Insulin Resistance Is Inversely Associated with the Status of Vitamin D in Both Diabetic and Non-Diabetic Populations
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis and Outcome Measures
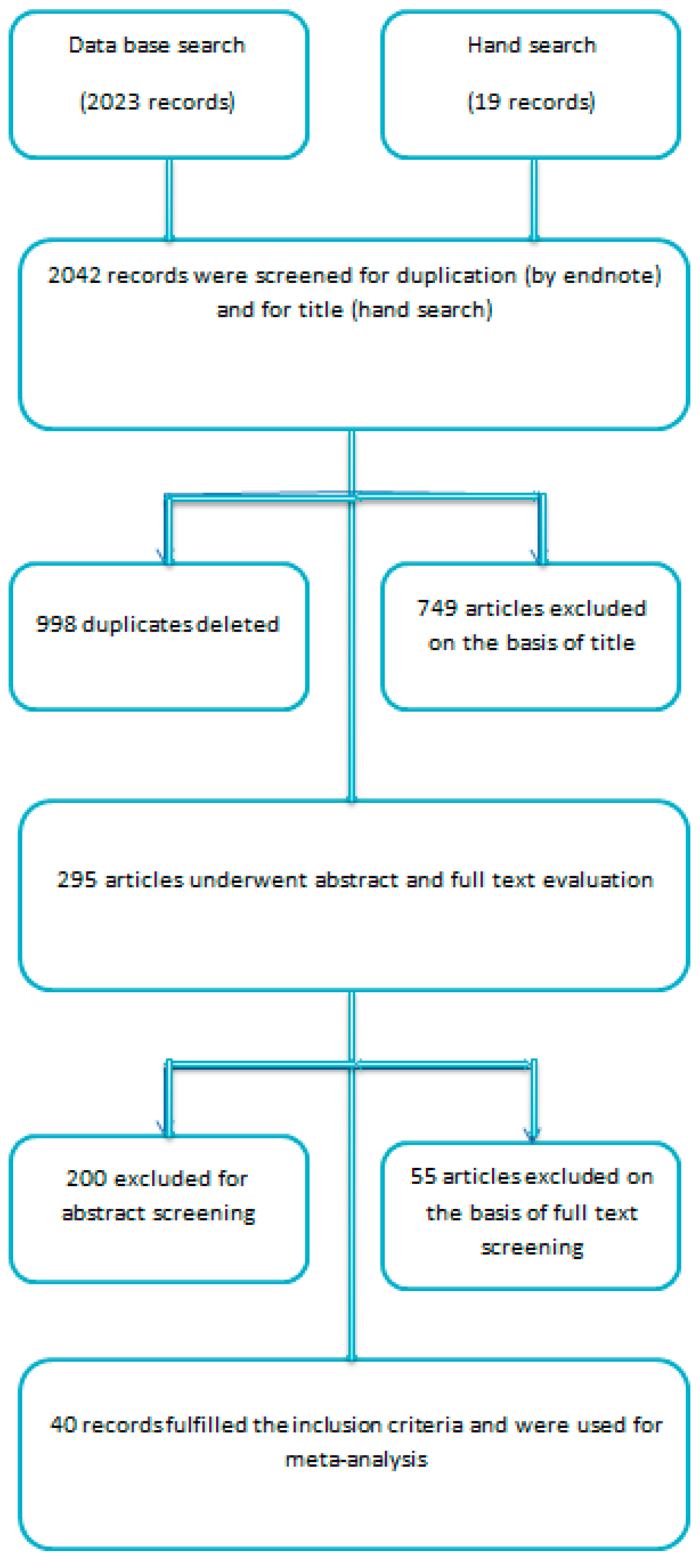
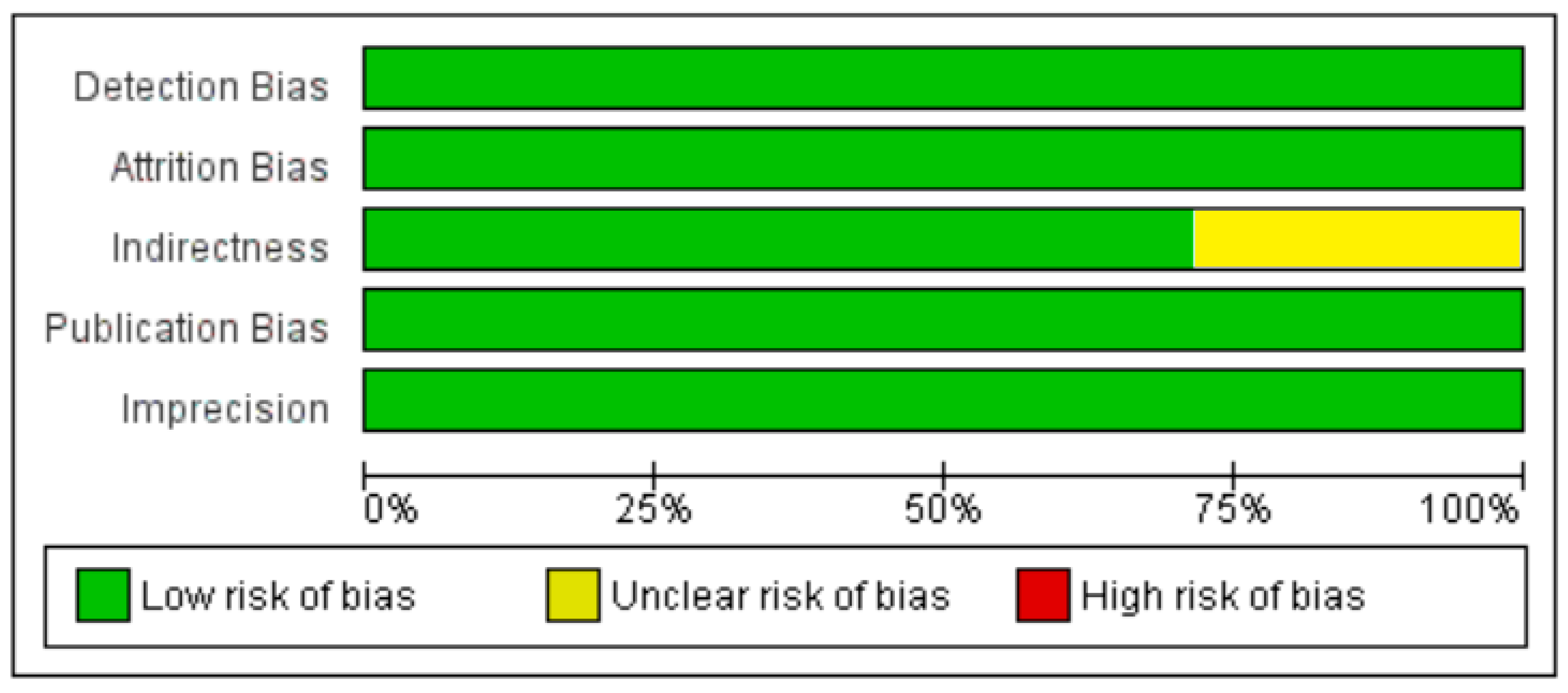
3. Results
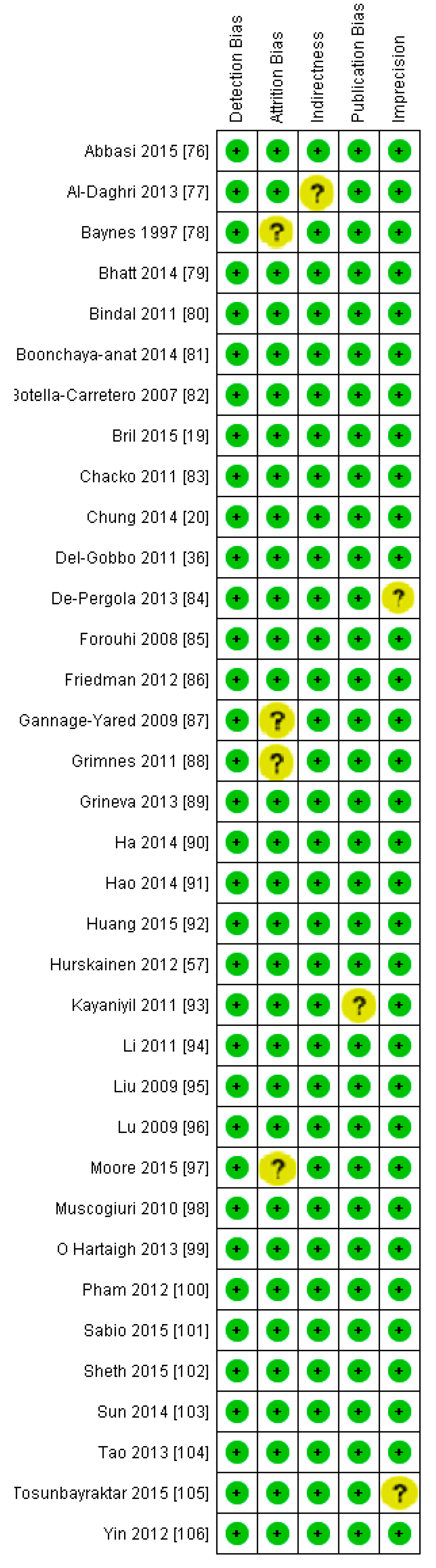
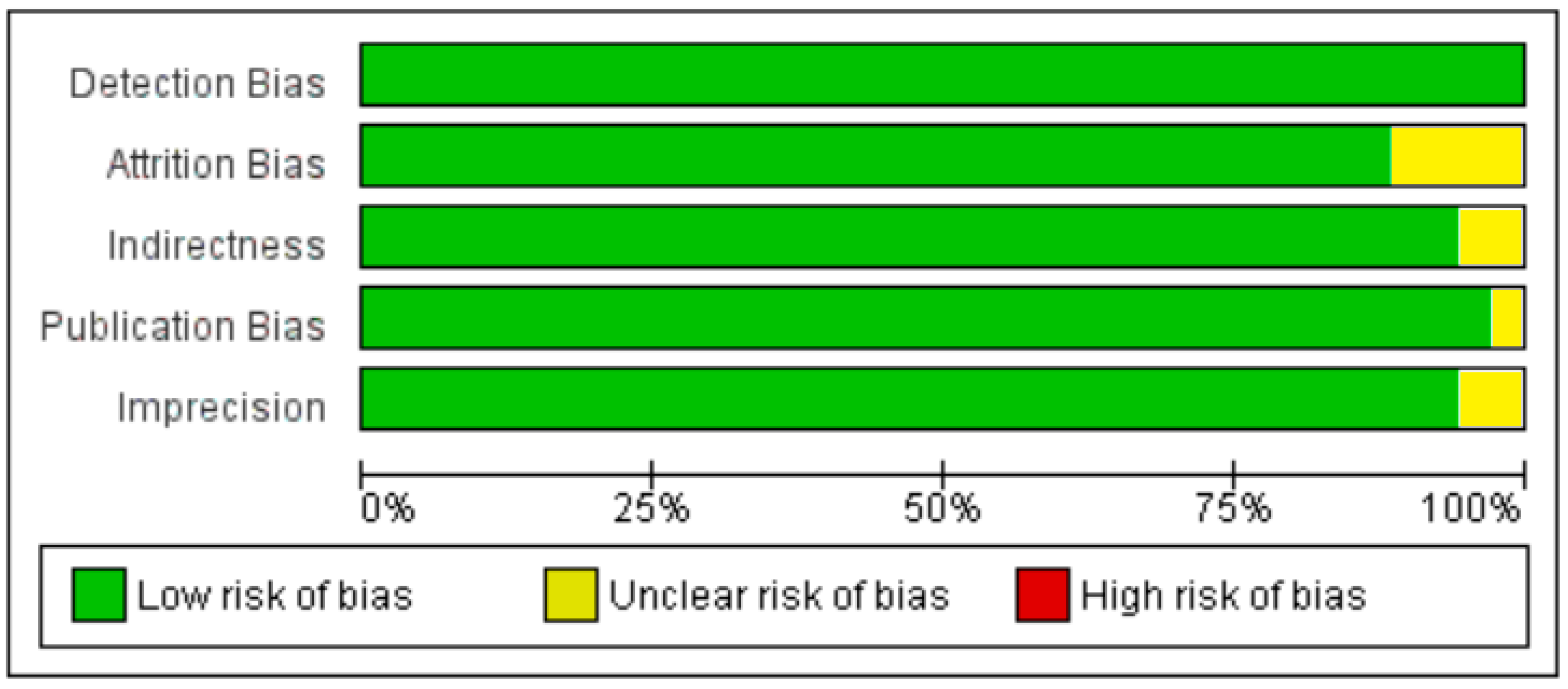
3.1. Excluded Studies on the Basis of Full Text Evaluation
3.2. Included Studies
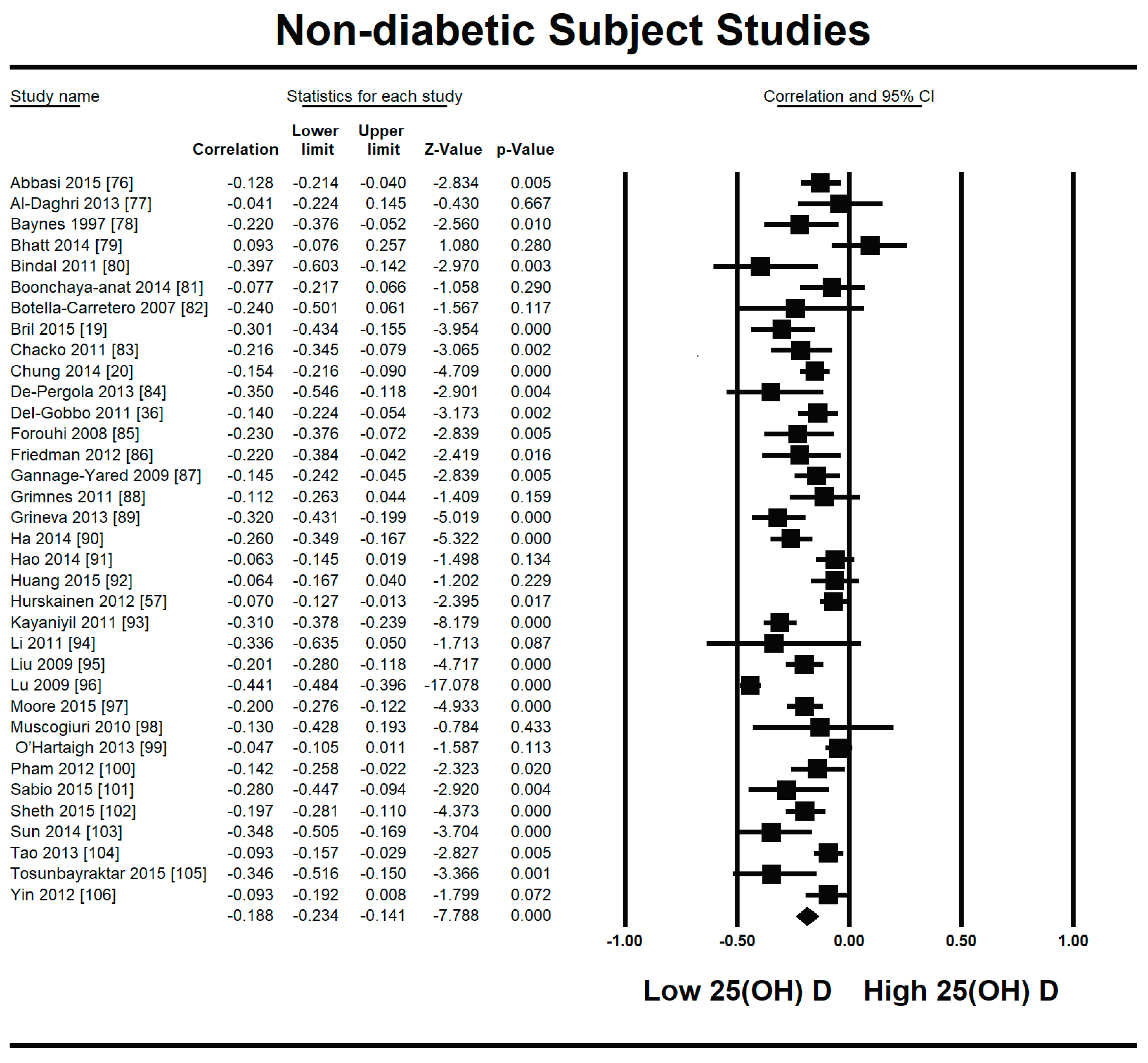
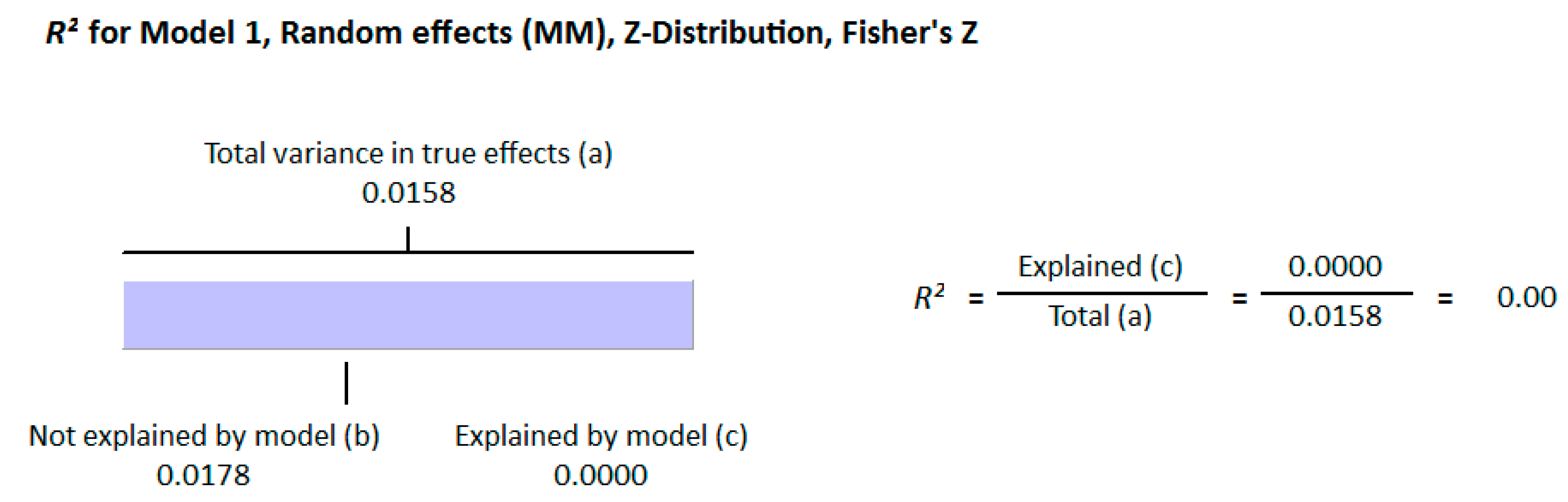
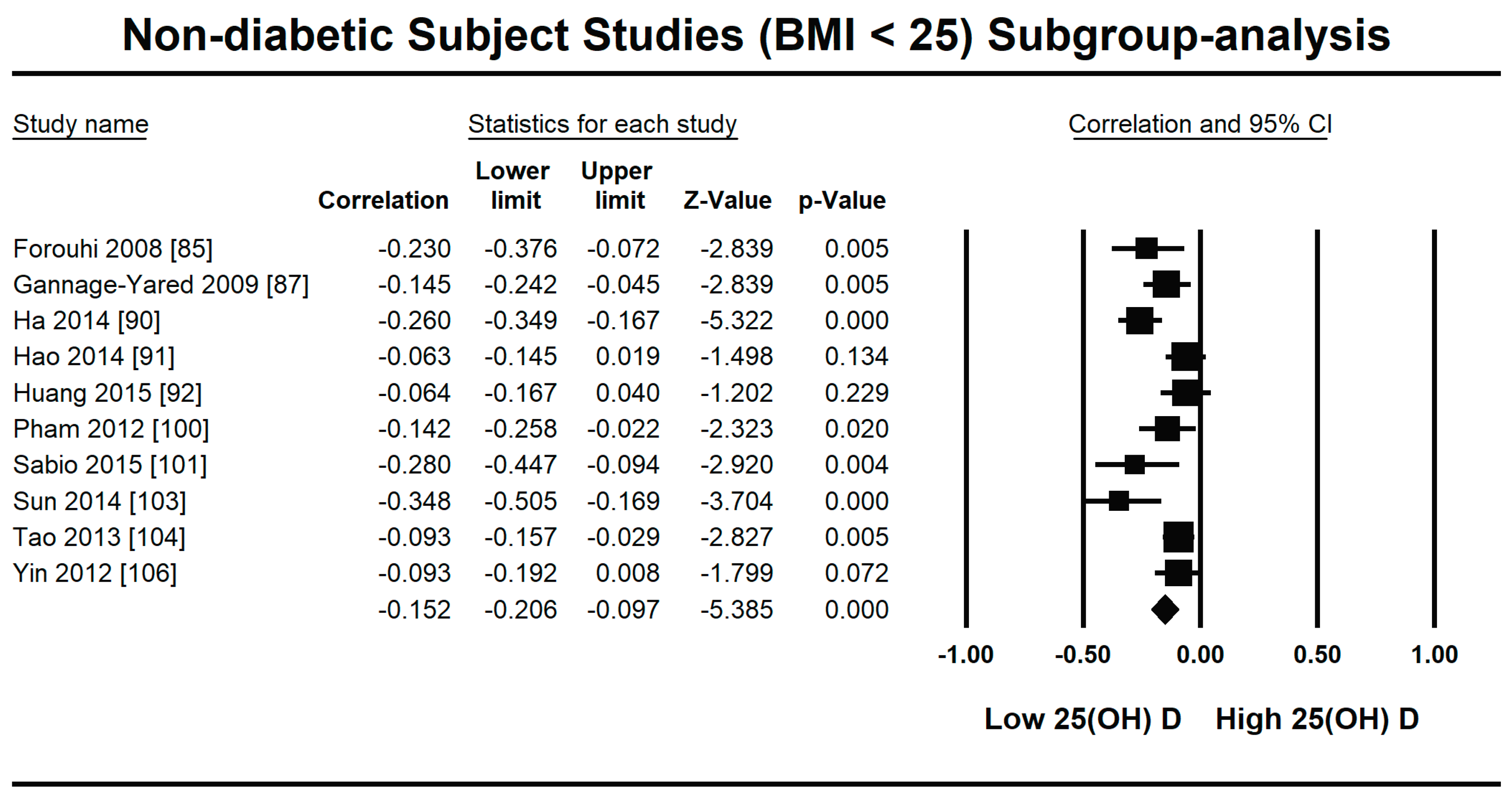
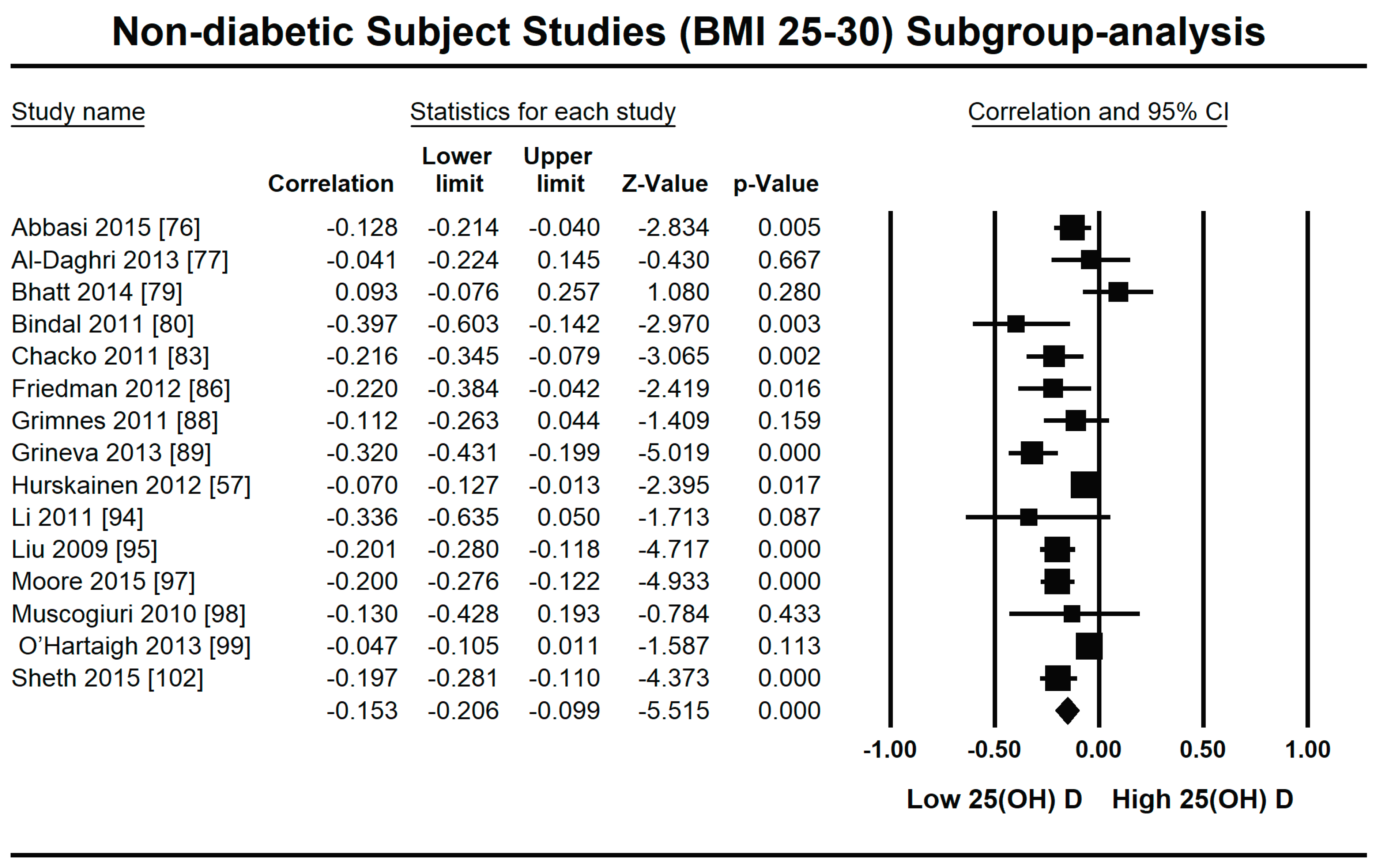
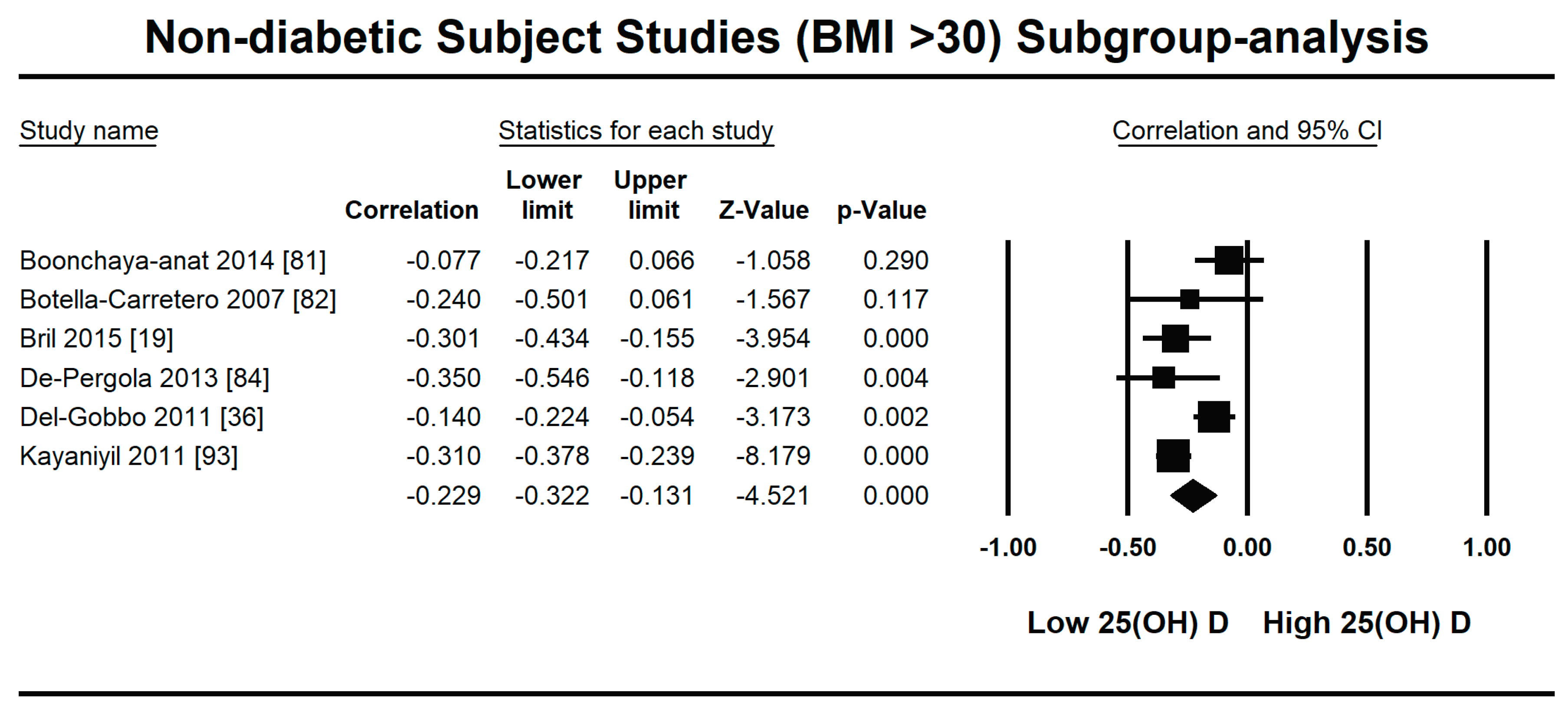
3.2.1. Meta-Analysis and Meta-Regression for Non-Diabetes Patient Studies
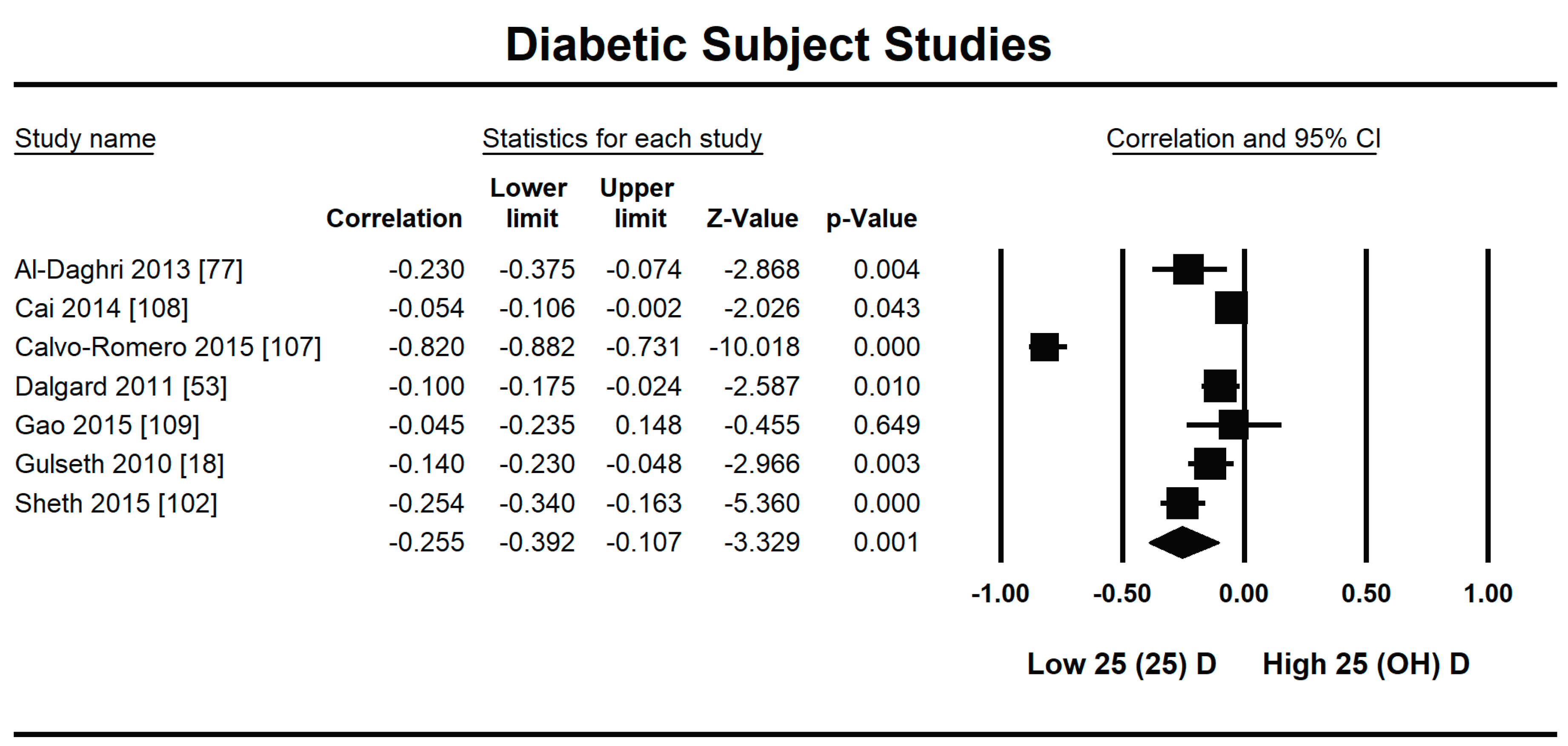
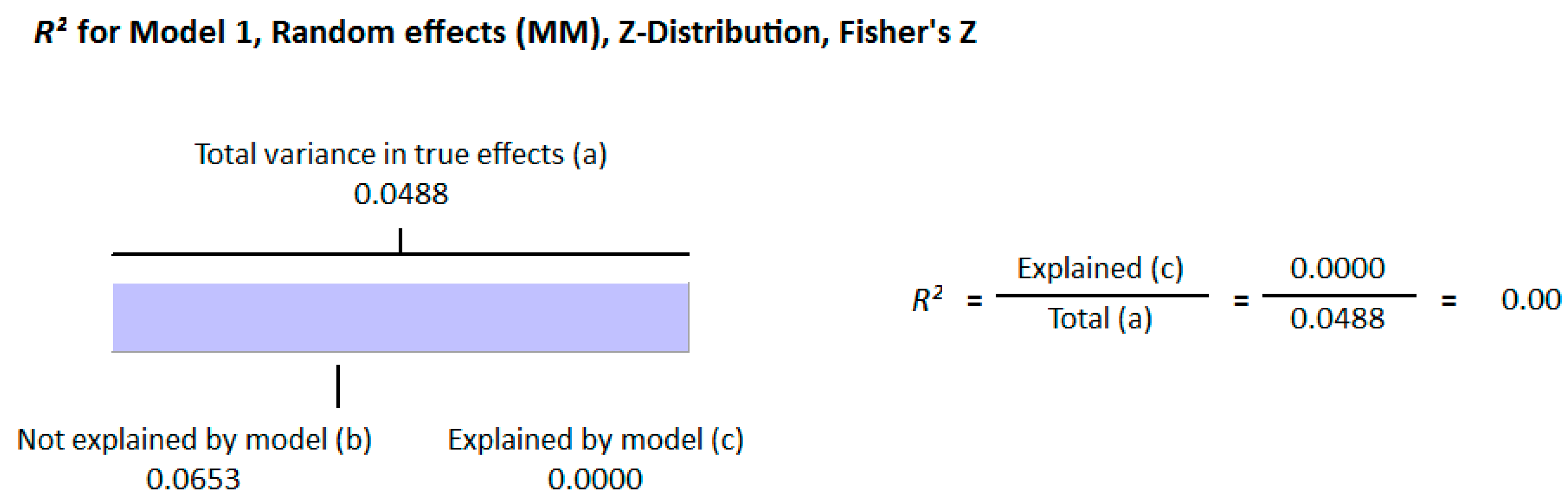
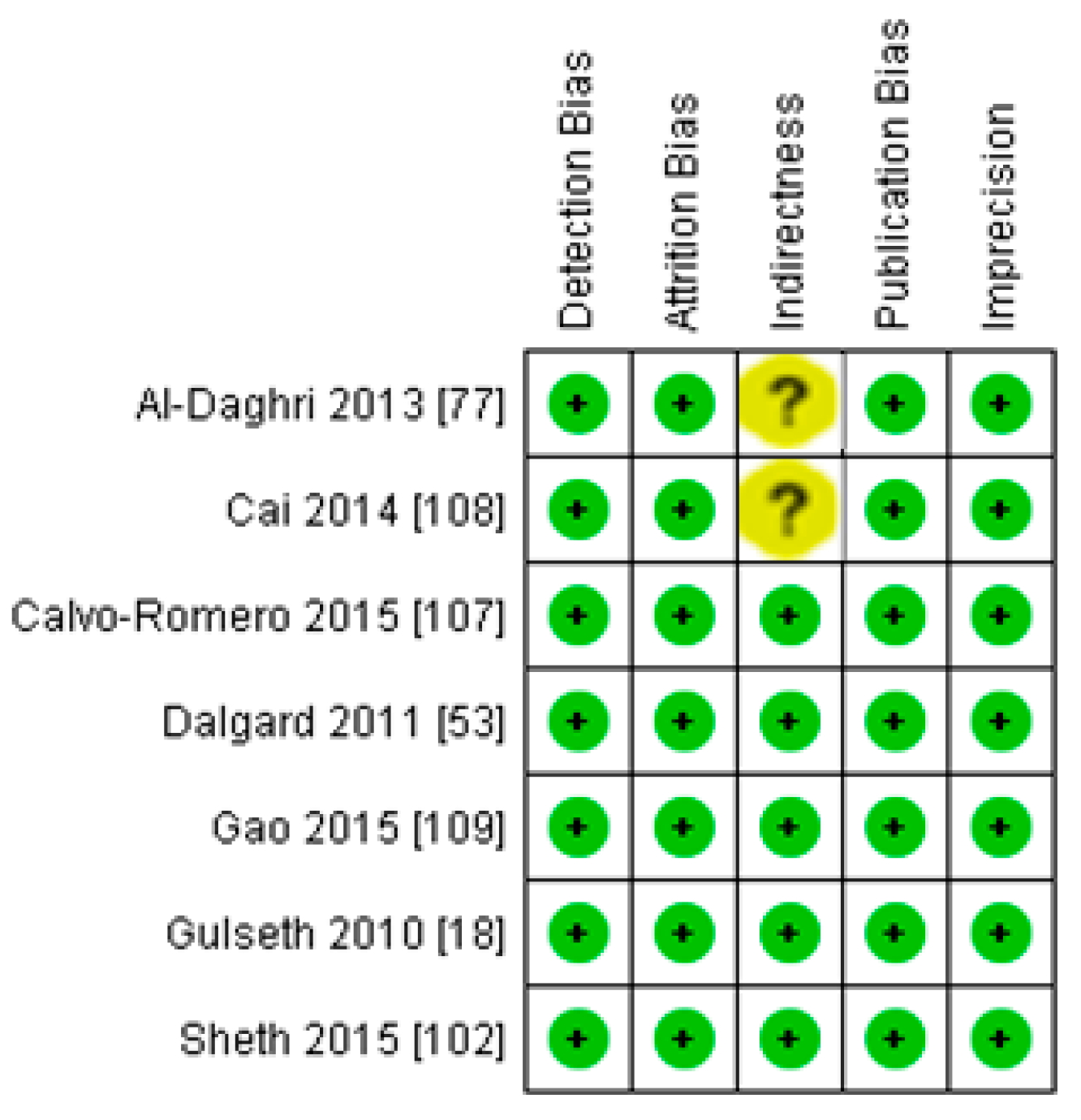
3.2.2. Meta-Analysis and Meta-Regression for Diabetes Patient Studies
4. Discussion
Strengths and Weaknesses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Ravier, M.A.; Rutter, G.A. Glucose or Insulin, but not Zinc Ions, Inhibit Glucagon Secretion from Mouse Pancreatic Alpha-cells. Diabetes 2006, 54, 1789–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, J.R.; Browning, E.T.; Olson, M. Interrelations between Fatty Acid Oxidation and the Control of Gluconeogenesis in Perfused Rat Liver. Adv. Enzym. Regul. 1968, 6, 67–100. [Google Scholar] [CrossRef]
- Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome: Mechanism and Implications for Pathogenesis. Endocr. Rev. 1997, 18, 774–800. [Google Scholar]
- Marchesini, G.; Brizi, M.; Morselli-Labate, A.M.; Bianchi, G.; Bugianesi, E.; McCullough, A.J.; Forlani, G.; Melchionda, N. Association of Nonalcoholic Fatty Liver Disease with Insulin Resistance. Am. J. Med. 1999, 107, 450–455. [Google Scholar] [CrossRef]
- Petersen, K.F.; Oral, E.A.; Dufour, S.; Befroy, D.; Ariyan, C.; Yu, C.; Cline, G.W.; DePaoli, A.M.; Taylor, S.I.; Gorden, P.; et al. Leptin Reverses Insulin Resistance and Hepatic Steatosis in Patients with Severe Lipodystrophy. J. Clin. Investig. 2002, 109, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasuga, M. Insulin Resistance and Pancreatic Beta Cell Failure. J. Clin. Investig. 2006, 116, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.S.; Epstein, S.; Corkey, B.E.; Grant, S.F.A.; Gavin, I.J.R.; Aguilar, R.B.; Herman, M.E. A Unified Pathophysiological Construct of Diabetes and its Complications. Trends Endocrinol. Metab. 2017, 28, 645–655. [Google Scholar] [CrossRef]
- Al Mheid, I.; Patel, R.S.; Tangpricha, V.; Quyyumi, A.A. Vitamin D and Cardiovascular Disease: Is the Evidence Solid? Eur. Heart J. 2013, 34, 3691–3698. [Google Scholar] [CrossRef] [Green Version]
- Tomson, J.; Emberson, J.; Hill, M.; Gordon, A.; Armitage, J.; Shipley, M. Vitamin D and Risk of Death from Vascular and Non-vascular Causes in the Whitehall Study and Meta-analyses of 12,000 Deaths. Eur. Heart J. 2013, 34, 1365–1374. [Google Scholar] [CrossRef]
- Seo, J.A.; Eun, C.R.; Cho, H.; Lee, S.K.; Yoo, H.J.; Kim, S.G. Low Vitamin D Status is Associated with Non-alcoholic Fatty Liver Disease Independent of Visceral Obesity in Korean Adults. PLoS ONE 2013, 8, e75197. [Google Scholar] [CrossRef] [Green Version]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nistico, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef]
- Chiu, K.C.; Chu, A.; Go, V.L.W.; Saad, M.F. Hypovitaminosis D is Associated with Insulin Resistance and Cell Dysfunction. Am. J. Clin. Nutr. 2004, 79, 820–825. [Google Scholar] [CrossRef] [Green Version]
- Pittas, A.G.; Lau, J.; Hu, F.B. Dawson-Hughes, B. The Role of Vitamin D and Calcium in Type 2 Diabetes. A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef]
- Kositsawat, J.; Freeman, V.; Gebber, B.; Geraci, S. Association of A1c Levels with Vitamin D Status in U.S. Adults. Diabetes Care 2010, 33, 1236–1238. [Google Scholar] [CrossRef] [Green Version]
- Hypponen, E.; Power, C. Vitamin D Status and Glucose Homeostasis in the 1958 British Bird Cohort: The role of obesity. Diabetes Care 2006, 29, 2244–2246. [Google Scholar] [CrossRef] [Green Version]
