heidenreich, A.; Lawrentschuk, N.; Tombal, B.; Pompeo, A.C.L.; Mendoza-Valdes, A.; Miller, K.; Debruyne, F.M.J.; Klotz, L. Androgen-targeted therapy in men with prostate cancer: Evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019, 22, 24–38. [Google Scholar] [CrossRef] [Green Version]
- Han, C.T.; Schoene, N.W.; Lei, K.Y. Influence of zinc deficiency on Akt-Mdm2-p53 and Akt-p21 signaling axes in normal and malignant human prostate cells. Am. J. Physiol. Cell Physiol. 2009, 297, C1188–C1199. [Google Scholar] [CrossRef]
- Uzzo, R.G.; Leavis, P.; Hatch, W.; Gabai, V.L.; Dulin, N.; Zvartau, N.; Kolenko, V.M. Zinc inhibits nuclear factor-kappa B activation and sensitizes prostate cancer cells to cytotoxic agents. Clin. Cancer Res. 2002, 8, 3579–3583. [Google Scholar]
- Golovine, K.; Uzzo, R.G.; Makhov, P.; Crispen, P.L.; Kunkle, D.; Kolenko, V.M. Depletion of intracellular zinc increases expression of tumorigenic cytokines VEGF, IL-6 and IL-8 in prostate cancer cells via NF-kappaB-dependent pathway. Prostate 2008, 68, 1443–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaz, C.V.; Alves, M.G.; Marques, R.; Moreira, P.I.; Oliveira, P.F.; Maia, C.J.; Socorro, S. Androgen-responsive and nonresponsive prostate cancer cells present a distinct glycolytic metabolism profile. Int. J. Biochem. Cell Biol. 2012, 44, 2077–2084. [Google Scholar] [CrossRef]
- Zaichick, V. A Systematic Review of the Zinc Content of the Normal Human Prostate Gland. Biol. Trace Elem. Res. 2020, 10.1007/s12011-020-02495-z. [Google Scholar] [CrossRef]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelleher, S.L.; McCormick, N.H.; Velasquez, V.; Lopez, V. Zinc in specialized secretory tissues: Roles in the pancreas, prostate, and mammary gland. Adv. Nutr. 2011, 2, 101–111. [Google Scholar] [CrossRef]
- Wakwe, V.C.; Odum, E.P.; Amadi, C. The impact of plasma zinc status on the severity of prostate cancer disease. Investig. Clin. Urol. 2019, 60, 162–168. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Q.; Hu, X.; Dong, X.; Wang, L.; Liu, Q.; Long, Z.; Li, L. Comparative study of serum zinc concentrations in benign and malignant prostate disease: A Systematic Review and Meta-Analysis. Sci. Rep. 2016, 6, 25778. [Google Scholar] [CrossRef] [Green Version]
- Costello, L.C.; Franklin, R.B.; Zou, J.; Feng, P.; Bok, R.; Swanson, M.G.; Kurhanewicz, J. Human prostate cancer ZIP1/zinc/citrate genetic/metabolic relationship in the TRAMP prostate cancer animal model. Cancer Biol. Ther. 2011, 12, 1078–1084. [Google Scholar] [CrossRef] [Green Version]
- To, P.K.; Do, M.H.; Cho, J.H.; Jung, C. Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. Int. J. Mol. Sci. 2020, 21, 2991. [Google Scholar] [CrossRef] [Green Version]
- Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, R.E. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal. Transduct. Target Ther. 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Kolenko, V.; Teper, E.; Kutikov, A.; Uzzo, R. Zinc and zinc transporters in prostate carcinogenesis. Nat. Rev. Urol. 2013, 10, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Milon, B.C.; Agyapong, A.; Bautista, R.; Costello, L.C.; Franklin, R.B. Ras responsive element binding protein-1 (RREB-1) down-regulates hZIP1 expression in prostate cancer cells. Prostate 2010, 70, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Mihelich, B.L.; Khramtsova, E.A.; Arva, N.; Vaishnav, A.; Johnson, D.N.; Giangreco, A.A.; Martens-Uzunova, E.; Bagasra, O.; Kajdacsy-Balla, A.; Nonn, L. miR-183-96-182 cluster is overexpressed in prostate tissue and regulates zinc homeostasis in prostate cells. J. Biol. Chem. 2011, 286, 44503–44511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.R.; Kim, I.J.; Kang, T.W.; Choi, C.; Kim, K.K.; Kim, M.S.; Nam, K.I.; Jung, C. HOXB13 downregulates intracellular zinc and increases NF-kappaB signaling to promote prostate cancer metastasis. Oncogene 2014, 33, 4558–4567. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Y.; Liu, Y.Y.; Zou, J.; Franklin, R.B.; Costello, L.C.; Feng, P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate 1999, 40, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Uzzo, R.G.; Crispen, P.L.; Golovine, K.; Makhov, P.; Horwitz, E.M.; Kolenko, V.M. Diverse effects of zinc on NF-kappaB and AP-1 transcription factors: Implications for prostate cancer progression. Carcinogenesis 2006, 27, 1980–1990. [Google Scholar] [CrossRef] [Green Version]
