Nutritional Treatment in Crohn’s Disease
Abstract
1. Introduction
2. Materials and Methods
3. Crohn’s Disease: Cause and Pathophysiology
4. Crohn’s Disease and Nutritional Deficiencies
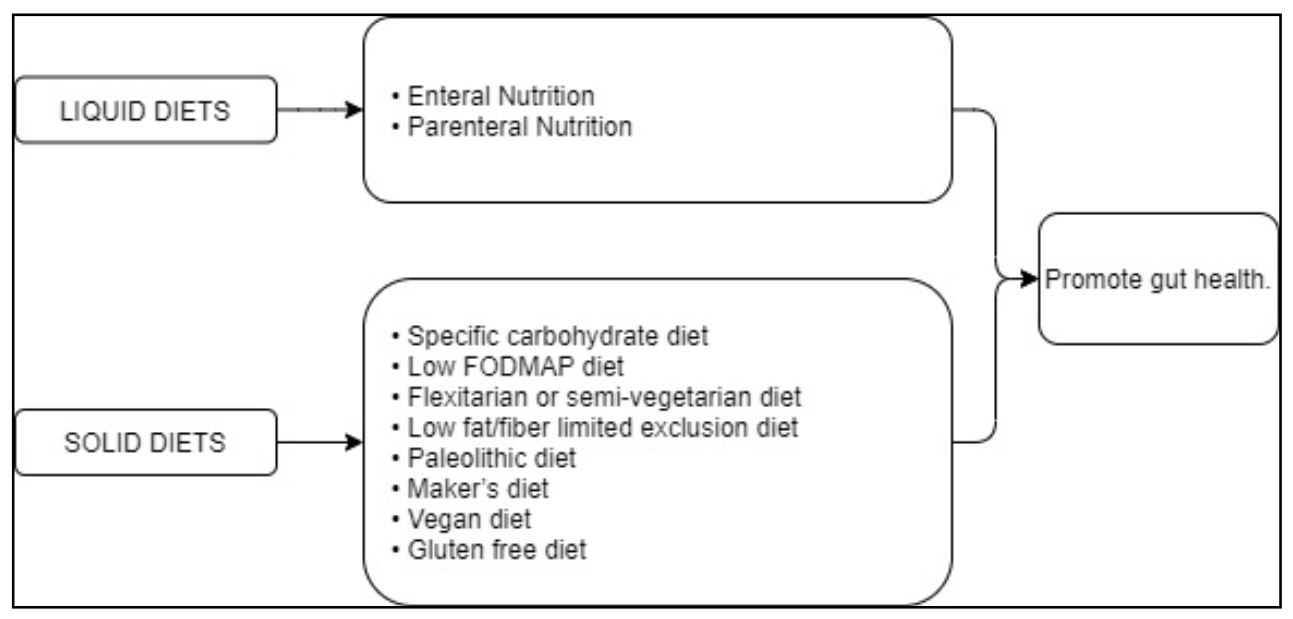
5. Diets for the Treatment of Crohn’s Disease
5.1. Liquid Diets: Enteral Nutrition and Parenteral Nutrition as Artificial Diets for the Preoperative Nutritional Optimization in CD
5.2. Enteral Nutrition
5.3. Parenteral Nutrition
5.4. Enteral Nutrition and Parenteral Nutrition for Safer Elective Surgery and Reduced Post-Operative Complications in Adults with CD
5.5. Enteral vs. Parenteral Nutrition
5.6. Specific Carbohydrate Diet (SCD)
5.7. Low FODMAP Diet
5.8. Semi-Vegetarian Diet (SVD)
5.9. Other Diets
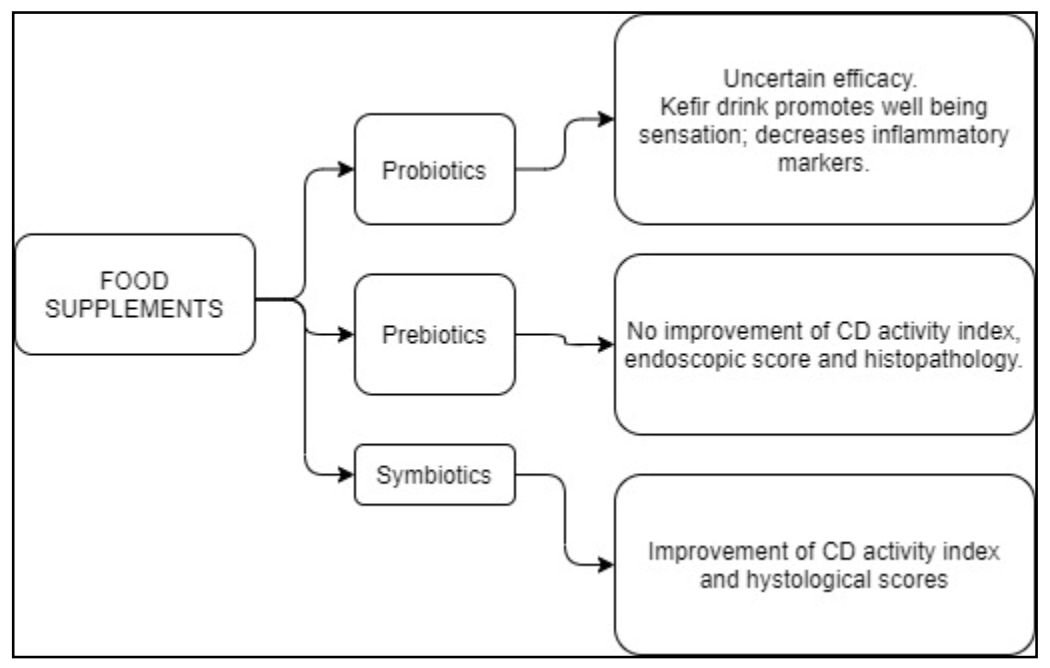
6. Probiotics, Prebiotics, and Symbiotics
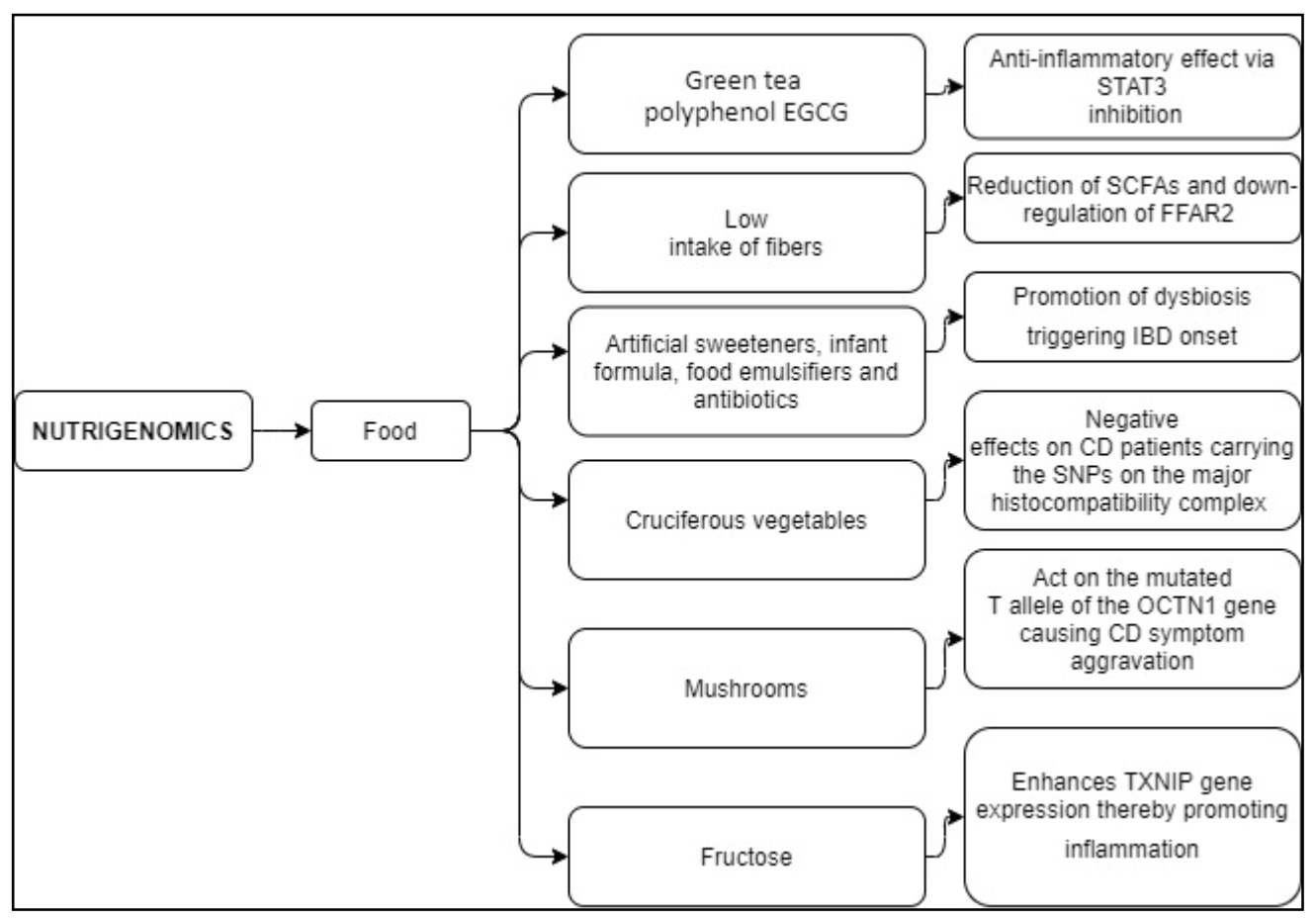
7. Nutrigenomics
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Damas, O.M.; Garces, L.; Abreu, M.T. Diet as Adjunctive Treatment for Inflammatory Bowel Disease: Review and Update of the Latest Literature. Curr. Treat. Options Gastroenterol. 2019, 17, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Cheifetz, A.S. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin. Proc. 2017, 92, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Veauthier, B.; Hornecker, J.R. Crohn’s Disease: Diagnosis and Management. Am. Fam. Physician 2018, 98, 661–669. [Google Scholar] [PubMed]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s Disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-Microbe Interactions Have Shaped the Genetic Architecture of Inflammatory Bowel Disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [PubMed]
- McGovern, D.P.B.; Kugathasan, S.; Cho, J.H. Genetics of Inflammatory Bowel Diseases. Gastroenterology 2015, 149, 1163–1176.e2. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The Microbiome in Inflammatory Bowel Disease: Current Status and the Future Ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium Prausnitzii Is an Anti-Inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Quévrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermúdez-Humarán, L.G.; Pigneur, B.; et al. Identification of an Anti-Inflammatory Protein from Faecalibacterium Prausnitzii, a Commensal Bacterium Deficient in Crohn’s Disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef]
- Bernink, J.H.; Peters, C.P.; Munneke, M.; te Velde, A.A.; Meijer, S.L.; Weijer, K.; Hreggvidsdottir, H.S.; Heinsbroek, S.E.; Legrand, N.; Buskens, C.J.; et al. Human Type 1 Innate Lymphoid Cells Accumulate in Inflamed Mucosal Tissues. Nat. Immunol. 2013, 14, 221–229. [Google Scholar] [CrossRef]
- Geremia, A.; Arancibia-Cárcamo, C.V.; Fleming, M.P.P.; Rust, N.; Singh, B.; Mortensen, N.J.; Travis, S.P.L.; Powrie, F. IL-23-Responsive Innate Lymphoid Cells Are Increased in Inflammatory Bowel Disease. J. Exp. Med. 2011, 208, 1127–1133. [Google Scholar] [CrossRef]
- Laube, R.; Liu, K.; Schifter, M.; Yang, J.L.; Suen, M.K.; Leong, R.W. Oral and Upper Gastrointestinal Crohn’s Disease. J. Gastroenterol. Hepatol. 2018, 33, 355–364. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Pizzoferrato, M.; Lopetuso, L.R.; Musca, T.; Ingravalle, F.; Sicignano, L.L.; Mentella, M.; Miggiano, G.; Mele, M.C.; Gaetani, E.; et al. Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide. Gastroenterol. Res. Pract. 2017, 2017, 8646495. [Google Scholar] [CrossRef]
- Laing, B.; Han, D.Y.; Ferguson, L.R. Candidate Genes Involved in Beneficial or Adverse Responses to Commonly Eaten Brassica Vegetables in a New Zealand Crohn’s Disease Cohort. Nutrients 2013, 5, 5046–5064. [Google Scholar] [CrossRef]
- Stein, J.; Bott, C. Diet and Nutrition in Crohn’s Disease and Ulcerative Colitis 20 Questions 20 Answers; Falk Foundation: Freiburg, Germany, 2008. [Google Scholar]
- Hartman, C.; Marderfeld, L.; Davidson, K.; Mozer-Glassberg, Y.; Poraz, I.; Silbermintz, A.; Zevit, N.; Shamir, R. Food Intake Adequacy in Children and Adolescents With Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 437–444. [Google Scholar] [CrossRef]
- Zoli, G.; Katelaris, P.H.; Garrow, J.; Gasbarrini, G.; Farthing, M.J.G. Increased Energy Expenditure in Growing Adolescents with Crohn’s Disease. Dig. Dis. Sci. 1996, 41, 1754–1759. [Google Scholar] [CrossRef]
- Heerasing, N.; Thompson, B.; Hendy, P.; Heap, G.A.; Walker, G.; Bethune, R.; Mansfield, S.; Calvert, C.; Kennedy, N.A.; Ahmad, T.; et al. Exclusive Enteral Nutrition Provides an Effective Bridge to Safer Interval Elective Surgery for Adults with Crohn’s Disease. Aliment. Pharmacol. Ther. 2017, 45, 660–669. [Google Scholar] [CrossRef]
- Cosnes, J. Crohn’s Disease Phenotype, Prognosis, and Long-Term Complications: What to Expect? Acta Gastro-Enterol. Belg. 2008, 71, 303–307. [Google Scholar]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and Natural History of Inflammatory Bowel Diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef]
- Ayoub, F.; Kamel, A.Y.; Ouni, A.; Chaudhry, N.; Ader, Y.; Tan, S.; Iqbal, A.; Zimmermann, E.M.; Glover, S.C. Pre-Operative Total Parenteral Nutrition Improves Post-Operative Outcomes in a Subset of Crohn’s Disease Patients Undergoing Major Abdominal Surgery. Gastroenterol. Rep. 2019, 7, 107–114. [Google Scholar] [CrossRef]