- Gulseth, H.L.; Gjelstad, I.M.F.; Tierney, A.C.; Lovengrove, J.A.; Defoort, C.; Blaak, E.E.; Lopez-Miranda, J.; Kiec-Wilk, B.; Ris, U.; Roshe, H.; et al. Serum Vitamin D Concentration Does Not Predict Insulin Action or Secretion in European Subjects with the Metabolic Syndrome. Diabetes Care 2010, 33, 923–925. [Google Scholar] [CrossRef] [Green Version]
- Bril, F.; Maximos, M.; Portillo-Sanchez, P.; Biernacki, D.; Lomonaco, R.; Subbarayan, S.; Correa, M.; Lo, M.; Suman, A.; Cusi, K. Relationship of Vitamin D with Insulin Resistance and Disease Severity in Non-alcoholic Steatohepatitis. J. Hepatol. 2015, 62, 405–411. [Google Scholar] [CrossRef]
- Chung, S.J.; Lee, Y.A.; Hong, H.; Kang, M.J.; Kwon, H.J.; Shin, C.H.; Yang, S.W. Inverse Relationship between Vitamin D Status and Insulin Resistance and the Risk of Impaired Fasting Glucose in Korean Children and Adolescents: The Korean National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Public Health Nutr. 2014, 17, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.A.; Ashraf, A. Role of Vitamin D in Insulin Secretion and Insulin Sensitivity for Glucose Homeostasis. Int. J. Endocrinol. 2010. [Google Scholar] [CrossRef] [Green Version]
- Dutta, D.; Maisnam, I.; Shrivastava, A.; Sinha, A.; Ghosh, S.; Mukhopadhyay, P.; Mukhopadhyay, S.; Chowdhury, S. Serum Vitamin-D Predicts Insulin Resistance in Individuals with Prediabetes. Indian J. Med. Res. 2013, 138, 853–860. [Google Scholar]
- Kabadi, S.M.; Lee, B.K.; Liu, L. Joint Effects of Obesity and Vitamin D Insufficiency on Insulin Resistance and Type 2 Diabetes: Results from the NHANES 2001–2006. Diabetes Care 2012, 35, 2048–2054. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Lim, J.; Kye, S.; Joung, H. Association between Vitamin D Status and Metabolic Syndrome Risk among Korean Population: Based on the Korean National Health and Nutrition Examination Survey IV-2, 2008. Diabetes Res. Clin. Pract. 2012, 96, 230–236. [Google Scholar] [CrossRef]
- Kobzaa, V.M.; Feet, J.C.; Zhoua, J.; Conley, T.B.; Peacock, M.; Reger, H.B.I.; Palmad, G.D.; Campbell, W.W. Vitamin D Status and Resistance Exercise Trainingindependently Affect Glucose Tolerance in Older Adults. Nutr. Res. 2013, 33, 349–357. [Google Scholar] [CrossRef]
- Liu, J.; Tan, J.; Jeynes, B. Serum 25(OH) Vitamin D Level, Femur Length, and Risk of Type 2 Diabetes among Adults. Appl. Physiol. Nutr. Metab. 2013, 6, 264–270. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Li, X.; Elli, E.F.; Ayloo, S.M.; Castellanos, K.J.; Fantuzzi, G.; Freels, S.; Braunschweig, C.L. Vitamin D, Inflammation, and Relations to Insulin Resistance in Premenopausal Women with Morbid Obesity. Obesity 2015, 23, 1591–1597. [Google Scholar] [CrossRef] [Green Version]
- Pannu, P.K.; Piers, L.S.; Soares, M.J.; Zhao, Y.; Ansari, Z. Vitamin D Status is Inversely Associated with Markers of Risk for Type 2 Diabetes: A Population Based Study in Victoria, Australia. PLoS ONE 2017, 12, e0178825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scragg, R.; Sowers, M.R.; Bell, C. Serum 25-Hydroxyvitamin D, Diabetes, and Ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004, 27, 2813–2818. [Google Scholar] [CrossRef] [Green Version]
- Weiler, H.A.; Lowea, J.; Krahnb, J.; William, D. LesliecOsteocalcin and Vitamin D Status are Inversely Associated with Homeostatic Model Assessment of Insulin Resistance in Canadian Aboriginal and White Women: The First Nations Bone Health Study. J. Nutr. Biochem. 2013, 24, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Alkharfy, K.M.; Al-Daghri, N.M.; Sabico, S.B.; Al-Othman, A.; Moharram, O.; Alokail, M.S.; Al-Saleh, Y.; Kumar, S.; Chrousos, G.P. Vitamin D Supplementation in Patients with Diabetes Mellitus Type 2 on Different Therapeutic Regimens: A One-year Prospective Study. Cardiovasc. Diabetol. 2013, 12, 113. [Google Scholar] [CrossRef] [Green Version]
- Al-Shoumer, K.A.; Al-Asoosi, A.A.; Ali, A.H.; Nair, V.S. Does Insulin Resistance in Type 2 Diabetes Alter Vitamin D Status? Prim. Care Diabetes 2013, 7, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Gedik, O.; Akalin, S. Effects of Vitamin D Deficiency and Repletion on Insulin and Glucagon Secretion in Man. Diabetologia 1986, 29, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Bardini, G.; Giannini, S.; Romano, D.; Rotella, C.M.; Mannucci, E. Lipid Accumulation Product and 25-OH-Vitamin D Deficiency in Type 2 Diabetes. Rev. Diabet. Stud. 2013, 10, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Nimitphong, H.; Chailurkit, L.; Chanprasertyothin, S.; Sritara, P.; Ongphiphadhanakul, B. The Association of Vitamin D Status and Fasting Glucose According to Body Fat Mass in Young Healthy Thais. Endocr. Disord. 2013, 13, 60. [Google Scholar] [CrossRef] [Green Version]