- Nardinocchi, L.; Pantisano, V.; Puca, R.; Porru, M.; Aiello, A.; Grasselli, A.; Leonetti, C.; Safran, M.; Rechavi, G.; Givol, D.; et al. Zinc downregulates HIF-1alpha and inhibits its activity in tumor cells in vitro and in vivo. PLoS ONE 2010, 5, e15048. [Google Scholar] [CrossRef] [Green Version]
- Feng, P.; Li, T.L.; Guan, Z.X.; Franklin, R.B.; Costello, L.C. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate 2002, 52, 311–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, T.; Kambe, T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef] [Green Version]
- Dambal, S.; Baumann, B.; McCray, T.; Williams, L.; Richards, Z.; Deaton, R.; Prins, G.S.; Nonn, L. The miR-183 family cluster alters zinc homeostasis in benign prostate cells, organoids and prostate cancer xenografts. Sci. Rep. 2017, 7, 7704. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.; Converse, A.; Berg, H.A. ZIP9, a novel membrane androgen receptor and zinc transporter protein. Gen. Comp. Endocrinol. 2018, 257, 130–136. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y.; Dong, J.; Berg, A.H. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology 2014, 155, 4250–4265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Z.; Choi, S.; Ouadid-Ahidouch, H.; Yang, J.M.; Beattie, J.H.; Korichneva, I. Zinc transporters and dysregulated channels in cancers. Front. Biosci. 2017, 22, 623–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagishi, T.; Hara, T.; Fukada, T. Recent Advances in the Role of SLC39A/ZIP Zinc Transporters In Vivo. Int. J. Mol. Sci. 2017, 18, 2708. [Google Scholar] [CrossRef] [Green Version]
- Cousins, R.J.; Liuzzi, J.P.; Lichten, L.A. Mammalian zinc transport, trafficking, and signals. J. Biol. Chem. 2006, 281, 24085–24089. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.K.; Malas, K.M.; Tydrick, C.; Siddiqui, I.A.; Iczkowski, K.A.; Ahmad, N. Analysis of Zinc-Exporters Expression in Prostate Cancer. Sci. Rep. 2016, 6, 36772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baltaci, A.K.; Yuce, K.; Mogulkoc, R. Zinc Metabolism and Metallothioneins. Biol. Trace Elem. Res. 2018, 183, 22–31. [Google Scholar] [CrossRef]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The multipurpose protein. Cell. Mol. Life Sci. 2002, 59, 627–647. [Google Scholar] [CrossRef]
- Wei, H.; Desouki, M.M.; Lin, S.; Xiao, D.; Franklin, R.B.; Feng, P. Differential expression of metallothioneins (MTs) 1, 2, and 3 in response to zinc treatment in human prostate normal and malignant cells and tissues. Mol. Cancer 2008, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Marr, M.T., 2nd; Isogai, Y.; Wright, K.J.; Tjian, R. Coactivator cross-talk specifies transcriptional output. Genes Dev. 2006, 20, 1458–1469. [Google Scholar] [CrossRef] [Green Version]
- Costello, L.C.; Franklin, R.B. Decreased zinc in the development and progression of malignancy: An important common relationship and potential for prevention and treatment of carcinomas. Expert Opin. Ther. Targets 2017, 21, 51–66. [Google Scholar] [CrossRef] [Green Version]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar] [PubMed]