- Heimann, T.M.; Greenstein, A.J.; Mechanic, L.; Aufses, A.H.J. Early Complications Following Surgical Treatment for Crohn’s Disease. Ann. Surg. 1985, 201, 494–498. [Google Scholar] [CrossRef]
- Fumery, M.; Seksik, P.; Auzolle, C.; Munoz-Bongrand, N.; Gornet, J.-M.; Boschetti, G.; Cotte, E.; Buisson, A.; Dubois, A.; Pariente, B.; et al. Postoperative Complications after Ileocecal Resection in Crohn’s Disease: A Prospective Study From the REMIND Group. Am. J. Gastroenterol. 2017, 112, 337–345. [Google Scholar] [CrossRef]
- Alves, A.; Panis, Y.; Bouhnik, Y.; Pocard, M.; Vicaut, E.; Valleur, P. Risk Factors for Intra-Abdominal Septic Complications after a First Ileocecal Resection for Crohn’s Disease: A Multivariate Analysis in 161 Consecutive Patients. Dis. Colon Rectum 2007, 50, 331–336. [Google Scholar] [CrossRef]
- Morar, P.S.; Hodgkinson, J.D.; Thalayasingam, S.; Koysombat, K.; Purcell, M.; Hart, A.L.; Warusavitarne, J.; Faiz, O. Determining Predictors for Intra-Abdominal Septic Complications Following Ileocolonic Resection for Crohn’s Disease-Considerations in Pre-Operative and Peri-Operative Optimisation Techniques to Improve Outcome. J. Crohns Colitis 2015, 9, 483–491. [Google Scholar] [CrossRef]
- Lindor, K.D.; Fleming, C.R.; Ilstrup, D.M. Preoperative Nutritional Status and Other Factors That Influence Surgical Outcome in Patients with Crohn’s Disease. Mayo Clin. Proc. 1985, 60, 393–396. [Google Scholar] [CrossRef]
- Yamamoto, T.; Allan, R.N.; Keighley, M.R. Risk Factors for Intra-Abdominal Sepsis after Surgery in Crohn’s Disease. Dis. Colon Rectum 2000, 43, 1141–1145. [Google Scholar] [CrossRef]
- Adamina, M.; Bonovas, S.; Raine, T.; Spinelli, A.; Warusavitarne, J.; Armuzzi, A.; Bachmann, O.; Bager, P.; Biancone, L.; Bokemeyer, B.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. J. Crohns Colitis 2020, 14, 155–168. [Google Scholar] [CrossRef]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN Guideline: Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. Edinb. Scotl. 2017, 36, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN Guideline: Clinical Nutrition in Surgery. Clin. Nutr. Edinb. Scotl. 2017, 36, 623–650. [Google Scholar] [CrossRef]
- Di Caro, S.; Fragkos, K.C.; Keetarut, K.; Koo, H.F.; Sebepos-Rogers, G.; Saravanapavan, H.; Barragry, J.; Rogers, J.; Mehta, S.J.; Rahman, F. Enteral Nutrition in Adult Crohn’s Disease: Toward a Paradigm Shift. Nutrients 2019, 11, 2222. [Google Scholar] [CrossRef]
- Yamamoto, T.; Shimoyama, T.; Kuriyama, M. Dietary and Enteral Interventions for Crohn’s Disease. Curr. Opin. Biotechnol. 2017, 44, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Nakahigashi, M.; Yamamoto, T.; Sacco, R.; Hanai, H.; Kobayashi, F. Enteral Nutrition for Maintaining Remission in Patients with Quiescent Crohn’s Disease: Current Status and Future Perspectives. Int. J. Colorectal Dis. 2016, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Critch, J.; Day, A.S.; Otley, A.; King-Moore, C.; Teitelbaum, J.E.; Shashidhar, H. Use of Enteral Nutrition for the Control of Intestinal Inflammation in Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Hirai, F.; Ishihara, H.; Yada, S.; Esaki, M.; Ohwan, T.; Nozaki, R.; Ashizuka, S.; Inatsu, H.; Ohi, H.; Aoyagi, K.; et al. Effectiveness of Concomitant Enteral Nutrition Therapy and Infliximab for Maintenance Treatment of Crohn’s Disease in Adults. Dig. Dis. Sci. 2013, 58, 1329–1334. [Google Scholar] [CrossRef]
- Nguyen, D.L.; Palmer, L.B.; Nguyen, E.T.; McClave, S.A.; Martindale, R.G.; Bechtold, M.L. Specialized Enteral Nutrition Therapy in Crohn’s Disease Patients on Maintenance Infliximab Therapy: A Meta-Analysis. Ther. Adv. Gastroenterol. 2015, 8, 168–175. [Google Scholar] [CrossRef]
- Hansen, T.; Duerksen, D.R. Enteral Nutrition in the Management of Pediatric and Adult Crohn’s Disease. Nutrients 2018, 10, 537. [Google Scholar] [CrossRef]
- Yamamoto, T.; Shiraki, M.; Nakahigashi, M.; Umegae, S.; Matsumoto, K. Enteral Nutrition to Suppress Postoperative Crohn’s Disease Recurrence: A Five-Year Prospective Cohort Study. Int. J. Colorectal Dis. 2013, 28, 335–340. [Google Scholar] [CrossRef]
- Whitten, K.E.; Leach, S.T.; Bohane, T.D.; Woodhead, H.J.; Day, A.S. Effect of Exclusive Enteral Nutrition on Bone Turnover in Children with Crohn’s Disease. J. Gastroenterol. 2010, 45, 399–405. [Google Scholar] [CrossRef]
- Werkstetter, K.J.; Schatz, S.B.; Alberer, M.; Filipiak-Pittroff, B.; Koletzko, S. Influence of Exclusive Enteral Nutrition Therapy on Bone Density and Geometry in Newly Diagnosed Pediatric Crohn’s Disease Patients. Ann. Nutr. Metab. 2013, 63, 10–16. [Google Scholar] [CrossRef]
- Green, N.; Miller, T.; Suskind, D.; Lee, D. A Review of Dietary Therapy for IBD and a Vision for the Future. Nutrients 2019, 11, 947. [Google Scholar] [CrossRef]
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, P.M.; Cucchiara, S. Polymeric Diet Alone versus Corticosteroids in the Treatment of Active Pediatric Crohn’s Disease: A Randomized Controlled Open-Label Trial. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2006, 4, 744–753. [Google Scholar] [CrossRef]