- Del-Gobbo, L.C.; Song, Y.; Dannenbaum, D.A.; Dewailly, E.; Egeland, G.M. Serum 25-Hydroxyvitamin D Is not Associated with Insulin Resistance or Beta Cell Function in Canadian Cree. J. Nutr. 2011, 141, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Diaz, G.M.; Gonza, L.; Ramos-Trautmann, G.; Marie, C.; Palacios, C. Vitamin D Status Is Associated with Metabolic Syndrome in a Clinic-Based Sample of Hispanic Adults. Metab. Syndr. Relat. Disord. 2016, 14, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Hidayat, R.; Setiati, S.; Soewondo, P. The Association Between Vitamin D Deficiency and Type 2 Diabetes Mellitus in Elderly Patients. Age 2010, 42, 123–129. [Google Scholar]
- Hirani, V.; Cumming, R.G.; Le Couteur, D.G.; Naganathan, V.; Blyth, F.; Handelsman, D.J.; Waite, L.M.; Seibel, M.J. Low Levels of 25-Hydroxy Vitamin D and Active 1,25-Dihydroxyvitamin D Independently Associated with Type 2 Diabetes Mellitus in Older Australian Men: The Concord Health and Ageing in Men Project. J. Am. Geriatr. Soc. 2014, 62, 1741–1747. [Google Scholar] [CrossRef]
- Husemoen, L.L.; Thuesen, B.H.; Fenger, M.; Jorgensen, T.; Glumer, C.; Svensson, J.; Ovesen, L.; Witte, D.R.; Linneberg, A. Serum 25(OH)D and Type 2 Diabetes Association in a General Population: A Prospective Study. Diabetes Care 2012, 35, 1695–1700. [Google Scholar] [CrossRef] [Green Version]
- Justice, J.N.; Pierpoint, L.A.; Mani, D.; Schwartz, R.S.; Enoka, R.M. Motor Function is Associated with 1,25(OH)2D and Indices of Insulin–glucose Dynamics in Non-diabetic Older Adults. Aging Clin. Exp. Res. 2014, 26, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Kabadi, S.M.; Liu, L.; Auchincloss, A.H.; Zakeri, I.F. Multivariate Path Analysis of Serum 25-hydroxyvitamin D Concentration, Inflammation, and Risk of Type 2 Diabetes Mellitus. Dis. Mark. 2013, 35, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Khor, G.L.; Chee, W.S.S.; Shariff, Z.M.; Poh, B.K.; Arumugam, M.; Rahman, J.A.; Theobaldb, H.E. High Prevalence of Vitamin D Insufficiency and Its Association with BMI-for-age among Primary School Children in Kuala Lumpur, Malaysia. BMC Public Health 2011, 1, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.; Park, S.; Kim, Y. Age- and Gender-specific Associations between Low Serum 25-hydroxyvitamin D Level and Type 2 Diabetes in the Korean General Population: Analysis of 2008–2009 Korean National Health and Nutrition Examination Survey data. Asia Pac. J. Clin. Nutr. 2012, 21, 536–546. [Google Scholar]
- Li, L.; Yin, X.; Yao, C.; Zhu, X.; Wu, X. Serum 25-hydroxyvitamin D, Parathyroid Hormone, and Their Association with Metabolic Syndrome in Chinese. Endocrine 2013, 44, 465–472. [Google Scholar] [CrossRef]
- Lu, L.; Wu, Y.; Qi, Q.; Liu, C.; Gan, W. Associations of Type 2 Diabetes with Common Variants in PPARD and the Modifying Effect of Vitamin D among Middle-Aged and Elderly Chinese. PLoS ONE 2012, 7, e34895. [Google Scholar] [CrossRef] [Green Version]
- Marques-Vidal, P.; Vollenweider, P.; Guessous, I.; Henry, H.; Boulat, O.; Waeber, G.; Jornayvaz, F.R. Serum Vitamin D Concentrations Are Not Associated with Insulin Resistance in Swiss Adults. J. Nutr. 2015, 145, 2117–2122. [Google Scholar] [CrossRef] [Green Version]
- Tsur, A.; Feldman, B.S.; Feldhammer, I.; Hoshen, M.B.; Leibowitz, G.; Balicer, R.D. Decreased Serum Concentrations of 25-hydroxycholecalciferol are Associated with Increased Risk of Progression to Impaired Fasting Glucose and Diabetes. Diabetes Care 2013, 36, 1361–1367. [Google Scholar] [CrossRef] [Green Version]
- Wright, O.R.L.; Hickman, I.J.; Petchey, W.G.; Sullivan, C.M.; Ong, C.; Rose, F.J.; Ng, C.; Prins, J.B.; Whitehead, J.P.; Moore-Sullivan, T.M. The Effect of 25-hydroxyvitamin D on Insulin Sensitivity in Obesity: Is It Mediated via Adiponectin? Can. J. Physiol. Pharmacol. 2013, 91, 496–501. [Google Scholar] [CrossRef]
