The Impact of Nutrition on the COVID-19 Pandemic and the Impact of the COVID-19 Pandemic on Nutrition
Abstract
:1. Introduction
2. Material and Methods
3. Predictions from Past Pandemics
4. Nutrition as a Strategy for Coronavirus Disease 2019 (COVID-19) Therapy
5. Food Security in the COVID-19 Pandemic
6. Changes in Dietary Behaviour during the Pandemic and Their Consequences
7. Conclusions
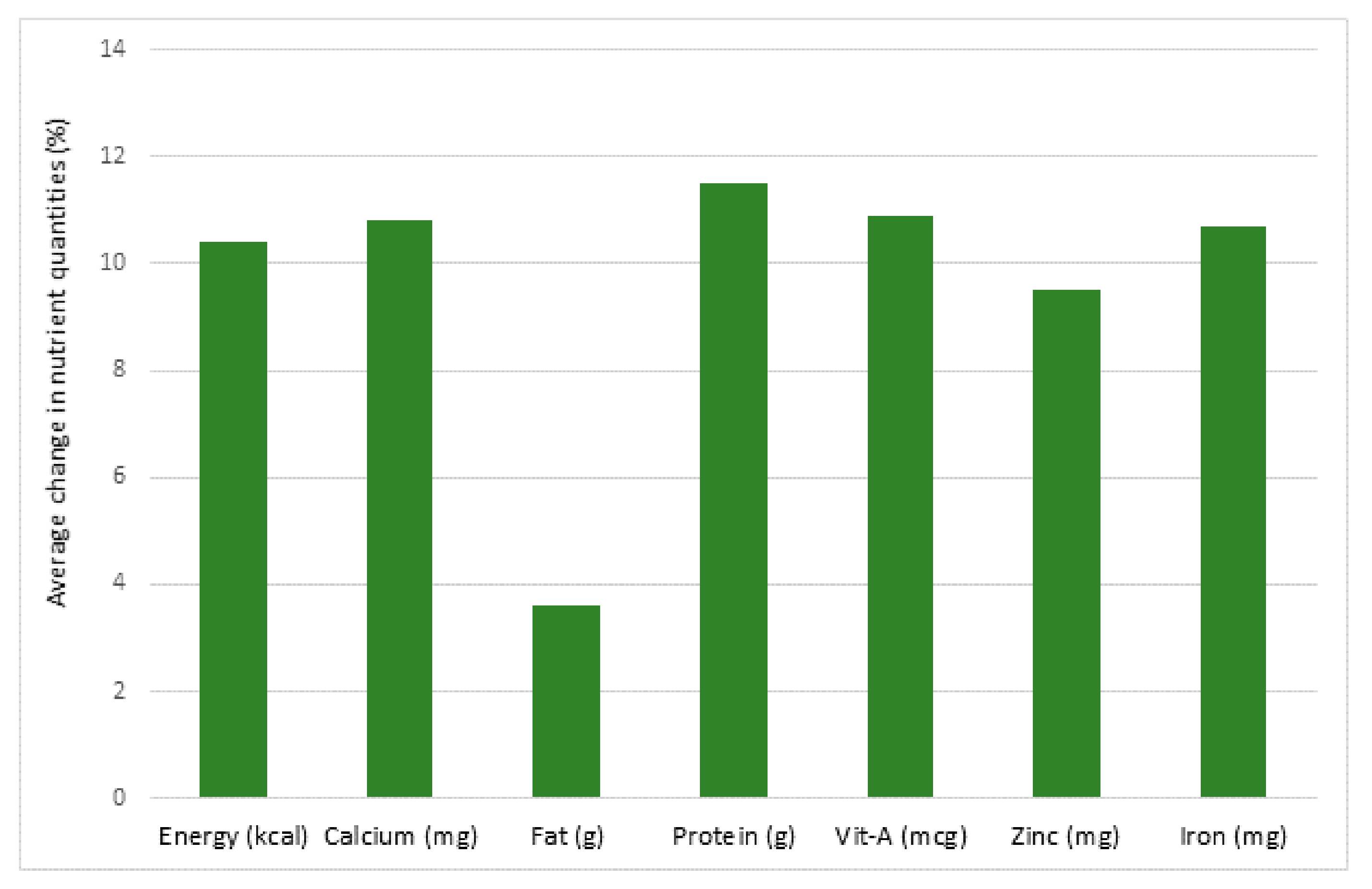
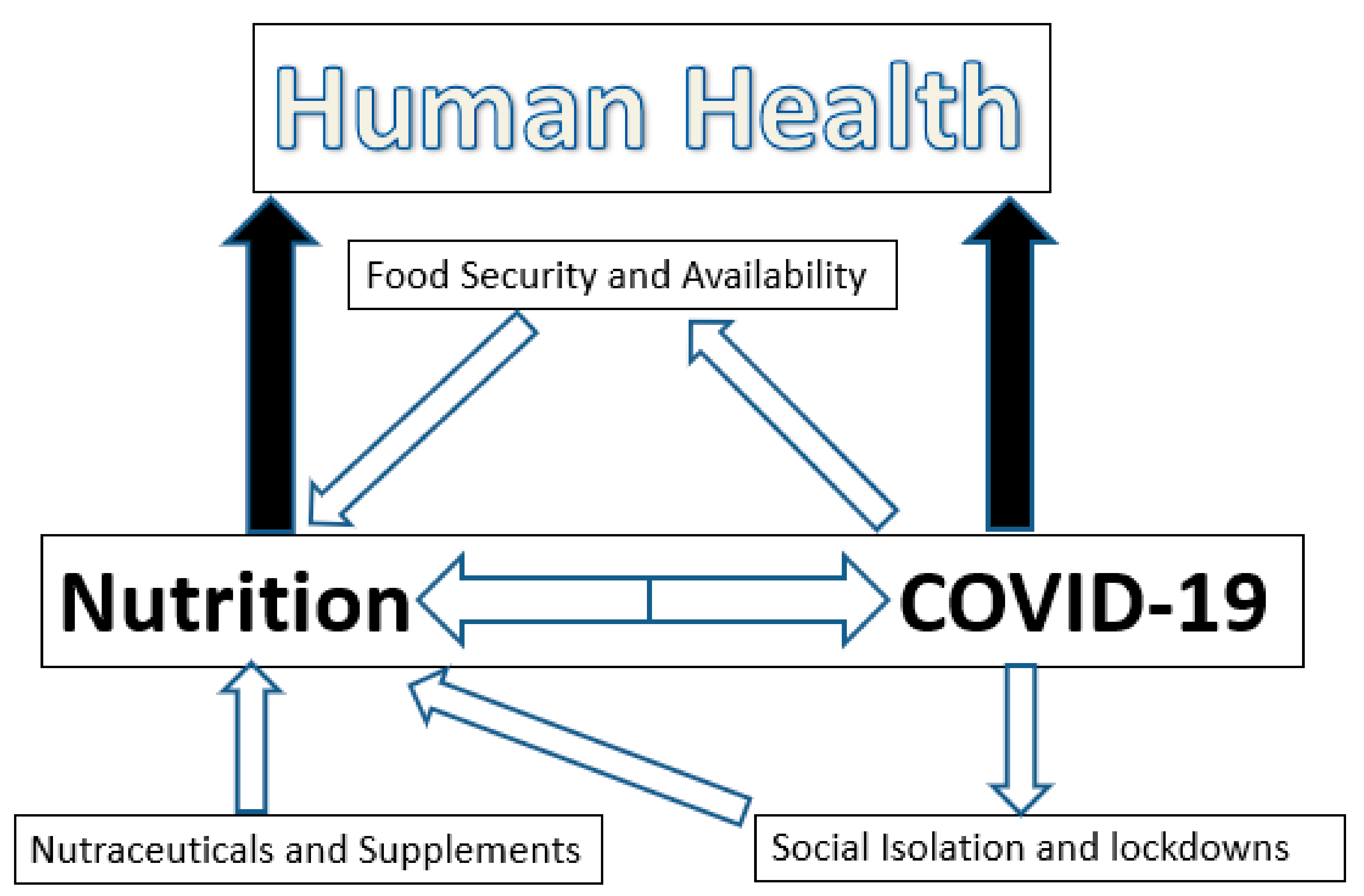
- The COVID-19 pandemic has caused changes in food supply. Special programs have been implemented in many countries to increase food availability and safety. Nonetheless, alterations in food availability due to the pandemic have occurred throughout the world. The severity depends upon the affluence and the economic status of the country. This has more serious implications particularly in third world countries as delays in food distribution, loss of food quality and quantity, impairments in food access and losses of income to purchase food have occurred. This has serious health implications acutely and, in view of the deleterious impacts that past pandemics have had on human growth and health, it is reasonable to predict that the current COVID-19 pandemic will induce nutritional deficiencies across the globe that will have a long-lasting negative impact on human health.