- Day, A.S.; Whitten, K.E.; Lemberg, D.A.; Clarkson, C.; Vitug-Sales, M.; Jackson, R.; Bohane, T.D. Exclusive Enteral Feeding as Primary Therapy for Crohn’s Disease in Australian Children and Adolescents: A Feasible and Effective Approach. J. Gastroenterol. Hepatol. 2006, 21, 1609–1614. [Google Scholar] [CrossRef]
- Gorard, D.A.; Hunt, J.B.; Payne-James, J.J.; Palmer, K.R.; Rees, R.G.; Clark, M.L.; Farthing, M.J.; Misiewicz, J.J.; Silk, D.B. Initial Response and Subsequent Course of Crohn’s Disease Treated with Elemental Diet or Prednisolone. Gut 1993, 34, 1198–1202. [Google Scholar] [CrossRef]
- Wall, C.L.; Day, A.S.; Gearry, R.B. Use of Exclusive Enteral Nutrition in Adults with Crohn’s Disease: A Review. World J. Gastroenterol. 2013, 19, 7652–7660. [Google Scholar] [CrossRef]
- Zachos, M.; Tondeur, M.; Griffiths, A.M. Enteral Nutritional Therapy for Induction of Remission in Crohn’s Disease. Cochrane Database Syst. Rev. 2007, CD000542. [Google Scholar] [CrossRef]
- Hu, D.; Ren, J.; Wang, G.; Li, G.; Liu, S.; Yan, D.; Gu, G.; Zhou, B.; Wu, X.; Chen, J.; et al. Exclusive Enteral Nutritional Therapy Can Relieve Inflammatory Bowel Stricture in Crohn’s Disease. J. Clin. Gastroenterol. 2014, 48, 790–795. [Google Scholar] [CrossRef]
- Yan, D.; Ren, J.; Wang, G.; Liu, S.; Li, J. Predictors of Response to Enteral Nutrition in Abdominal Enterocutaneous Fistula Patients with Crohn’s Disease. Eur. J. Clin. Nutr. 2014, 68, 959–963. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Day, A.S. The Impact of Exclusive Enteral Nutrition on the Intestinal Microbiota in Inflammatory Bowel Disease. AIMS Microbiol. 2018, 4, 584–593. [Google Scholar] [CrossRef]
- Vindigni, S.M.; Zisman, T.L.; Suskind, D.L.; Damman, C.J. The Intestinal Microbiome, Barrier Function, and Immune System in Inflammatory Bowel Disease: A Tripartite Pathophysiological Circuit with Implications for New Therapeutic Directions. Ther. Adv. Gastroenterol. 2016, 9, 606–625. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Bertz, M.; Hanske, L.; Junick, J.; Biskou, O.; Aguilera, M.; Garrick, V.; Russell, R.K.; Blaut, M.; McGrogan, P.; et al. Decline in Presumptively Protective Gut Bacterial Species and Metabolites Are Paradoxically Associated with Disease Improvement in Pediatric Crohn’s Disease during Enteral Nutrition. Inflamm. Bowel Dis. 2014, 20, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Schwerd, T.; Frivolt, K.; Clavel, T.; Lagkouvardos, I.; Katona, G.; Mayr, D.; Uhlig, H.H.; Haller, D.; Koletzko, S.; Bufler, P. Exclusive Enteral Nutrition in Active Pediatric Crohn Disease: Effects on Intestinal Microbiota and Immune Regulation. J. Allergy Clin. Immunol. 2016, 138, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Quince, C.; Ijaz, U.Z.; Loman, N.; Eren, A.M.; Saulnier, D.; Russell, J.; Haig, S.J.; Calus, S.T.; Quick, J.; Barclay, A.; et al. Extensive Modulation of the Fecal Metagenome in Children With Crohn’s Disease During Exclusive Enteral Nutrition. Am. J. Gastroenterol. 2015, 110, 1718–1729, quiz 1730. [Google Scholar] [CrossRef] [PubMed]
- Logan, M.; Clark, C.M.; Ijaz, U.Z.; Gervais, L.; Duncan, H.; Garrick, V.; Curtis, L.; Buchanan, E.; Cardigan, T.; Armstrong, L.; et al. The Reduction of Faecal Calprotectin during Exclusive Enteral Nutrition Is Lost Rapidly after Food Re-Introduction. Aliment. Pharmacol. Ther. 2019, 50, 664–674. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Day, A.S.; Leach, S.T.; Lemberg, D.A.; Nielsen, S.; Mitchell, H.M. Effect of Exclusive Enteral Nutrition on the Microbiota of Children with Newly Diagnosed Crohn’s Disease. Clin. Transl. Gastroenterol. 2015, 6, e71. [Google Scholar] [CrossRef]
- Ruemmele, F.M.; Veres, G.; Kolho, K.L.; Griffiths, A.; Levine, A.; Escher, J.C.; Amil Dias, J.; Barabino, A.; Braegger, C.P.; Bronsky, J.; et al. Consensus Guidelines of ECCO/ESPGHAN on the Medical Management of Pediatric Crohn’s Disease. J. Crohns Colitis 2014, 8, 1179–1207. [Google Scholar] [CrossRef]
- Zoli, G.; Carè, M.; Parazza, M.; Spanò, C.; Biagi, P.L.; Bernardi, M.; Gasbarrini, G. A Randomized Controlled Study Comparing Elemental Diet and Steroid Treatment in Crohn’s Disease. Aliment. Pharmacol. Ther. 1997, 11, 735–740. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Wolf, A.; Parian, A.M. Nutritional Interventions in the Patient with Inflammatory Bowel Disease. Gastroenterol. Clin. North Am. 2018, 47, 155–177. [Google Scholar] [CrossRef]
- Comeche, J.M.; Comino, I.; Altavilla, C.; Tuells, J.; Gutierrez-Hervas, A.; Caballero, P. Parenteral Nutrition in Patients with Inflammatory Bowel Disease Systematic Review, Meta-Analysis and Meta-Regression. Nutrients 2019, 11, 2865. [Google Scholar] [CrossRef]
- Adamina, M.; Gerasimidis, K.; Sigall-Boneh, R.; Zmora, O.; de Buck van Overstraeten, A.; Campmans-Kuijpers, M.; Ellul, P.; Katsanos, K.; Kotze, P.G.; Noor, N.; et al. Perioperative Dietary Therapy in Inflammatory Bowel Disease. J. Crohns Colitis 2020, 14, 431–444. [Google Scholar] [CrossRef]
- Braga, M.; Ljungqvist, O.; Soeters, P.; Fearon, K.; Weimann, A.; Bozzetti, F. ESPEN Guidelines on Parenteral Nutrition: Surgery. Clin. Nutr. Edinb. Scotl. 2009, 28, 378–386. [Google Scholar] [CrossRef]
- McIntyre, P.B.; Powell-Tuck, J.; Wood, S.R.; Lennard-Jones, J.E.; Lerebours, E.; Hecketsweiler, P.; Galmiche, J.P.; Colin, R. Controlled Trial of Bowel Rest in the Treatment of Severe Acute Colitis. Gut 1986, 27, 481–485. [Google Scholar] [CrossRef]