- Wright, C.S.; Weinheimer-Haus, E.M.; Fleet, J.C.; Peacock, M.; Campbell, W.W. The Apparent Relation between Plasma 25-Hydroxyvitamin D and Insulin Resistance Is Largely Attributable to Central Adiposity in Overweight and Obese Adults. J. Nutr. 2015, 145, 2683–2689. [Google Scholar] [CrossRef] [Green Version]
- Zoppini, G.; Galletti, A.; Targher, G.; Brangani, C.; Pichiri, I.; Negri, C.; Stoico, V.; Cacciatori, V.; Bonora, E. Glycated Haemoglobin Is Inversely Related to Serum Vitamin D Levels in Type 2 Diabetic Patients. PLoS ONE 2013, 8, e82733. [Google Scholar] [CrossRef]
- Bellan, M.; Guzzaloni, G.; Rinaldi, M.; Merlotti, E.; Ferrari, C.; Tagliaferri, A.; Pirisi, M.; Aimaretti, G.; Scacchi, M.; Marzullo, P. Altered Glucose Metabolism Rather Than Naïve Type 2 Diabetes Mellitus (T2DM) is Related to Vitamin D Status in Severe Obesity. Cardiovasc. Diabetol. 2014, 13, 57. [Google Scholar] [CrossRef] [Green Version]
- Dalgard, C.; Skaalum, M.; Weihe, P.P.; Grandjean, P. Vitamin D Status in Relation to Glucose Metabolism and Type 2 Diabetes in Septuagenarians. Diabetes Care 2011, 34, 1284–1288. [Google Scholar] [CrossRef] [Green Version]
- Heras, J.D.L.; Rajkumar, K.; Lee, S.; Bacha, F.; Holick, M.F.; Arslanian, S.A. 25-Hydroxyvitamin D in Obese Youth Across theSpectrumofGlucose Tolerance from Normal to Prediabetes to Type 2 Diabetes. Diabetes Care 2013, 36, 2048–2053. [Google Scholar] [CrossRef] [Green Version]
- Esteghamatia, A.; Aryanab, B.; Esteghamatia, A.; Nakhjavania, M. Differences in Vitamin D Concentration between Metabolically Healthy Andunhealthy Obese Adults: Associations with Inflammatory and Cardiometabolicmarkers in 4391 Subjects. Diabetes Metab. 2014, 40, 347–355. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Wang, M.; Ning, H.; Lima, A.; Li, Y.; Sun, C. Lipoprotein lipase links vitamin D, insulin resistance, and type 2 diabetes: A cross-sectional epidemiological study. Cardiovasc. Diabetol. 2013, 12, 17. [Google Scholar] [CrossRef] [Green Version]
- Hurskainen, A.R.; Virtanen, J.K.; Tuomainen, T.P.; Nurmi, T.; Voutilainen, S. Association of Serum 25-hydroxyvitamin D with Type 2 Diabetes and Markers of Insulin Resistance in a General Older Population in Finland. Diabetes Metab. Res. Rev. 2012, 28, 418–423. [Google Scholar] [CrossRef]
- Jiang, H.; Peng, S. The Relationship between Serum Vitamin D and HOMA-IR in Overweight Elderly Patients. Int. J. Cardiol. 2014, 177, 1100–1102. [Google Scholar] [CrossRef]
- Park, H.Y.; Lim, Y.; Kim, J.H.; Bae, S.; Oh, S.; Hong, Y. Association of Serum 25-Hydroxyvitamin D Levels with Markers for Metabolic Syndrome in the Elderly: A Repeated Measure Analysis. J. Korean Med. Sci. 2012, 27, 653–660. [Google Scholar] [CrossRef]
- Kavadar, G.; Demircioglu, D.T.; Ozgonenel, L.; Emre, T.Y. The Relationship between Vitamin D Status, Physical Activity and Insulin Resistance in Overweight and Obese Subjects. Bosn. J. Basic Med. Sci. 2015, 15, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Kim, J. Association between Serum Vitamin D, Parathyroid Hormone and Metabolic Syndrome in Middle-aged and Older Korean Adults. Eur. J. Clin. Nutr. 2015, 69, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kang, M.I.; Oh, K.W.; Kwon, H.S.; Lee, J.H.; Lee, W.C.; Yoon, K.; Ho, Y. The Association of Serum Vitamin D Level with Presence of Metabolic Syndrome and Hypertension in Middle-aged Korean Subjects. Clin. Endocrinol. 2010, 73, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, M.J.; Choi, S.H.; Shin, C.S.; Park, K.S.; Jang, H.C.; Billings, L.K.; Meigs, J.B. Association of Vitamin D Deficiency with Incidence of Type 2 Diabetes in High-risk Asian Subjects. Am. J. Clin. Nutr. 2013, 97, 524–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morisset, A.; Tardio, V.; Weisnagel, J.; Lemieux, S.; Bergeron, J.; Gagnon, C. Associations Between Serum 25-Hydroxyvitamin D, Insulin Sensitivity, Insulin Secretion, and B-Cell Function According to Glucose Tolerance Status. Metab. Syndr. Relat. Disord. 2015, 13, 208–214. [Google Scholar] [CrossRef]
- Nielsen, N.O.; Bjerregaard, P.; Rønn, P.F.; Friis, H.; Andersen, S.; Melbye, M. Associations between Vitamin D Status and Type 2 Diabetes Measures among Inuit in Greenland May Be Affected by Other Factors. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Pinelli, N.R.; Jaber, L.A.; Brown, M.B.; Herman, W.H. 3Serum 25-Hydroxy Vitamin D and Insulin Resistance, Metabolic Syndrome, and Glucose Intolerance Among Arab Americans. Diabetes Care 2010, 33, 373–1375. [Google Scholar] [CrossRef] [Green Version]