- Franklin, R.B.; Ma, J.; Zou, J.; Guan, Z.; Kukoyi, B.I.; Feng, P.; Costello, L.C. Human ZIP1 is a major zinc uptake transporter for the accumulation of zinc in prostate cells. J. Inorg. Biochem. 2003, 96, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Darago, A.; Sapota, A.; Nasiadek, M.; Klimczak, M.; Kilanowicz, A. The Effect of Zinc and Selenium Supplementation Mode on Their Bioavailability in the Rat Prostate. Should Administration Be Joint or Separate? Nutrients 2016, 8, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cragg, R.A.; Phillips, S.R.; Piper, J.M.; Varma, J.S.; Campbell, F.C.; Mathers, J.C.; Ford, D. Homeostatic regulation of zinc transporters in the human small intestine by dietary zinc supplementation. Gut 2005, 54, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Sapota, A.; Darago, A.; Skrzypinska-Gawrysiak, M.; Nasiadek, M.; Klimczak, M.; Kilanowicz, A. The bioavailability of different zinc compounds used as human dietary supplements in rat prostate: A comparative study. Biometals 2014, 27, 495–505. [Google Scholar] [CrossRef]
- Jarrard, D.F. Does zinc supplementation increase the risk of prostate cancer? Arch. Ophthalmol. 2005, 123, 102–103. [Google Scholar] [CrossRef] [Green Version]
- Epstein, M.M.; Kasperzyk, J.L.; Andren, O.; Giovannucci, E.L.; Wolk, A.; Hakansson, N.; Andersson, S.O.; Johansson, J.E.; Fall, K.; Mucci, L.A. Dietary zinc and prostate cancer survival in a Swedish cohort. Am. J. Clin. Nutr. 2011, 93, 586–593. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.M.; Al-Alem, U.; Dabbous, F.; Ali, M.M.; Batai, K.; Shah, E.; Kittles, R.A. Zinc Intake and Risk of Prostate Cancer: Case-Control Study and Meta-Analysis. PLoS ONE 2016, 11, e0165956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyad, M.A. Zinc for prostate disease and other conditions: A little evidence, a lot of hype, and a significant potential problem. Urol. Nurs. 2004, 24, 49–52. [Google Scholar] [PubMed]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.R.; Kriedt, C.L.; Lents, N.H.; Hoyer, M.K.; Jamaluddin, N.; Klein, C.; Baldassare, J. Direct intra-tumoral injection of zinc-acetate halts tumor growth in a xenograft model of prostate cancer. J. Exp. Clin. Cancer Res. 2009, 28, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Che, X.; Wang, J.; Chen, F.; Wang, X.; Zhang, Z.; Fan, B.; Yang, D.; Song, X. Zinc sensitizes prostate cancer cells to sorafenib and regulates the expression of Livin. Acta Biochim. Biophys. Sin. 2013, 45, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, H.; Tsutsui, T. Infants and elderlies are susceptible to zinc deficiency. Sci. Rep. 2016, 6, 21850. [Google Scholar] [CrossRef] [Green Version]
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66 (Suppl. S2), 22–33. [Google Scholar] [CrossRef]
- Wagner, S.E.; Burch, J.B.; Hussey, J.; Temples, T.; Bolick-Aldrich, S.; Mosley-Broughton, C.; Liu, Y.; Hebert, J.R. Soil zinc content, groundwater usage, and prostate cancer incidence in South Carolina. Cancer Causes Control CCC 2009, 20, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Taitt, H.E. Global Trends and Prostate Cancer: A Review of Incidence, Detection, and Mortality as Influenced by Race, Ethnicity, and Geographic Location. Am. J. Mens Health 2018, 12, 1807–1823. [Google Scholar] [CrossRef] [Green Version]
- Jemal, A.; Center, M.M.; DeSantis, C.; Ward, E.M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010, 19, 1893–1907. [Google Scholar] [CrossRef] [Green Version]
- Rebbeck, T.R.; Devesa, S.S.; Chang, B.L.; Bunker, C.H.; Cheng, I.; Cooney, K.; Eeles, R.; Fernandez, P.; Giri, V.N.; Gueye, S.M.; et al. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of african descent. Prostate Cancer 2013, 2013, 560857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, R.M.; Gilliland, F.D.; Eley, J.W.; Harlan, L.C.; Stephenson, R.A.; Stanford, J.L.; Albertson, P.C.; Hamilton, A.S.; Hunt, W.C.; Potosky, A.L. Racial and ethnic differences in advanced-stage prostate cancer: The Prostate Cancer Outcomes Study. J. Natl. Cancer Inst. 2001, 93, 388–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.K.; Check, D.P.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Ferlay, J.; Bray, F.; Cook, M.B.; Devesa, S.S. Prostate cancer incidence in 43 populations worldwide: An analysis of time trends overall and by age group. Int. J. Cancer 2016, 138, 1388–1400. [Google Scholar] [CrossRef]