- Jones, V.A. Comparison of Total Parenteral Nutrition and Elemental Diet in Induction of Remission of Crohn’s Disease. Long-Term Maintenance of Remission by Personalized Food Exclusion Diets. Dig. Dis. Sci. 1987, 32, 100S–107S. [Google Scholar] [CrossRef]
- Jacobson, S. Early Postoperative Complications in Patients with Crohn’s Disease given and Not given Preoperative Total Parenteral Nutrition. Scand. J. Gastroenterol. 2012, 47, 170–177. [Google Scholar] [CrossRef]
- Fasth, S.; Hultén, L.; Magnusson, O.; Nordgren, S.; Warnold, I. The Immediate and Long-Term Effects of Postoperative Total Parenteral Nutrition on Body Composition. Int. J. Colorectal Dis. 1987, 2, 139–145. [Google Scholar] [CrossRef]
- Abad-Lacruz, A.; González-Huix, F.; Esteve, M.; Fernández-Bañares, F.; Cabré, E.; Boix, J.; Acero, D.; Humbert, P.; Gassull, M.A. Liver Function Tests Abnormalities in Patients with Inflammatory Bowel Disease Receiving Artificial Nutrition: A Prospective Randomized Study of Total Enteral Nutrition vs Total Parenteral Nutrition. JPEN J. Parenter. Enteral Nutr. 1990, 14, 618–621. [Google Scholar] [CrossRef]
- Eisenberg, P.G.; Gianino, S.; Clutter, W.E.; Fleshman, J.W. Abrupt Discontinuation of Cycled Parenteral Nutrition Is Safe. Dis. Colon Rectum 1995, 38, 933–939. [Google Scholar] [CrossRef]
- Greenberg, G.R.; Fleming, C.R.; Jeejeebhoy, K.N.; Rosenberg, I.H.; Sales, D.; Tremaine, W.J. Controlled Trial of Bowel Rest and Nutritional Support in the Management of Crohn’s Disease. Gut 1988, 29, 1309–1315. [Google Scholar] [CrossRef]
- Wright, R.A.; Adler, E.C. Peripheral Parenteral Nutrition Is No Better than Enteral Nutrition in Acute Exacerbation of Crohn’s Disease: A Prospective Trial. J. Clin. Gastroenterol. 1990, 12, 396–399. [Google Scholar] [CrossRef]
- Ockenga, J.; Borchert, K.; Stüber, E.; Lochs, H.; Manns, M.P.; Bischoff, S.C. Glutamine-Enriched Total Parenteral Nutrition in Patients with Inflammatory Bowel Disease. Eur. J. Clin. Nutr. 2005, 59, 1302–1309. [Google Scholar] [CrossRef]
- Lauro, A.; D’Amico, F.; Gondolesi, G. The Current Therapeutic Options for Crohn’s Disease: From Medical Therapy to Intestinal Transplantation. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nakahigashi, M.; Shimoyama, T.; Umegae, S. Does Preoperative Enteral Nutrition Reduce the Incidence of Surgical Complications in Patients with Crohn’s Disease? A Case-Matched Study. Colorectal Dis. 2020, 22, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ren, J.; Wang, G.; Hu, D.; Gu, G.; Liu, S.; Ren, H.; Wu, X.; Li, J. Preoperative Exclusive Enteral Nutrition Reduces the Postoperative Septic Complications of Fistulizing Crohn’s Disease. Eur. J. Clin. Nutr. 2014, 68, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Montalvo, M.; MacDonald, S.; Keefe, L.; Su, X.Y.; Drover, J.W. Total Parenteral Nutrition in the Surgical Patient: A Meta-Analysis. Can. J. Surg. J. Can. Chir. 2001, 44, 102–111. [Google Scholar]
- Braunschweig, C.L.; Levy, P.; Sheean, P.M.; Wang, X. Enteral Compared with Parenteral Nutrition: A Meta-Analysis. Am. J. Clin. Nutr. 2001, 74, 534–542. [Google Scholar] [CrossRef]
- Peter, J.V.; Moran, J.L.; Phillips-Hughes, J. A Metaanalysis of Treatment Outcomes of Early Enteral versus Early Parenteral Nutrition in Hospitalized Patients. Crit. Care Med. 2005, 33, 213–220, discussion 260–261. [Google Scholar] [CrossRef]
- Klek, S.; Forbes, A.; Gabe, S.; Holst, M.; Wanten, G.; Irtun, Ø.; Damink, S.O.; Panisic-Sekeljic, M.; Pelaez, R.B.; Pironi, L. Management of Acute Intestinal Failure: A Position Paper from the European Society for Clinical Nutrition and Metabolism (ESPEN) Special Interest Group. Clin. Nutr. Edinb. Scotl. 2016, 35, 1209–1218. [Google Scholar] [CrossRef]
- Kakodkar, S.; Mutlu, E.A. Diet as a Therapeutic Option for Adult Inflammatory Bowel Disease. Gastroenterol. Clin. North. Am. 2017, 46, 745–767. [Google Scholar] [CrossRef]
- Kakodkar, S.; Farooqui, A.J.; Mikolaitis, S.L.; Mutlu, E.A. The Specific Carbohydrate Diet for Inflammatory Bowel Disease: A Case Series. J. Acad. Nutr. Diet. 2015, 115, 1226–1232. [Google Scholar] [CrossRef]
- Cohen, S.A.; Gold, B.D.; Oliva, S.; Lewis, J.; Stallworth, A.; Koch, B.; Eshee, L.; Mason, D. Clinical and Mucosal Improvement with Specific Carbohydrate Diet in Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 516–521. [Google Scholar] [CrossRef]
- Obih, C.; Wahbeh, G.; Lee, D.; Braly, K.; Giefer, M.; Shaffer, M.L.; Nielson, H.; Suskind, D.L. Specific Carbohydrate Diet for Pediatric Inflammatory Bowel Disease in Clinical Practice within an Academic IBD Center. Nutrition 2016, 32, 418–425. [Google Scholar] [CrossRef]
- Burgis, J.C.; Nguyen, K.; Park, K.T.; Cox, K. Response to Strict and Liberalized Specific Carbohydrate Diet in Pediatric Crohn’s Disease. World J. Gastroenterol. 2016, 22, 2111–2117. [Google Scholar] [CrossRef]
- Suskind, D.L.; Cohen, S.A.; Brittnacher, M.J.; Wahbeh, G.; Lee, D.; Shaffer, M.L.; Braly, K.; Hayden, H.S.; Klein, J.; Gold, B.; et al. Clinical and Fecal Microbial Changes With Diet Therapy in Active Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2018, 52, 155–163. [Google Scholar] [CrossRef]