- Rhee, S.Y.; Hwang, Y.C.; Chung, H.Y.; Woo, J.T. Vitamin D and Diabetes in Koreans: Analyses Based on the Fourth Korea National Health and Nutrition Examination Survey (KNHANES), 2008–2009. Diabetic Med. 2012, 1003–1010. [Google Scholar] [CrossRef] [Green Version]
- Esteghamati, A.; Aryan, Z.; Esteghamati, A.R.; Nakhjavani, M. Vitamin D Deficiency is Associated with Insulin Resistance in Nondiabetics and Reduced Insulin Production in Type 2 Diabetics. Horm. Metab. Res. 2015, 47, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Al-Daghri, N.M.; Al-Attas, O.S.; Al-Okail, M.S.; Alkharfy, K.M.; Al-Yousef, M.A.; Nadhrah, H.M.; Sabico, S.B.; Chrousos, G.P. Severe Hypovitaminosis D is Widespread and more Common in Non-diabetics Than Diabetics in Saudi Adults. Saudi Med. J. 2010, 31, 775–780. [Google Scholar]
- Eraslan, S.; Kizilgul, M.; Uzunlulu, M.; Colak, Y.; Ozturk, O.; Tuncer, I. Frequency of Metabolic Syndrome and 25-hydroxyvitamin D3 Levels in Patients with Non-alcoholic Fatty Liver Disease. Minerva Med. 2013, 104, 447–453. [Google Scholar]
- Hutchinson, M.S.; Figenschau, Y.; Almås, B.; Njølstad, I.; Jorde, R. Serum 25-hydroxyvitamin D Levels in Subjects with Reduced Glucose Tolerance and Type 2 Diabetes–The Tromsø OGTT-study. Int. J Vitam. Nutr. Res. 2011, 81, 317–327. [Google Scholar] [CrossRef]
- Imura, H.; Seino, Y.; Ishida, H. Osteopenia and Circulating Levels of Vitamin D Metabolites in Diabetes Mellitus. J. Nutr. Sci. Vitaminol. 1985, 31, 27–32. [Google Scholar] [CrossRef]
- Nomata, S.; Kadowaki, S.; Yamatani, T.; Fukase, M.; Fujita, T. Effect of 1 Alpha (OH)-vitamin D3 on Insulin Secretion in Diabetes Mellitus. Bone Miner. 1986, 1, 187–192. [Google Scholar]
- Mhatre, M.; Hall, M. Student Forum: Does Calcium and Vitamin D Intake Affect Incidence of Type 2 Diabetes Mellitus and Insulin Resistance Syndrome? Consult. Pharm. 2010, 25, 379–381. [Google Scholar] [CrossRef]
- Mirzaei, K.; Hossein-Nezhad, A.; Keshavarz, S.A.; Eshaghi, S.M.; Koohdani, F.; Saboor-Yaraghi, A.A.; Hosseini, S.; Tootee, A.; Djalali, M. Insulin Resistance via Modification of PGC1α Function Identifying a Possible Preventive Role of Vitamin D Analogues in Chronic Inflammatory State of Obesity. A Double Blind Clinical Trial Study. Minerva Med. 2014, 105, 63–78. [Google Scholar]
- Abbasi, F.; Blasey, C.; Feldman, D.; Caulfield, M.P.; Hantash, F.M.; Reaven, G.M. Low Circulating 25-Hydroxyvitamin D Concentrations Are Associated with Defects in Insulin Action and Insulin Secretion in Persons with Prediabetes. J. Nutr. 2015, 145, 714–719. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Al-Othman, A.; Draz, H.M.; Yakout, S.M.; Al-Saleh, Y.; Al-Yousef, M.; Sabico, S. Hypovitaminosis D Associations with Adverse Metabolic Parameters are Accentuated in Patients with Type 2 Diabetes Mellitus: A Body Mass Index-independent Role of Adiponectin? J. Endocrinol. Investig. 2013, 36, 1–6. [Google Scholar]
- Baynes, K.C.R.; Boucher, B.J.; Feskens, E.J.M.; Kromhout, D. Vitamin D Glucose Tolerance and Insulinaemia in Elderly Men. Diabetologia 1997, 40, 344–347. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, S.P.; Misra, A.; Sharma, M.; Guleria, R.; Pandey, R.M.; Luthra, K.; Vikram, N.K. Vitamin D Insufficiency is Associated with Abdominal Obesity in Urban Asian Indians without Diabetes in North India. Diabetes Technol. Ther. 2014, 16, 392–396. [Google Scholar] [CrossRef]
- Bindal, M.E.; Taskapan, H. Hypovitaminosis D and Insulin Resistance in Peritoneal Dialysis Patients. Int. Urol. Nephrol. 2011, 43, 527–534. [Google Scholar] [CrossRef]
- Boonchaya-anant, P.; Holick, F.M.; Caroline, M. ApovianSerum 25-Hydroxyvitamin D Levels and Metabolic Health Status in Extremely Obese Individuals. Obesity 2014, 22, 2539–2543. [Google Scholar] [PubMed] [Green Version]
- Botella-Carretero, J.I.; Alvarez-Blasco, F.; Villafruela, J.J.; Balsa, J.A.; Vazquez, C.; Escobar-Morreale, H.F. Vitamin D Deficiency is Associated with the Metabolic Syndrome in Morbid Obesity. Clin. Nutr. 2007, 26, 573–580. [Google Scholar] [CrossRef]