- There is no evidence that the COVID-19 virus has been transmitted through foods or food packaging. This does not negate the importance of using appropriate precautionary measures in the food industry with, for example, the use of appropriate personal protective equipment, hand hygiene and disinfectants.
- The nutritional status of a person can modulate infectious disease and the inflammatory processes associated with it positively or negatively by altering the immune system. Malnutrition in disadvantaged populations and in the elderly clearly leaves these populations more susceptible to COVID-19 infections and more severe clinical symptoms and outcomes. However, although infectivity rates and the severity of the clinical symptoms associated with a coronavirus infection may be modulated, it is highly unlikely that strong viral transmission can be fully prevented by following a healthy diet or supplementing the diet with nutraceuticals. The impact of vitamin D and zinc status in COVID-19 patients regarding viral transmission and its clinical symptoms remains unclear and requires further research.
- COVID-19 has had a significant impact in some populations through altered eating behaviors. The impact of social isolation and lockdowns on eating behaviors during the COVID-19 pandemic should not be underestimated as it has already had acute effects and will likely produce long-term deleterious effects on population health as well. Poor nutritional choices sustained over extended periods of time will have increased plasma risk factors for cardiovascular disease, diabetes and cancer.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1345–1422. [Google Scholar] [CrossRef] [Green Version]
- Palloni, A.; McEniry, M.; Huangfu, Y.; Beltran-Sanchez, H. Impacts of the 1918 flu on survivors’ nutritional status: A double quasi-natural experiment. PLoS ONE 2020, 15, e0232805. [Google Scholar] [CrossRef]
- Mazumder, B.; Almond, D.; Park, K.; Crimmins, E.M.; Finch, C.E. Lingering prenatal effects of the 1918 influenza pandemic on cardiovascular disease. J. Dev. Orig. Health Dis. 2010, 1, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiyar, A.; Pingali, P. Pandemics and food systems–towards a proactive food safety approach to disease prevention and management. Food Secur. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Kodish, S.R.; Roher, F.; Beauliere, J.-M.; Daffe, M.; Ayoya, M.A.; Wirth, J.P.; Ngnie-Teta, I. Implications of the Ebola virus disease outbreak in Guinea: Qualitative findings to inform future health and nutrition-related responses. PLoS ONE 2018, 13, e0202468. [Google Scholar]
- Kodish, S.R.; Simen-Kapeu, A.; Beauliere, J.-M.; Ngnie-Teta, I.; Jalloh, M.B.; Pyne-Bailey, S.; Schwartz, H.; Wirth, J.P. Consensus building around nutrition lessons from the 2014-16 Ebola virus disease outbreak in Guinea and Sierra Leone. Health Policy Plan. 2019, 34, 83–91. [Google Scholar] [CrossRef]
- Ververs, M.; Gabra, M. Nutritional care for patients with Ebola virus disease. Emerg. Infect. Dis. 2020, 26, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Ververs, M.; Vorfeld, C. Guidance materials from 2014-2019 on nutritional care for Ebola patients in Ebola treatment units: An analysis. Public Health Nutr. 2021, 24, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kamara, M.H.; Najjemba, R.; van Griensven, J.; Yorpoi, D.; Jimissa, A.S.; Chan, A.K.; Mishra, S. Increase in acute malnutrition in children following the 2014-2015 Ebola outbreak in rural Sierra Leone. Public Health Action 2017, 7 (Suppl. 1), S27–S33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Jung, Y.-J.; Kim, K.-H.; Kwon, Y.; Kim, Y.-J.; Zhang, Z.; Kang, H.