- Comparing Two Diets for Patients with Crohn’s Disease. Available online: https://www.pcori.org/research-results/2016/comparing-two-diets-patients-crohn%E2%80%99s-disease. (accessed on 29 March 2021).
- Prince, A.C.; Myers, C.E.; Joyce, T.; Irving, P.; Lomer, M.; Whelan, K. Fermentable Carbohydrate Restriction (Low FODMAP Diet) in Clinical Practice Improves Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 1129–1136. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Consistent Prebiotic Effect on Gut Microbiota With Altered FODMAP Intake in Patients with Crohn’s Disease: A Randomised, Controlled Cross-Over Trial of Well-Defined Diets. Clin. Transl. Gastroenterol. 2016, 7, e164. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhan, Y.-A.; Dai, S.-X. Is a Low FODMAP Diet Beneficial for Patients with Inflammatory Bowel Disease? A Meta-Analysis and Systematic Review. Clin. Nutr. Edinb. Scotl. 2018, 37, 123–129. [Google Scholar] [CrossRef]
- Simrén, M.; Axelsson, J.; Gillberg, R.; Abrahamsson, H.; Svedlund, J.; Björnsson, E.S. Quality of Life in Inflammatory Bowel Disease in Remission: The Impact of IBS-like Symptoms and Associated Psychological Factors. Am. J. Gastroenterol. 2002, 97, 389–396. [Google Scholar] [CrossRef]
- Caio, G.; Riegler, G.; Patturelli, M.; Facchiano, A.; Laura, D.E.M.; Sapone, A. Pathophysiology of Non-Celiac Gluten Sensitivity: Where Are We Now? Minerva Gastroenterol. Dietol. 2017, 63, 16–21. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients With Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef]
- Bennet, S.M.P.; Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Öhman, L.; Simrén, M. Multivariate Modelling of Faecal Bacterial Profiles of Patients with IBS Predicts Responsiveness to a Diet Low in FODMAPs. Gut 2018, 67, 872–881. [Google Scholar] [CrossRef]
- Chiba, M.; Abe, T.; Tsuda, H.; Sugawara, T.; Tsuda, S.; Tozawa, H.; Fujiwara, K.; Imai, H. Lifestyle-Related Disease in Crohn’s Disease: Relapse Prevention by a Semi-Vegetarian Diet. World J. Gastroenterol. 2010, 16, 2484–2495. [Google Scholar] [CrossRef] [PubMed]
- Sandefur, K.; Kahleova, H.; Desmond, A.N.; Elfrink, E.; Barnard, N.D. Crohn’s Disease Remission with a Plant-Based Diet: A Case Report. Nutrients 2019, 11, 1385. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Tsuji, T.; Nakane, K.; Komatsu, M. High Amount of Dietary Fiber Not Harmful but Favorable for Crohn Disease. Perm. J. 2015, 19, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Walker, M.; Burger, D.; Martin, N.; von Wulffen, M.; Koloski, N.; Jones, M.; Talley, N.J.; Holtmann, G.J. Link Between Celiac Disease and Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2019, 53, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.N.; Herfarth, H. Gluten-Free Diet in IBD: Time for a Recommendation? Mol. Nutr. Food Res. 2020, 65, 1901274. [Google Scholar] [CrossRef] [PubMed]
- Herfarth, H.H.; Martin, C.F.; Sandler, R.S.; Kappelman, M.D.; Long, M.D. Prevalence of a Gluten-Free Diet and Improvement of Clinical Symptoms in Patients with Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2014, 20, 1194–1197. [Google Scholar] [CrossRef]
- Schreiner, P.; Yilmaz, B.; Rossel, J.-B.; Franc, Y.; Misselwitz, B.; Scharl, M.; Zeitz, J.; Frei, P.; Greuter, T.; Vavricka, S.R.; et al. Vegetarian or Gluten-Free Diets in Patients with Inflammatory Bowel Disease Are Associated with Lower Psychological Well-Being and a Different Gut Microbiota, but No Beneficial Effects on the Course of the Disease. United Eur. Gastroenterol. J. 2019, 7, 767–781. [Google Scholar] [CrossRef]
- Triantafillidis, J.K.; Tzouvala, M.; Triantafyllidi, E. Enteral Nutrition Supplemented with Transforming Growth Factor-β, Colostrum, Probiotics, and Other Nutritional Compounds in the Treatment of Patients with Inflammatory Bowel Disease. Nutrients 2020, 12, 1048. [Google Scholar] [CrossRef]
- Pace, F.; Pace, M.; Quartarone, G. Probiotics in Digestive Diseases: Focus on Lactobacillus GG. Minerva Gastroenterol. Dietol. 2015, 61, 273–292. [Google Scholar]
- Astó, E.; Méndez, I.; Audivert, S.; Farran-Codina, A.; Espadaler, J. The Efficacy of Probiotics, Prebiotic Inulin-Type Fructans, and Synbiotics in Human Ulcerative Colitis: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 293. [Google Scholar] [CrossRef]
- Peng, L.; Zhong, Y.; Wang, A.; Jiang, Z. Probiotics Combined with Aminosalicylic Acid Affiliates Remission of Ulcerative Colitis: A Meta-Analysis of Randomized Controlled Trial. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Qiu, Z.-W.; Tang, H.-M.; Zhuang, K.-H.; Cai, Q.-Q.; Chen, X.-L.; Li, H.-B. Efficacy and Safety of Bifid Triple Viable plus Aminosalicylic Acid for the Treatment of Ulcerative Colitis: A Systematic Review and Meta-Analysis. Medicine 2019, 98, e17955. [Google Scholar] [CrossRef]
- Bjarnason, I.; Sission, G.; Hayee, B. A Randomised, Double-Blind, Placebo-Controlled Trial of a Multi-Strain Probiotic in Patients with Asymptomatic Ulcerative Colitis and Crohn’s Disease. Inflammopharmacology 2019, 27, 465–473. [Google Scholar] [CrossRef]
- Derwa, Y.; Gracie, D.J.; Hamlin, P.J.; Ford, A.C. Systematic Review with Meta-Analysis: The Efficacy of Probiotics in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2017, 46, 389–400. [Google Scholar] [CrossRef]