- Chacko, S.A.; Song, Y.; Manson, J.E.; Horn, L.V.; Eaton, C.; Martin, L.W.; McTiernan, A.; Curb, J.D.; Wylie-Rosett, J.; Phillips, L.S. Serum 25-hydroxyvitamin D Concentrations in Relation to Cardiometabolic Risk Factors and Metabolic Syndrome in Postmenopausal Women. Am. J. Clin. Nutr. 2011, 94, 209–217. [Google Scholar] [CrossRef] [PubMed]
- De-Pergola, G.; Nitti, A.; Bartolomeo, N.; Gesuita, A.; Giagulli, V.A.; Triggiani, V.; Guastamacchia, E.; Silvestris, F. Possible Role of Hyperinsulinemia and Insulin Resistance in Lower Vitamin D Levels in Overweight and Obese Patients. BioMed Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Luan, J.; Cooper, A.; Boucher, B.J.; Wareham, N.J. Baseline Serum 25-hydroxy Vitamin D is Predictive of Future Glycemic Status and Insulin Resistance. Diabetes 2008, 57, 2619–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, D.J.; Bhatt, N.; Hayman, N.S.; Nichols, B.J.; Herman, M.; Nikolaev, N.; Danziger, J. Impact of Activated Vitamin D on Insulin Resistance in Nondiabetic Chronic Kidney Disease Patients. Clin. Endocrinol. 2012, 77, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Gannage-Yared, M.L.; Chedid, R.; Khalife, S.; Azzi, E.; Zoghbi, F.; Halaby, G. Vitamin D in Relation to Etabolic Risk Factors, Insulin Sensitivity and Adiponectin in a Young Middle-Eastern Population. Eur. J. Endocrinol. 2009, 160, 965–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimnes, G.; Figenschau, Y.; Almås, B.; Jorde, R. Vitamin D, Insulin Secretion, Sensitivity, and Lipids Results from a Case-Control Study and a Randomized Controlled Trial Using Hyperglycemic Clamp Technique. Diabetes 2011, 60, 2748–2757. [Google Scholar] [CrossRef] [Green Version]
- Grineva, E.N.; Karonova, T.; Micheeva, E.; Belyaeva, O.; Nikitina, I.L. Vitamin D Deficiency is a Risk Factor for Obesity and Diabetes Type 2 in Women at Late Reproductive Age. Aging 2013, 5, 575–581. [Google Scholar]
- Ha, C.; Han, T.; Lee, S.; Cho, J.; Kang, H. Association between Serum Vitamin D Status and Metabolic Syndrome in Korean Young Men. Epidemiology 2014, 46, 513–519. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, X.; Shen, Y.; Ni, J.; Luo, Y.; Xiao, Y.; Bao, Y.; Jia, W. Associations of Serum 25-Hydroxyvitamin D3 Levels with Visceral Adipose Tissue in Chinese Men with Normal Glucose Tolerance. PLoS ONE 2014, 9, e86773. [Google Scholar] [CrossRef]
- Huang, C.; Chang, H.; Lu, C.; Tseng, F.; Lee, L.; Huang, K. Vitamin D Status and Risk of Metabolic Syndrome among Non-diabetic Young Adults. Clin. Nutr. 2015, 34, 484–489. [Google Scholar] [CrossRef]
- Kayaniyil, S.; Vieth, R.; Harris, S.B.; Retnakaran, R.; Knight, J.A.; Gerstein, H.C.; Perkins, B.A.; Zinman, B.; Hanley, A.J. Association of 25(OH)D and PTH with Metabolic Syndrome and Its Traditional and Nontraditional Components. J. Clin. Endocrinol. Metab. 2011, 96, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Li, H.W.R.; Brereton, R.E.; Anderson, R.A.; Wallace, A.M.; Ho, C.K.M. Vitamin D Deficiency is Common and Associated with Metabolic Risk Factors in Patients with Polycystic Ovary Syndrome. Metab. Clin. Exp. 2011, 60, 1475–1481. [Google Scholar] [CrossRef]
- Liu, E.; Meigs, J.B.; Pittas, A.G.; McKeown, N.M.; Economos, C.D.; Booth, S.L.; Jacques, P.F. Plasma 25-Hydroxyvitamin D Is Associated with Markers of the Insulin Resistant Phenotype in Nondiabetic Adults. J. Nutr. 2009, 139, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Yu, Z.; Pan, A.; Hu, F.B.; Franco, O.H.; Li, H.; Li, X.; Yang, X.; Chen, Y.; Lin, X. Plasma 25-hydroxyvitamin D Concentration and Metabolic Syndrome among Middle-aged and Elderly Chinese Individuals. Diabetes Care 2009, 32, 1278–1283. [Google Scholar] [CrossRef] [Green Version]
- Moore, A.; Hochner, H.; Sitlani, C.M.; Williams, M.A.; Hoofnagle, A.N.; de Boer, I.H.; Kestenbaum, B.; Siscovic, D.S.; Friedlander, Y.; Enquobahrie, D.A. Plasma Vitamin D is Associated with Fasting Insulin and Homeostatic Model Assessment of Insulin Resistance in Young Adult Males, but Not Females, of the Jerusalem Perinatal Study. Public Health Nutr. 2015, 18, 1324–1331. [Google Scholar] [CrossRef] [Green Version]
- Muscogiuri, G.; Sorice, G.P.; Prioletta, A.; Policola, C.; Casa, S.D.; Pontecorvi, A.; Giaccari, A. 25-Hydroxyvitamin D Concentration Correlates with Insulin-Sensitivity and BMI in Obesity. Obesity 2010, 18, 1906–1910. [Google Scholar] [CrossRef]