-S.; Wang, B.-Z.; Quan, F.-S.; Kang, S.-M. Antiviral activity of fermented ginseng extracts against a broad range of influenza viruses. Viruses 2018, 1, 471. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, A.; Suzuki, K.; Asayama, Y.; Konno, M.; Saito, K.; Yamazaki, N.; Takimoto, H. Oral administration of Euglena gracilis Z and its carbohydrate storage substance provides survival protection against influenza virus infection in mice. Biochem. Biophys. Res. Commun. 2017, 9, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Tanaka, K.; Hirota, Y. Immune response to influenza vaccine in healthy adults and the elderly: Association with nutritional status. Vaccine 2005, 23, 1457–1463. [Google Scholar] [CrossRef]
- Lordan, R.; Rando, H.M.; COVID-19 Review Consortium; Greene, C.S. Dietary supplements and nutraceuticals under investigation for COVID-19 prevention and treatment. mSystems 2021, 6, e00122-21. [Google Scholar] [CrossRef]
- Sundaram, M.E.; Meydani, S.N.; Vandermause, M.; Shay, D.K.; Coleman, L.A. Vitamin E, vitamin A, and zinc status are not related to serologic response to influenza vaccine in older adults: An observational prospective cohort study. Nutr. Res. 2014, 34, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Yam, D.; Aluisio, A.R.; Perera, S.M.; Peters, J.L.; Cho, D.K.; Kennedy, S.B.; Massaquoi, M.; Sahr, F.; Smit, M.A.; Locks, L.; et al. Association between multivitamin supplementation and mortality among patients with Ebola virus disease: An international multisite cohort study. Afr. J. Emerg. Med. 2020, 10, 23–29. [Google Scholar] [CrossRef]
- Butler, M.J.; Barrientos, R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav. Immun. 2020, 87, 53–54. [Google Scholar] [CrossRef]
- Aman, F.; Masood, S. How nutrition can help to fight against COVID-19 pandemic. Pak. J. Med. Sci. 2020, 36, S121–S123. [Google Scholar] [CrossRef] [PubMed]
- Bold, J.; Harris, M.; Fellows, L.; Chouchane, M. Nutrition, the digestive system and immunity in COVID-19 infection. Gastroenterol. Hepatol. Bed Bench. 2020, 13, 331–340. [Google Scholar]
- Virgens, I.P.A.; Santana, N.M.; Lima, S.C.V.C.; Fayh, A.P.T. Can COVID-19 be a risk for cachexia for patients during intensive care? Narrative review and nutritional recommendations. Br. J. Nutr. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Mossink, J.P. Zinc as nutritional intervention and prevention measure for COVID-19 disease. BMJ Nutr. Prev. Health 2020, 3, 111–117. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Aldawoud, T.M.S.; Rizou, M.; Rowan, N.J.; Ibrahim, S.A. Food ingredients and active compounds against the coronavirus disease (COVID-19) pandemic: A comprehensive review. Foods 2020, 9, 1701. [Google Scholar] [CrossRef]
- McAuliffe, S.; Ray, S.; Fallon, E.; Bradfield, J.; Eden, T.; Kohlmeier, M. Dietary micronutrients in the wake of COVID-19: An appraisal of evidence with a focus on high-risk groups and preventative healthcare. BMJ Nutr. Prev. Health 2020, 3, 93–99. [Google Scholar] [CrossRef]
- Paoli, A.; Gorini, S.; Caprio, M. The dark side of the spoon glucose, ketones and COVID-19: A possible role for ketogenic diet? J. Transl. Med. 2020, 20, 441. [Google Scholar] [CrossRef]
- Khubber, S.; Hashemifesharaki, R.; Mohammadi, M.; Gharibzahedi, S.M.T. Garlic (Allium sativum L.): A potential unique therapeutic food rich in organosulfur and flavonoid compounds to fight with COVID-19. Nutr. J. 2020, 19, 124. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Orhan, C.; Uckun, F.M.; Sahin, K. Clinical impact potential of supplemental nutrients as adjuncts of therapy in high-risk COVID-19 for obese patients. Front. Nutr. 2020, 7, 580504. [Google Scholar] [CrossRef]