- Wyatt, N.J.; Milne, A.; Woodward, E.M.S.; Rees, A.P.; Browning, T.J.; Bouman, H.A.; Worsfold, P.J.; Lohan, M.C. Biogeochemical cycling of dissolved zinc along the GEOTRACES South Atlantic transect GA10 at 40°S. Global Biogeochem. Cycles 2014, 28, 44–56. [Google Scholar] [CrossRef]
- Rishi, I.; Baidouri, H.; Abbasi, J.A.; Bullard-Dillard, R.; Kajdacsy-Balla, A.; Pestaner, J.P.; Skacel, M.; Tubbs, R.; Bagasra, O. Prostate cancer in African American men is associated with downregulation of zinc transporters. Appl. Immunohistochem. Mol. Morphol. 2003, 11, 253–260. [Google Scholar] [CrossRef]
- Sreenivasulu, K.; Raghu, P.; Nair, K.M. Polyphenol-rich beverages enhance zinc uptake and metallothionein expression in Caco-2 cells. J. Food Sci. 2010, 75, H123–H128. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Dinsmore, W.W.; Callender, M.E.; McMaster, D.; Love, A.H. The absorption of zinc from a standardized meal in alcoholics and in normal volunteers. Am. J. Clin. Nutr. 1985, 42, 688–693. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [Green Version]
- Wapnir, R.A.; Stiel, L. Zinc intestinal absorption in rats: Specificity of amino acids as ligands. J. Nutr. 1986, 116, 2171–2179. [Google Scholar] [CrossRef]
- Pillay, D.; Gathiram, P.; Ubbink, J.B. Zinc status in vitamin B6 deficiency. Int. J. Vitam. Nutr. Res. 1997, 67, 22–26. [Google Scholar]
- Rosado, J.L.; Diaz, M.; Gonzalez, K.; Griffin, I.; Abrams, S.A.; Preciado, R. The addition of milk or yogurt to a plant-based diet increases zinc bioavailability but does not affect iron bioavailability in women. J. Nutr. 2005, 135, 465–468. [Google Scholar] [CrossRef]
- Hashimoto, A.; Ohkura, K.; Takahashi, M.; Kizu, K.; Narita, H.; Enomoto, S.; Miyamae, Y.; Masuda, S.; Nagao, M.; Irie, K.; et al. Soybean extracts increase cell surface ZIP4 abundance and cellular zinc levels: A potential novel strategy to enhance zinc absorption by ZIP4 targeting. Biochem. J. 2015, 472, 183–193. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wu, M.; Schoene, N.W.; Cheng, W.H.; Wang, T.T.; Alshatwi, A.A.; Alsaif, M.; Lei, K.Y. Effect of resveratrol and zinc on intracellular zinc status in normal human prostate epithelial cells. Am. J. Physiol. Cell Physiol. 2009, 297, C632–C644. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Ognik, K.; Thoene, M.; Rawicka, A.; Juskiewicz, J. Resveratrol modulates the blood plasma levels of Cu and Zn, the antioxidant status and the vascular response of thoracic arteries in copper deficient Wistar rats. Toxicol. Appl. Pharmacol. 2020, 390, 114877. [Google Scholar] [CrossRef] [PubMed]
- Dias, K.; Nikolaou, S. Does the combination of resveratrol with Al (III) and Zn (II) improve its antioxidant activity? Nat. Prod. Commun. 2011, 6, 1673–1676. [Google Scholar] [CrossRef] [Green Version]
- Dabbagh-Bazarbachi, H.; Clergeaud, G.; Quesada, I.M.; Ortiz, M.; O’Sullivan, C.K.; Fernandez-Larrea, J.B. Zinc ionophore activity of quercetin and epigallocatechin-gallate: From Hepa 1-6 cells to a liposome model. J. Agric. Food Chem. 2014, 62, 8085–8093. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Tan, X. Epigallocatechin-3-gallate and zinc provide anti-apoptotic protection against hypoxia/reoxygenation injury in H9c2 rat cardiac myoblast cells. Mol. Med. Rep. 2015, 12, 1850–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesada, I.M.; Bustos, M.; Blay, M.; Pujadas, G.; Ardevol, A.; Salvado, M.J.; Blade, C.; Arola, L.; Fernandez-Larrea, J. Dietary catechins and procyanidins modulate zinc homeostasis in human HepG2 cells. J. Nutr. Biochem. 2011, 22, 153–163. [Google Scholar] [CrossRef]