- Fedorak, R.N.; Feagan, B.G.; Hotte, N.; Leddin, D.; Dieleman, L.A.; Petrunia, D.M.; Enns, R.; Bitton, A.; Chiba, N.; Paré, P.; et al. The Probiotic VSL#3 Has Anti-Inflammatory Effects and Could Reduce Endoscopic Recurrence after Surgery for Crohn’s Disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2015, 13, 928–935.e2. [Google Scholar] [CrossRef]
- Gupta, P.; Andrew, H.; Kirschner, B.S.; Guandalini, S. Is Lactobacillus GG Helpful in Children with Crohn’s Disease? Results of a Preliminary, Open-Label Study. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 453–457. [Google Scholar] [CrossRef]
- Bousvaros, A.; Guandalini, S.; Baldassano, R.N.; Botelho, C.; Evans, J.; Ferry, G.D.; Goldin, B.; Hartigan, L.; Kugathasan, S.; Levy, J.; et al. A Randomized, Double-Blind Trial of Lactobacillus GG versus Placebo in Addition to Standard Maintenance Therapy for Children with Crohn’s Disease. Inflamm. Bowel Dis. 2005, 11, 833–839. [Google Scholar] [CrossRef]
- Yılmaz, İ.; Dolar, M.E.; Özpınar, H. Effect of Administering Kefir on the Changes in Fecal Microbiota and Symptoms of Inflammatory Bowel Disease: A Randomized Controlled Trial. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2019, 30, 242–253. [Google Scholar] [CrossRef]
- Benjamin, J.L.; Hedin, C.R.H.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Hart, A.L.; Kamm, M.A.; Sanderson, J.D.; Knight, S.C.; Forbes, A.; et al. Randomised, Double-Blind, Placebo-Controlled Trial of Fructo-Oligosaccharides in Active Crohn’s Disease. Gut 2011, 60, 923–929. [Google Scholar] [CrossRef]
- Steed, H.; Macfarlane, G.T.; Blackett, K.L.; Bahrami, B.; Reynolds, N.; Walsh, S.V.; Cummings, J.H.; Macfarlane, S. Clinical Trial: The Microbiological and Immunological Effects of Synbiotic Consumption—A Randomized Double-Blind Placebo-Controlled Study in Active Crohn’s Disease. Aliment. Pharmacol. Ther. 2010, 32, 872–883. [Google Scholar] [CrossRef]
- Ferguson, J.F.; Allayee, H.; Gerszten, R.E.; Ideraabdullah, F.; Kris-Etherton, P.M.; Ordovás, J.M.; Rimm, E.B.; Wang, T.J.; Bennett, B.J. Nutrigenomics, the Microbiome, and Gene-Environment Interactions: New Directions in Cardiovascular Disease Research, Prevention, and Treatment: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Genet. 2016, 9, 291–313. [Google Scholar] [CrossRef]
- Ferguson, L.R. Nutritional Modulation of Gene Expression: Might This Be of Benefit to Individuals with Crohn’s Disease? Front. Immunol. 2015, 6, 467. [Google Scholar] [CrossRef]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Han, D.Y.; Fraser, A.G.; Ferguson, L.R. Transcriptomics to Study the Effect of a Mediterranean-Inspired Diet on Inflammation in Crohn’s Disease Patients. Hum. Genom. 2013, 7, 24. [Google Scholar] [CrossRef]
- Coughlin, S.S. Toward a Road Map for Global -Omics: A Primer on -Omic Technologies. Am. J. Epidemiol. 2014, 180, 1188–1195. [Google Scholar] [CrossRef]
- Bennike, T.; Birkelund, S.; Stensballe, A.; Andersen, V. Biomarkers in Inflammatory Bowel Diseases: Current Status and Proteomics Identification Strategies. World J. Gastroenterol. 2014, 20, 3231–3244. [Google Scholar] [CrossRef]
- Goyette, P.; Boucher, G.; Mallon, D.; Ellinghaus, E.; Jostins, L.; Huang, H.; Ripke, S.; Gusareva, E.S.; Annese, V.; Hauser, S.L.; et al. High-Density Mapping of the MHC Identifies a Shared Role for HLA-DRB1*01:03 in Inflammatory Bowel Diseases and Heterozygous Advantage in Ulcerative Colitis. Nat. Genet. 2015, 47, 172–179. [Google Scholar] [CrossRef]
- Fenech, M.F. Nutriomes and Nutrient Arrays—The Key to Personalised Nutrition for DNA Damage Prevention and Cancer Growth Control. Genome Integr. 2010, 1, 11. [Google Scholar] [CrossRef]
- Fenech, M.F. Dietary Reference Values of Individual Micronutrients and Nutriomes for Genome Damage Prevention: Current Status and a Road Map to the Future. Am. J. Clin. Nutr. 2010, 91, 1438S–1454S. [Google Scholar] [CrossRef]
- Gruber, L.; Lichti, P.; Rath, E.; Haller, D. Nutrigenomics and Nutrigenetics in Inflammatory Bowel Diseases. J. Clin. Gastroenterol. 2012, 46, 735–747. [Google Scholar] [CrossRef]
- Naderi, N.; Farnood, A.; Habibi, M.; Derakhshan, F.; Balaii, H.; Motahari, Z.; Agah, M.R.; Firouzi, F.; Rad, M.G.; Aghazadeh, R.; et al. Association of Vitamin D Receptor Gene Polymorphisms in Iranian Patients with Inflammatory Bowel Disease. J. Gastroenterol. Hepatol. 2008, 23, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.D.; Mullighan, C.; Welsh, K.I.; Jewell, D.P. Vitamin D Receptor Gene Polymorphism: Association with Crohn’s Disease Susceptibility. Gut 2000, 47, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chao, L.-K.; Hung, P.-H.; Chen, Y.-J. EGCG Inhibits the Growth and Tumorigenicity of Nasopharyngeal Tumor-Initiating Cells through Attenuation of STAT3 Activation. Int. J. Clin. Exp. Pathol. 2014, 7, 2372–2381. [Google Scholar] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of Inflammatory Responses by Gut Microbiota and Chemoattractant Receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Gurav, A.; Paschall, A.V.; Coe, G.L.; Chaudhary, K.; Cai, Y.; Kolhe, R.; Martin, P.; Browning, D.; Huang, L.; et al. An Essential Role of Ffar2 (Gpr43) in Dietary Fibre-Mediated Promotion of Healthy Composition of Gut Microbiota and Suppression of Intestinal Carcinogenesis. Oncogenesis 2016, 5, e238. [Google Scholar] [CrossRef]