- O’Hartaigh, B.; Thomas, G.N.; Silbernagel, G.N.; Bosch, J.A.; Pilz, S.; Loerbroks, A.; Kleber, M.E.; Grammer, T.B.; Bohm, B.O.; Marz, W. Association of 25-hydroxyvitamin D with Type 2 Diabetes among Patients Undergoing Coronary Angiography: Cross-sectional Findings from the LUdwigshafen Risk and Cardiovascular Health (LURIC) Study. Clin. Endocrinol. 2013, 79, 192–198. [Google Scholar] [CrossRef]
- Pham, N.M.; Akter, S.; Kurotani, K.; Nanri, A.; Sato, M.; Hayabuchi, H.; Yasuda, K.; Mizoue, T. Serum 25-hydroxyvitamin D and Markers of Insulin Resistance in a Japanese Working Population. Eur. J. Clin. Nutr. 2012, 66, 1323–1328. [Google Scholar] [CrossRef]
- Sabio, J.M.; Vargas-Hitos, J.A.; Martinez-Bordonado, J.; Navarrete-Navarrete, N.; Diaz-Chamorro, A.; Olvera-Porcel, C.; Zamora, M.; Jiménez-Alonso, J. Association between Low 25-hydroxyvitamin D, Insulin Resistance and Arterial Stiffness in Nondiabetic Women with Systemic Lupus Erythematosus. Lupus 2015, 24, 155–163. [Google Scholar] [CrossRef]
- Sheth, J.J.; Shah, A.; Sheth, F.J.; Trivedi, S.; Lele, M.; Shah, N.; Thakor, P.; Vaidya, R. Does Vitamin D Play a Significant Role in Type 2 Diabetes? BMC Endocr. Disord. 2015, 15, 5. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Cao, Z.; Tanisawa, K.; Ito, T.; Oshima, S.; Higuchi, M. The Relationship between Serum 25-Hydroxyvitamin D Concentration, Cardiorespiratory Fitness, and Insulin Resistance in Japanese Men. Nutrients 2014, 7, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Tao, M.; Zhang, Z.; Yao-hua, K.E.; Jin-wei, H.E.; Wen-zhen, F.U.; Chang-qing, Z.; Zhen-lin, Z. Association of Serum 25-hydroxyvitamin D with Insulin Resistance and β-cell Function in a Healthy Chinese Female Population. Acta Pharmacol. Sin. 2013, 34, 1070–1074. [Google Scholar] [CrossRef] [Green Version]
- Tosunbayraktar, G.; Bas, M.; Kut, A.; Buyukkaragoz, A.H. Low Serum 25(OH)D Levels are Assocıated to Higher BMI and Metabolic Syndrome Parameters in Adults Subjects in Turkey. Afr. Health Sci. 2015, 15, 1161–1169. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Sun, Q.; Zhang, X.; Lu, Y.; Sun, C.; Cui, Y.; Wang, S. Serum 25(OH)D is Inversely Associated with Metabolic Syndrome Risk Profile among Urban Middle-aged Chinese Population. Nutr. J. 2012, 11, 68. [Google Scholar] [CrossRef] [Green Version]
- Calvo-Romero, J.M.; Ramiro-Lozano, J.M. Vitamin D Levels in Patients with Type 2 Diabetes Mellitus. J. Investig. Med. 2015, 63, 921–923. [Google Scholar] [CrossRef]
- Cai, X.; Hu, Z.; Chen, L.; Han, X.; Ji, L. Analysis of the Associations between Vitamin D and Albuminuria or Beta-Cell Function in Chinese Type 2 Diabetes. BioMed Res. 2014. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Fu, Q.; Li, Y.; Yang, T.; Tang, W. The Relationship between Serum 25-hydroxyvitamin D and Insulin Sensitivity and Beta Cell Function in Newly Diagnosed Type 2 Diabetes. J. Diabetes Res. 2015. [Google Scholar] [CrossRef]
- Rosen, C.J.; Adams, J.S.; Bikle, D.D.; Black, D.M.; Demay, M.B.; Manson, J.E.; Murad, M.H.; Kovacs, C.S. The Nonskeletal Effects of Vitamin D: An Endocrine Society Scientific Statement. Endocr. Rev. 2012, 33, 456–492. [Google Scholar] [CrossRef] [Green Version]
- Palomer, X.; González-Clemente, J.M.; Blanco-Vaca, F.; Mauricio, D. Role of Vitamin D in the Pathogenesis of Type 2 Diabetes Mellitus. Diabetes Obes. Metab. 2008, 10, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; Strain, G.; Sinha, N.; Ortiz, D.; Pomp, A.; Dakin, G.; McMahon, D.J.; Bockman, R.; Silverberg, S.J. Vitamin D Insufficiency Prior to Bariatric Surgery: Risk Factors and a Pilot Treatment Study. Clin. Endocrinol. 2009, 71, 176–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wamberg, L.; Cullberg, K.B.; Rejnmark, L.; Richelsen, B.; Pedersen, S.B. Investigations of the Anti-inflammatory Effects of Vitamin D in Adipose Tissue: Results from an in vitro Study and a Randomized Controlled Trial. Horm. Metab. Res. 2013, 45, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q. Chronic Inflammation in Fat Plays a Crucial Role in the Development of Obesity-related Insulin Resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Sasso, F.C.; Pafundi, P.C.; Marfella, R.; Calabrò, P.; Piscione, F.; Furbatto, F.; Esposito, G.; Galiero, R.; Gragnano, F.; Rinaldi, L.; et al. Adiponectin and Insulin Resistance are Related to Restenosis and Overall New PCI in Subjects with Normal Glucose Tolerance: The Prospective AIRE Study. Cardiovasc. Diabetol. 2019, 18, 24. [Google Scholar] [CrossRef]