- Malhotra, A.; Nair, P.; Dhawan, D.K. Curcumin and resveratrol synergistically stimulate p21 and regulate cox-2 by maintaining adequate zinc levels during lung carcinogenesis. Eur. J. Cancer Prev. 2011, 20, 411–416. [Google Scholar] [CrossRef]
- Bel-Serrat, S.; Stammers, A.L.; Warthon-Medina, M.; Moran, V.H.; Iglesia-Altaba, I.; Hermoso, M.; Moreno, L.A.; Lowe, N.M.; Network, E. Factors that affect zinc bioavailability and losses in adult and elderly populations. Nutr. Rev. 2014, 72, 334–352. [Google Scholar] [CrossRef]
- Udechukwu, M.C.; Collins, S.A.; Udenigwe, C.C. Prospects of enhancing dietary zinc bioavailability with food-derived zinc-chelating peptides. Food Funct. 2016, 7, 4137–4144. [Google Scholar] [CrossRef]
- Singh, C.K.; George, J.; Ahmad, N. Resveratrol-based combinatorial strategies for cancer management. Ann. N. Y. Acad. Sci. 2013, 1290, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, C.K.; Ndiaye, M.A.; Ahmad, N. Resveratrol and cancer: Challenges for clinical translation. Biochim. Biophys. Acta 2015, 1852, 1178–1185. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.K.; Liu, X.; Ahmad, N. Resveratrol, in its natural combination in whole grape, for health promotion and disease management. Ann. N. Y. Acad. Sci. 2015, 1348, 150–160. [Google Scholar] [CrossRef]
- Singh, C.K.; Siddiqui, I.A.; El-Abd, S.; Mukhtar, H.; Ahmad, N. Combination chemoprevention with grape antioxidants. Mol. Nutr. Food Res. 2016, 60, 1406–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, C.K.; Mintie, C.A.; Ndiaye, M.A.; Chhabra, G.; Dakup, P.P.; Ye, T.; Yu, M.; Ahmad, N. Chemoprotective Effects of Dietary Grape Powder on UVB Radiation-Mediated Skin Carcinogenesis in SKH-1 Hairless Mice. J. Investig. Dermatol. 2019, 139, 552–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, C.K.; Chhabra, G.; Ahmad, N. Chapter 7: Resveratrol and Cancer Cell Biology. In Resveratrol: State of the Art Science and Health Applications; Actionable Targets and Mechanisms of Resveratrol; Wu, J.M., Hsieh, T.-C., Eds.; World Scientific Publishing Co Pte Ltd: Singapore, 2018; pp. 183–207. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Mintie, C.A.; Ahmad, N. Grape chemopreventive agents against angiogenesis and metastasis. In Natural Products for Cancer Chemoprevention: Single Compounds and Combinations; Pezzuto, J.M., Vang, O., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 375–400. [Google Scholar] [CrossRef]
- Harper, C.E.; Patel, B.B.; Wang, J.; Arabshahi, A.; Eltoum, I.A.; Lamartiniere, C.A. Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis 2007, 28, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Seeni, A.; Takahashi, S.; Takeshita, K.; Tang, M.; Sugiura, S.; Sato, S.Y.; Shirai, T. Suppression of prostate cancer growth by resveratrol in the transgenic rat for adenocarcinoma of prostate (TRAP) model. Asian Pac. J. Cancer Prev. 2008, 9, 7–14. [Google Scholar]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Siddiqui, I.A.; Panackal, J.E.; Mintie, C.A.; Ahmad, N. Quercetin-Resveratrol Combination for Prostate Cancer Management in TRAMP Mice. Cancers 2020, 12, 2141. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [Green Version]
- Sharmila, G.; Athirai, T.; Kiruthiga, B.; Senthilkumar, K.; Elumalai, P.; Arunkumar, R.; Arunakaran, J. Chemopreventive effect of quercetin in MNU and testosterone induced prostate cancer of Sprague-Dawley rats. Nutr. Cancer 2014, 66, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jiang, X.; Song, L.; Wang, H.; Mei, Z.; Xu, Z.; Xing, N. Quercetin inhibits angiogenesis through thrombospondin-1 upregulation to antagonize human prostate cancer PC-3 cell growth in vitro and in vivo. Oncol. Rep. 2016, 35, 1602–1610. [Google Scholar] [CrossRef] [Green Version]