- Petermann, I.; Triggs, C.M.; Huebner, C.; Han, D.Y.; Gearry, R.B.; Barclay, M.L.; Demmers, P.S.; McCulloch, A.; Ferguson, L.R. Mushroom Intolerance: A Novel Diet-Gene Interaction in Crohn’s Disease. Br. J. Nutr. 2009, 102, 506–508. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, W.S.; Kim, D.O.; Byun, J.-E.; Huy, H.; Lee, S.Y.; Song, H.Y.; Park, Y.-J.; Kim, T.-D.; Yoon, S.R.; et al. Macrophage Migration Inhibitory Factor Interacts with Thioredoxin-Interacting Protein and Induces NF-ΚB Activity. Cell. Signal. 2017, 34, 110–120. [Google Scholar] [CrossRef]
- Dotimas, J.R.; Lee, A.W.; Schmider, A.B.; Carroll, S.H.; Shah, A.; Bilen, J.; Elliott, K.R.; Myers, R.B.; Soberman, R.J.; Yoshioka, J.; et al. Diabetes Regulates Fructose Absorption through Thioredoxin-Interacting Protein. eLife 2016, 5. [Google Scholar] [CrossRef]
- Park, M.-J.; Kim, D.-I.; Lim, S.-K.; Choi, J.-H.; Kim, J.-C.; Yoon, K.-C.; Lee, J.-B.; Lee, J.-H.; Han, H.-J.; Choi, I.-P.; et al. Thioredoxin-Interacting Protein Mediates Hepatic Lipogenesis and Inflammation via PRMT1 and PGC-1α Regulation in Vitro and in Vivo. J. Hepatol. 2014, 61, 1151–1157. [Google Scholar] [CrossRef]
- Segata, N. Gut Microbiome: Westernization and the Disappearance of Intestinal Diversity. Curr. Biol. 2015, 25, R611–R613. [Google Scholar] [CrossRef]
- Broussard, J.L.; Devkota, S. The Changing Microbial Landscape of Western Society: Diet, Dwellings and Discordance. Mol. Metab. 2016, 5, 737–742. [Google Scholar] [CrossRef]
- Cooney, J.M.; Barnett, M.P.G.; Brewster, D.; Knoch, B.; McNabb, W.C.; Laing, W.A.; Roy, N.C. Proteomic Analysis of Colon Tissue from Interleukin-10 Gene-Deficient Mice Fed Polyunsaturated Fatty Acids with Comparison to Transcriptomic Analysis. J. Proteome Res. 2012, 11, 1065–1077. [Google Scholar] [CrossRef]
- Hume, G.E.; Fowler, E.V.; Griffiths, L.R.; Doecke, J.D.; Radford-Smith, G.L. Common PPARγ Variants C161T and Pro12Ala Are Not Associated with Inflammatory Bowel Disease in an Australian Cohort. J. Gastrointest. Liver Dis. JGLD 2012, 21, 349–355. [Google Scholar]
- Bassaganya-Riera, J.; Hontecillas, R. Dietary Conjugated Linoleic Acid and N-3 Polyunsaturated Fatty Acids in Inflammatory Bowel Disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 569–573. [Google Scholar] [CrossRef]
- Barua, A.B.; Olson, J.A. Beta-Carotene Is Converted Primarily to Retinoids in Rats in Vivo. J. Nutr. 2000, 130, 1996–2001. [Google Scholar] [CrossRef]
- Leung, W.C.; Hessel, S.; Méplan, C.; Flint, J.; Oberhauser, V.; Tourniaire, F.; Hesketh, J.E.; von Lintig, J.; Lietz, G. Two Common Single Nucleotide Polymorphisms in the Gene Encoding Beta-Carotene 15,15’-Monoxygenase Alter Beta-Carotene Metabolism in Female Volunteers. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 1041–1053. [Google Scholar] [CrossRef]
- Laing, B.B.; Lim, A.G.; Ferguson, L.R. A Personalised Dietary Approach-A Way Forward to Manage Nutrient Deficiency, Effects of the Western Diet, and Food Intolerances in Inflammatory Bowel Disease. Nutrients 2019, 11, 1532. [Google Scholar] [CrossRef]
- Fernández-Ponce, C.; Navarro Quiroz, R.; Díaz Perez, A.; Aroca Martinez, G.; Cadena Bonfanti, A.; Acosta Hoyos, A.; Gómez Escorcia, L.; Hernández Agudelo, S.; Orozco Sánchez, C.; Villarreal Camacho, J.; et al. MicroRNAs Overexpressed in Crohn’s Disease and Their Interactions with Mechanisms of Epigenetic Regulation Explain Novel Aspects of Crohn’s Disease Pathogenesis. Clin. Epigenetics 2021, 13, 39. [Google Scholar] [CrossRef]
- Kalla, R.; Ventham, N.T.; Kennedy, N.A.; Quintana, J.F.; Nimmo, E.R.; Buck, A.H.; Satsangi, J. MicroRNAs: New Players in IBD. Gut 2015, 64, 504–517. [Google Scholar] [CrossRef]
- Krissansen, G.W.; Yang, Y.; McQueen, F.M.; Leung, E.; Peek, D.; Chan, Y.C.; Print, C.; Dalbeth, N.; Williams, M.; Fraser, A.G. Overexpression of MiR-595 and MiR-1246 in the Sera of Patients with Active Forms of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 520–530. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 Fatty Acids in Health and Disease and in Growth and Development. Am. J. Clin. Nutr. 1991, 54, 438–463. [Google Scholar] [CrossRef]
- Razack, R.; Seidner, D.L. Nutrition in Inflammatory Bowel Disease. Curr. Opin. Gastroenterol. 2007, 23, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Thomas, C.J.; Radcliffe, J.; Itsiopoulos, C. Omega-3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer’s Disease. BioMed Res. Int. 2015, 2015, 172801. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-M.; Kwon, S.-H.; Han, Y.-M.; Hahm, K.-B.; Kim, E.-H. Omega-3 Polyunsaturated Fatty Acids as Potential Chemopreventive Agent for Gastrointestinal Cancer. J. Cancer Prev. 2013, 18, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.C.; Singh, S.; Craig, J.P.; Downie, L.E. Omega-3 Fatty Acids and Eye Health: Opinions and Self-Reported Practice Behaviors of Optometrists in Australia and New Zealand. Nutrients 2020, 12, 1179. [Google Scholar] [CrossRef]
- Eaton, S.B.; Konner, M. Paleolithic Nutrition. A Consideration of Its Nature and Current Implications. N. Engl. J. Med. 1985, 312, 283–289. [Google Scholar] [CrossRef]
- Leaf, A.; Weber, P.C. A New Era for Science in Nutrition. Am. J. Clin. Nutr. 1987, 45, 1048–1053. [Google Scholar] [CrossRef]