- Mathieu, C.; Van Etten, E.; Gysemans, C. In vitro and in vivo Analysis of the Immune System of Vitamin D Receptor Knockout Mice. J. Bone Miner. Res. 2001, 16, 2057–2065. [Google Scholar] [CrossRef]
- Healy, K.D.; Frahm, M.A.; DeLuca, H.F. 1,25-Dihydroxyvitamin D3 Up-regulates the Renal Vitamin D Receptor through Indirect Gene Activation and Receptor Stabilization. Arch. Biochem. Biophys. 2005, 433, 466–473. [Google Scholar] [CrossRef]
- Cade, C.; Norman, A.W. Rapid Normalization/Stimulation by 1,25-dihydroxyvitamin D3 of Insulin Secretion and Glucose Tolerance in the Vitamin D-deficient Rat. Endocrinology 1987, 120, 1490–1497. [Google Scholar] [CrossRef]
- Rafiq, S.; Jeppesen, P.B. Is Hypovitaminosis D Related to Incidence of Type 2 Diabetes and High Fasting Glucose Level in Healthy Subjects: A Systematic Review and Meta-analysis of Observational Studies. Nutrients 2018, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- Alkharfy, K.M.; Al-Daghri, N.M.; Yakout, S.M. Calcitriol Attenuates Weight-related Systemic Inflammation and Ultrastructural Changes of the Liver in a Rodent Model. Basic Clin. Pharmacol. Toxicol. 2012, 112, 42–49. [Google Scholar] [CrossRef]
- Barnard, W.F.; Saxena, V.K.; Wenny, B.N.; DeLuisi, J.J. Daily Surface UV Exposure and Its Relationship to Surface Pollutant Measurements. J. Air Waste Manag. Assoc. 2003, 53, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Elminir, H.K. Sensitivity of Ultraviolet Solar Radiation to Anthropogenic Air Pollutants and Weather Conditions. Atmos. Res. 2007, 84, 250–264. [Google Scholar] [CrossRef]
- Alam, U.; Arul-Devah, V.; Javed, S. Vitamin D and Diabetic Complications: True or False Prophet? Diabetes Ther. 2016, 7, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Kaul, K.; Hodgkinson, A.; Tarr, J.M.; Kohner, E.M.; Chibber, R. Is Inflammation a Common Retinal-renal-nerve Pathogenic Link in Diabetes? Curr. Diabetes Rev. 2010, 6, 294–303. [Google Scholar] [CrossRef]
- Burton, J.M.; Kimball, S.; Vieth, R. A Phase I/II Dose-escalation Trial of Vitamin D3 and Calcium in Multiple Sclerosis. Neurology 2010, 74, 1852–1859. [Google Scholar] [CrossRef] [Green Version]
- Holmøy, T.; Moen, S.M. Assessing Vitamin D in the Central Nervous System. Acta Neurol. Scand. Suppl. 2010, 122, 88–92. [Google Scholar] [CrossRef]
- Simon, K.C.; Munger, K.L.; Ascherio, A. Vitamin D and Multiple Sclerosis: Epidemiology, Immunology, and Genetics. Curr. Opin. Neurol. 2012, 25, 246–251. [Google Scholar] [CrossRef]
- Fex, S.A.; Dahlin, L.B. Repair of the Peripheral Nerve—Remyelination That Works. Brain Sci. 2013, 3, 1182–1197. [Google Scholar]
- Neveu, I.; Naveilhan, P.; Jehan, F. 1,25-Dihydroxyvitamin D3 Regulates the Synthesis of Nerve Growth Factor in Primary Cultures of Glial Cells. Brain Res. Mol. Brain Res. 1994, 24, 70–76. [Google Scholar] [CrossRef]
- Ito, S.; Ohtsuki, S.; Nezu, Y.; Koitabashi, Y.; Murata, S.; Terasaki, T. 1,25-Dihydroxyvitamin D3 Enhances Cerebral Clearance of Human Amyloid-b Peptide (1-40) from Mouse Brain Across the Blood-brain Barrier. Fluids Barriers CNS 2011, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Generini, S.; Tuveri, M.A.; Matucci Cerinic, M.; Mastinu, F.; Manni, L.; Aloe, L. Topical Application of Nerve Growth Factor in Human Diabetic Foot Ulcers. A Study of Three Cases. Exp. Clin. Endocrinol. Diabetes 2004, 112, 542–544. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafiq, S.; Jeppesen, P.B. Insulin Resistance Is Inversely Associated with the Status of Vitamin D in Both Diabetic and Non-Diabetic Populations. Nutrients 2021, 13, 1742. https://doi.org/10.3390/nu13061742
Rafiq S, Jeppesen PB. Insulin Resistance Is Inversely Associated with the Status of Vitamin D in Both Diabetic and Non-Diabetic Populations. Nutrients. 2021; 13(6):1742. https://doi.org/10.3390/nu13061742
Chicago/Turabian StyleRafiq, Shamaila, and Per Bendix Jeppesen. 2021. "Insulin Resistance Is Inversely Associated with the Status of Vitamin D in Both Diabetic and Non-Diabetic Populations" Nutrients 13, no. 6: 1742. https://doi.org/10.3390/nu13061742