- Butters, D.; Whitehouse, M. COVID-19 and nutraceutical therapies, especially using zinc to supplement antimicrobials. Inflammopharmacology 2020, 16, 1–5. [Google Scholar]
- Chowdhury, P.; Barooah, A.K. Tea bioactive modulate innate immunity: In perception to COVID-19 pandemic. Front. Immunol. 2020, 11, 590716. [Google Scholar] [CrossRef] [PubMed]
- De Araujo Morais, A.H.; Passos, T.S.; de Lima Vale, S.H.; da Silva Maia, J.K.; Maciel, B.L. Obesity and the increased risk for COVID-19: Mechanisms and nutritional management. Nutr. Res. Rev. 2020, 13, 1–13. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids and their metabolites in the pathobiology of (coronavirus disease 2019) COVID-19. Nutrition 2021, 82, 111052. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, F.; Sessa, F.; Valenzano, A.; Polito, R.; Monda, V.; Cibelli, G.; Villano, I.; Pisanelli, D.; Perrella, M.; Daniele, A.; et al. COVID-19: Role of nutrition and supplementation. Nutrients 2021, 13, 976. [Google Scholar] [CrossRef]
- Bedock, D.; Lassen, P.B.; Mathian, A.; Moreau, P.; Couffignal, J.; Ciangura, C.; Poitou-Bernert, C.; Jeannin, A.-C.; Mosbah, H.; Fadlallah, J.; et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin. Nutr. ESPEN 2020, 40, 214–219. [Google Scholar] [CrossRef]
- Allard, L.; Ouedraogo, E.; Molleville, J.; Bihan, H.; Giroux-Leprieur, B.; Sutton, A.; Baudry, C.; Josse, C.; Didier, M.; Deutsch, D.; et al. Malnutrition: Percentage and association with prognosis in patients hospitalized for coronavirus disease 2019. Nutrients 2020, 12, 3679. [Google Scholar] [CrossRef]
- Formisano, E.; Di Maio, P.; Ivaldi, C.; Sferrazzo, E.; Arieta, L.; Bongiovanni, S.; Panizzi, L.; Valentino, E.; Pasta, A.; Giudice, M.; et al. Nutritional therapy for patients with coronavirus disease 2019 (COVID-19): Practical protocol from a single center highly affected by outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Nutrition 2021, 82, 111048. [Google Scholar] [CrossRef] [PubMed]
- Damayanthi, H.D.W.T.; Prabani, K.I.P. Nutritional determinants and COVID-19 outcomes of older patients with COVID-19: A systematic review. Arch. Gerontol. Geriatr. 2021, 95, 104411. [Google Scholar] [CrossRef] [PubMed]
- Anuk, A.T.; Polat, N.; Akdas, S.; Erol, S.A.; Tanacan, A.; Biriken, D.; Keskin, H.L.; Tekin, O.M.; Yazihan, N.; Sahin, D. The relationship between trace element status (zinc, copper, magnesium) and clinical outcomes in COVID-19 infection during pregnancy. Biol. Trace Elem. Res. 2020, 24, 1–10. [Google Scholar]
- Huang, F.; Zhang, C.; Liu, Q.; Zhao, Y.; Zhang, Y.; Qin, Y.; Li, X.; Li, C.; Zhou, C.; Jin, N.; et al. Identification of amitriptyline HCl, flavin adenine dinucleotide, azacitidine and calcitriol as repurposing drugs for influenza A H5N1 virus-induced lung injury. PLoS Pathog. 2020, 16, e1008341. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, H. Mini-review on the roles of Vitamin C, Vitamin D, and selenium in the immune system against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Griffin, G.; Hewison, M.; Hopkin, J.; Kenny, R.A.; Quinton, R.; Rhodes, J.; Subramanian, S.; Thickett, D. Preventing vitamin D deficiency during the COVID-19 pandemic: UK definitions of vitamin D sufficiency and recommended supplement dose are set too low. Clin. Med. 2021, 21, e48–e51. [Google Scholar] [CrossRef]
- Lanham-New, S.A.; Webb, A.R.; Cashman, K.D.; Buttriss, J.L.; Fallowfield, J.L.; Masud, T.; Hewison, M.; Mathers, J.C.; Kiely, M.; A Welch, A.; et al. Vitamin D and SARS-CoV-2 virus/COVID-19 disease. BMJ Nutr. Prev. Health 2020, 3, 106–110. [Google Scholar] [CrossRef]