- Ward, A.B.; Mir, H.; Kapur, N.; Gales, D.N.; Carriere, P.P.; Singh, S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018, 16, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, R.; Penso, N.E.C.; Hackman, R.M.; Wang, Y.; Mackenzie, G.G. Epigallocatechin-3-Gallate (EGCG) Suppresses Pancreatic Cancer Cell Growth, Invasion, and Migration partly through the Inhibition of Akt Pathway and Epithelial-Mesenchymal Transition: Enhanced Efficacy when Combined with Gemcitabine. Nutrients 2019, 11, 1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.G.; Yu, H.N.; Sun, S.L.; Zhang, L.C.; He, G.Q.; Das, U.N.; Ruan, H.; Shen, S.R. Epigallocatechin-3-gallate affects the growth of LNCaP cells via membrane fluidity and distribution of cellular zinc. J. Zhejiang Univ. Sci. B 2009, 10, 411–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.H.; Han, D.H.; Kim, S.W.; Kim, M.J.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; et al. A randomized, double-blind, placebo-controlled trial to evaluate the role of curcumin in prostate cancer patients with intermittent androgen deprivation. Prostate 2019, 79, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.A.; Molana, S.H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of Curcumin Supplementation During Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr. Cancer 2016, 68, 77–85. [Google Scholar] [CrossRef]
- Hunt, J.R.; Beiseigel, J.M. Dietary calcium does not exacerbate phytate inhibition of zinc absorption by women from conventional diets. Am. J. Clin. Nutr. 2009, 89, 839–843. [Google Scholar] [CrossRef] [Green Version]
- Foster, M.; Karra, M.; Picone, T.; Chu, A.; Hancock, D.P.; Petocz, P.; Samman, S. Dietary fiber intake increases the risk of zinc deficiency in healthy and diabetic women. Biol. Trace Elem. Res. 2012, 149, 135–142. [Google Scholar] [CrossRef]
- Galic, J.; Simunovic, D. Prostate disease prevalence with epidemiological and hormonal analysis in randomly selected male population in Croatia. Coll. Antropol. 2008, 32, 1195–1202. [Google Scholar] [PubMed]
- Christudoss, P.; Selvakumar, R.; Fleming, J.J.; Gopalakrishnan, G. Zinc status of patients with benign prostatic hyperplasia and prostate carcinoma. Indian J. Urol. 2011, 27, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, D.; Cyrus, A.; Baghinia, M.R.; Kazemifar, A.M.; Shirincar, M. The efficacy of zinc for treatment of chronic prostatitis. Acta Med. Indones. 2013, 45, 259–264. [Google Scholar] [PubMed]
- Yang, X.; Li, H.; Zhang, C.; Lin, Z.; Zhang, X.; Zhang, Y.; Yu, Y.; Liu, K.; Li, M.; Zhang, Y.; et al. Serum quantitative proteomic analysis reveals potential zinc-associated biomarkers for nonbacterial prostatitis. Prostate 2015, 75, 1538–1555. [Google Scholar] [CrossRef] [PubMed]
| Dietary Phytochemicals | Effect on Zn signaling | References |
|---|---|---|
| Green tea, red wine and grape juice |
| [77] |
| Soybean extracts and soyasaponin Bb |
| [84] |
| Resveratrol |
| [85,86,87] |
| Quercetin |
| [77,88] |
| Epigallocatechin-3-gallate (EGCG) |
| [89,90] |
| Grape-seed procyanidin extract (GSPE) |
| [90] |
| Curcumin |
| [91] |
| Phytate |
| [92,93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, C.K.; Chhabra, G.; Patel, A.; Chang, H.; Ahmad, N. Dietary Phytochemicals in Zinc Homeostasis: A Strategy for Prostate Cancer Management. Nutrients 2021, 13, 1867. https://doi.org/10.3390/nu13061867
Singh CK, Chhabra G, Patel A, Chang H, Ahmad N. Dietary Phytochemicals in Zinc Homeostasis: A Strategy for Prostate Cancer Management. Nutrients. 2021; 13(6):1867. https://doi.org/10.3390/nu13061867
Chicago/Turabian StyleSingh, Chandra K., Gagan Chhabra, Arth Patel, Hao Chang, and Nihal Ahmad. 2021. "Dietary Phytochemicals in Zinc Homeostasis: A Strategy for Prostate Cancer Management" Nutrients 13, no. 6: 1867. https://doi.org/10.3390/nu13061867