- Goncalves, T.J.M.; Goncalves, S.E.A.B.; Guarnieri, A.; Risegato, R.C.; Guimaraes, M.P.; Cabral de Freitas, D.; Razuk-Filho, A.; Junior, P.B.B.; Parrillo, E.F. Prevalence of obesity and hypovitaminosis D in elderly with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Nutr. 2020, 40, 110–114. [Google Scholar] [CrossRef]
- Cereda, E.; Bogliolo, L.; Klersy, C.; Lobascio, F.; Masi, S.; Crotti, S.; De Stefano, L.; Bruno, R.; Corsico, A.G.; Di Sabatino, A.; et al. Vitamin D 25OH deficiency in COVID-19 patients admitted to a tertiary referral hospital. Clin. Nutr. 2020, 40, 2469–2472. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, M. Avoidance of vitamin D deficiency to slow the Covid-19 pandemic. BMJ Nutr. Prev. Health 2020, 3, e000096. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Bogliolo, L.; Lobascio, F.; Barichella, M.; Zecchinelli, A.L.; Pezzoli, G.; Caccialanza, R. Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy, Italy. Nutrition 2021, 82, 111055. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture. Definitions of Food Security. Economic Research Service. 2020. Available online: https://ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/definitions-of-food-security.aspx (accessed on 18 January 2021).
- World Health Organization; Food and Agriculture Organization of the United Nations. COVID-19 and Food Safety: Guidance for Competent Authorities Responsible for National Food Safety Control Systems; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization; Food and Agriculture Organization of the United Nations. COVID-19 and Food Safety: Guidance for Food Business; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Mardones, F.O.; Rich, K.M.; Boden, L.A.; Moreno-Switt, A.I.; Caipo, M.L.; Zimin-Veselkoff, N.; Alateeqi, A.M.; Baltenweck, I. The COVID-19 pandemic and global food security. Front. Vet. Sci. 2020, 7, 578508. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID-19 and Animals. Available online: https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/animals.html (accessed on 18 January 2021).
- Gurgel Ado, M.; Silva Dos Santos, C.C.; de Souza Alves, K.P.; Araujo, J.M.; Leal, V.S. Government strategies to ensure the human right to adequate and healthy food facing the COVID-19 pandemic in Brazil. Cien Saude Colet. 2020, 25, 4945–4956. [Google Scholar] [CrossRef]
- Nicoletti, C.F.; Esteves, G.P.; Genario, R.; Santo, M.A.; de Cleva, R.; Gualano, B.; Roschel, H. Nutritional inadequacies among post-bariatric patients during COVID-19 quarantine in Sao Paulo, Brazil. Obes. Surg. 2020, 24, 1–5. [Google Scholar]
- Nathan, I.; Benon, M. COVID-19 relief food distribution: Impact and lessons for Uganda. Pan. Afr. Med. J. 2020, 35 (Suppl. 2), 142. [Google Scholar] [CrossRef]
- Elsahoryi, N.; Al-Sayyed, H.; Odeh, M.; McGrattan, A.; Hammad, F. Effect of COVID-19 on food security: A cross-sectional survey. Clin. Nutr. 2020, 40, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nourozi, S.; Acharya, L.; Thapa, S. Estimating the potential effects of COVID-19 pandemic on food commodity prices and nutrition security in Nepal. J. Nutr. Sci. 2020, 9, e51. [Google Scholar] [CrossRef] [PubMed]
- Soldavini, J.; Andrew, H.; Berner, M. Characteristics associated with changes in food security status among college students during the COVID-19 pandemic. Transl. Behav. Med. 2021, 11, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Niles, M.T.; Bertmann, F.; Belarmino, E.H.; Wentworth, T.; Biehl, E.; Neff, R. The early food insecurity impacts of COVID-19. Nutrients 2020, 12, 2096. [Google Scholar] [CrossRef] [PubMed]
- Zack, R.M.; Weil, R.; Babbin, M.; Lynn, C.D.; Velez, D.S.; Travis, L.; Taitelbaum, D.J.; Fiechtner, L. An overburdened charitable food system: Making the case for increased government support during COVID-19 crisis. Am. J. Public Health 2021, 111, 804–807. [Google Scholar] [CrossRef]
- Bertrand, L.; Shaw, K.A.; Ko, J.; Deprez, D.; Chilibeck, P.D.; Zello, G.A. The impact of the coronavirus disease 2019 (COVID-19) pandemic on university students’ dietary intake, physical activity, and sedentary behaviour. Appl. Physiol. Nutr. Metab. 2021, 15, 1–8. [Google Scholar]
- Visser, M.; Schaap, L.A.; Wijnhoven, H.A.H. Self-reported impact of the COVID-19 pandemic on nutrition and physical activity behaviour in Dutch older adults living independently. Nutrients 2020, 12, 3708. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, D.; Rešetar, J.; Kljusurić, J.G.; Krbavčić, I.P.; Bender, D.V.; Rodríguez-Pérez, C.; Ruíz-López, M.D.; Šatalić, Z. Cooking at home and adherence to the Mediterranean diet during COVID-19 confinement: The experience from the Croatian COVIDiet Study. Front. Nutr. 2021, 8, 617721. [Google Scholar] [CrossRef]
- Vandevijvere, S.; De Ridder, S.; Drieskens, S.; Charafeddine, R.; Berete, F.; Demarest, S. Food insecurity and its association with changes in nutritional habits among adults during the COVID-19 confinement measures in Belgium. Public Health Nutr. 2020, 9, 1–12. [Google Scholar]
- Huber, B.C.; Steffen, J.; Schichtiger, J.; Brunner, S. Altered nutrition behaviour during COVID-19 pandemic lockdown in young adults. Eur. J. Nutr. 2020, 1, 1–10. [Google Scholar]
- Zarah, A.B.; Enriquez-Marulanda, J.; Andrade, J.M. Relationship between dietary habits, food attitudes and food security status among adults living within the United States three months post-mandated quarantine: A cross-sectional study. Nutrients 2020, 12, 3468. [Google Scholar] [CrossRef] [PubMed]
- Amatori, S.; Zeppa, S.D.; Preti, A.; Gervasi, M.; Gobbi, E.; Ferrini, F.; Rocchi, M.B.L.; Baldari, C.; Perroni, F.; Piccoli, G.; et al. Dietary Habits and Psychological States during COVID-19 Home Isolation in Italian College Students: The Role of Physical Exercise. Nutrients 2020, 12, 3660. [Google Scholar] [CrossRef]
- Galali, Y. The impact of COVID-19 confinement on the eating habits and lifestyle changes: A cross sectional study. Food Sci. Nutr. 2021, 9, 2105–2113. [Google Scholar] [CrossRef]
- Sulejmani, E.; Hyseni, A.; Xhabiri, G.; Rodríguez-Pérez, C. Relationship in dietary habits variations during COVID-19 lockdown in Kosovo: The COVIDiet study. Appetite 2021, 164, 105244. [Google Scholar] [CrossRef] [PubMed]
| High food security: no reported indications of food-access problems or limitations; |
| Marginal food security: one or two reported indications—typically of anxiety over food sufficiency or shortage of food in the house. Little or no indication of changes in diets or food intake; |
| Low food security: reports of reduced quality, variety, or desirability of diet. Little or no indication of reduced food intake; |
| Very low food security: multiple indications of disrupted eating patterns and reduced food intake. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Leyva, D.; Pierce, G.N. The Impact of Nutrition on the COVID-19 Pandemic and the Impact of the COVID-19 Pandemic on Nutrition. Nutrients 2021, 13, 1752. https://doi.org/10.3390/nu13061752
Rodriguez-Leyva D, Pierce GN. The Impact of Nutrition on the COVID-19 Pandemic and the Impact of the COVID-19 Pandemic on Nutrition. Nutrients. 2021; 13(6):1752. https://doi.org/10.3390/nu13061752
Chicago/Turabian StyleRodriguez-Leyva, Delfin, and Grant N. Pierce. 2021. "The Impact of Nutrition on the COVID-19 Pandemic and the Impact of the COVID-19 Pandemic on Nutrition" Nutrients 13, no. 6: 1752. https://doi.org/10.3390/nu13061752