- Wacklin, P.; Mäkivuokko, H.; Alakulppi, N.; Nikkilä, J.; Tenkanen, H.; Räbinä, J.; Partanen, J.; Aranko, K.; Mättö, J. Secretor Genotype (FUT2 Gene) Is Strongly Associated with the Composition of Bifidobacteria in the Human Intestine. PLoS ONE 2011, 6, e20113. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. MMBR 2017, 81. [Google Scholar] [CrossRef]
- Sloan, T.J.; Jalanka, J.; Major, G.A.D.; Krishnasamy, S.; Pritchard, S.; Abdelrazig, S.; Korpela, K.; Singh, G.; Mulvenna, C.; Hoad, C.L.; et al. A Low FODMAP Diet Is Associated with Changes in the Microbiota and Reduction in Breath Hydrogen but Not Colonic Volume in Healthy Subjects. PLoS ONE 2018, 13, e0201410. [Google Scholar] [CrossRef]
- Mishkin, S. Dairy Sensitivity, Lactose Malabsorption, and Elimination Diets in Inflammatory Bowel Disease. Am. J. Clin. Nutr. 1997, 65, 564–567. [Google Scholar] [CrossRef]
- Enattah, N.S.; Sahi, T.; Savilahti, E.; Terwilliger, J.D.; Peltonen, L.; Järvelä, I. Identification of a Variant Associated with Adult-Type Hypolactasia. Nat. Genet. 2002, 30, 233–237. [Google Scholar] [CrossRef]
- Nolan, D.J.; Han, D.Y.; Lam, W.J.; Morgan, A.R.; Fraser, A.G.; Tapsell, L.C.; Ferguson, L.R. Genetic Adult Lactase Persistence Is Associated with Risk of Crohn’s Disease in a New Zealand Population. BMC Res. Notes 2010, 3, 339. [Google Scholar] [CrossRef]
| Dietary Treatment | Putative Action | Clinical Impact | ||
|---|---|---|---|---|
| Enteral nutrition (EN) | Promotes gut health [29] | EN promotes CD remission [33] | ||
| Parenteral nutrition (PN) | Promotes gut health [63] | PN favors CD remission. This diet is particularly indicated for malnourished patients during an acute inflammatory phase or post-operative complications affecting gastrointestinal function [61,62] | ||
| Specific carbohydrate diet (SCD) | Promotes gut health. “Forbidden” foods are sucrose, maltose, isomaltose, lactose, potatoes, okra, corn, fluid milk, soy, cheeses with a high amount of lactose such as fresh cheese, food additives, and preservatives [79] | SCD improves symptoms and quality of life and, in some cases, maintains remission with no need of medications [79]. In children, SCD promotes mucosal healing [81]. SCD normalizes inflammatory markers, e.g., CRP and fecal calprotectin, and serum albumin [82,83,84] | ||
| Low FODMAP diet | Promotes gut health. “Forbidden” foods are fermentables, oligosaccharides, disaccharides, monosaccharides, and polyols [86] | Improved gastrointestinal symptoms [1,86]; no evidence that calpotectin levels or luminal inflammation ameliorate [87] | ||
| Flexitarian or semi-vegetarian diet (SVD) | It promotes gut health. Limited amounts of meat and fish are allowed [93] | SVD is effective in preventing CD relapse [93] | ||
| Low fat/fiber limited exclusion diet (LOFFLEX) | Elemental formula followed by an exclusion diet in a well-structured protocol [79] | Possible induction of CD remission although its efficacy is not yet fully demonstrated [79] | ||
| Paleolithic diet | Maker’s diet | Vegan diet | Elimination diets [79] | Efficacy not demonstrated yet [79] |
| Gluten free diet | Absence of gluten intake [79] | Contrasting data [79] | ||
| Food Supplement | Mechanism of Action | Clinical Impact |
|---|---|---|
| Probiotics | Mainly bacteria able to reach the small intestine and the colon alive, providing positive interaction with gut microbiota of the host. Probiotics may exert various beneficial effects, including antimicrobial action, mucosal integrity, and enhancing the host immune response [100] | Uncertain clinical efficacy in CD patients. Kefir drink, a probiotic mix, improves abdominal pain, bloating, and inflammatory markers, along with increasing wellbeing sensation [110] |
| Prebiotics | Indigestible dietary compounds fueling beneficial bacteria of the gut microbiota | No major improvement of CD activity index, endoscopic score, or histopayhology [87,111] |
| Symbiotics | Combination of probiotics and prebiotics | A symbiotic containing Bifidobacterium longum and Synergy1 improved CD activity and histological scores [112] |
| Food/Dietary Component | Putative Mechanism | Effects |
|---|---|---|
| The green tea polyphenol EGCG | Limits the activation of the STAT3 pathway [114,124] | Anti-inflammatory effect [114,124] |
| Low intake of fibers | Reduced SCFAs production by dietary fiber fermenting bacteria, down-regulating the FFAR2 signaling [125,126] | FFAR2 mutations worsen fiber tolerance in CD patients [126] |
| Artificial sweeteners, infant formula, food emulsifiers, and antibiotics | Promote dysbiosis [131,132] | Increased risk of IBD onset [131,132] |
| Cruciferous vegetables | Antioxidant effects [14] | Detrimental effects on CD patients carrying the SNPs on the major histocompatibility complex [14] |
| Mushrooms | Act on the mutated T allele of the OCTN1 (c. 1672 C > T) gene [127] | People suffering from CD and carrying the genetic mutation show mushroom sensitivity [127] |
| Fructose | Enhances TXNIP gene expression [128,129,130] | Promotes inflammation in endothelial cells, eliciting hepatic inflammation, and contributes to NF-κB regulation [128,129,130] |
| Genetic Abnormality | Mechanism of Action | Effects |
|---|---|---|
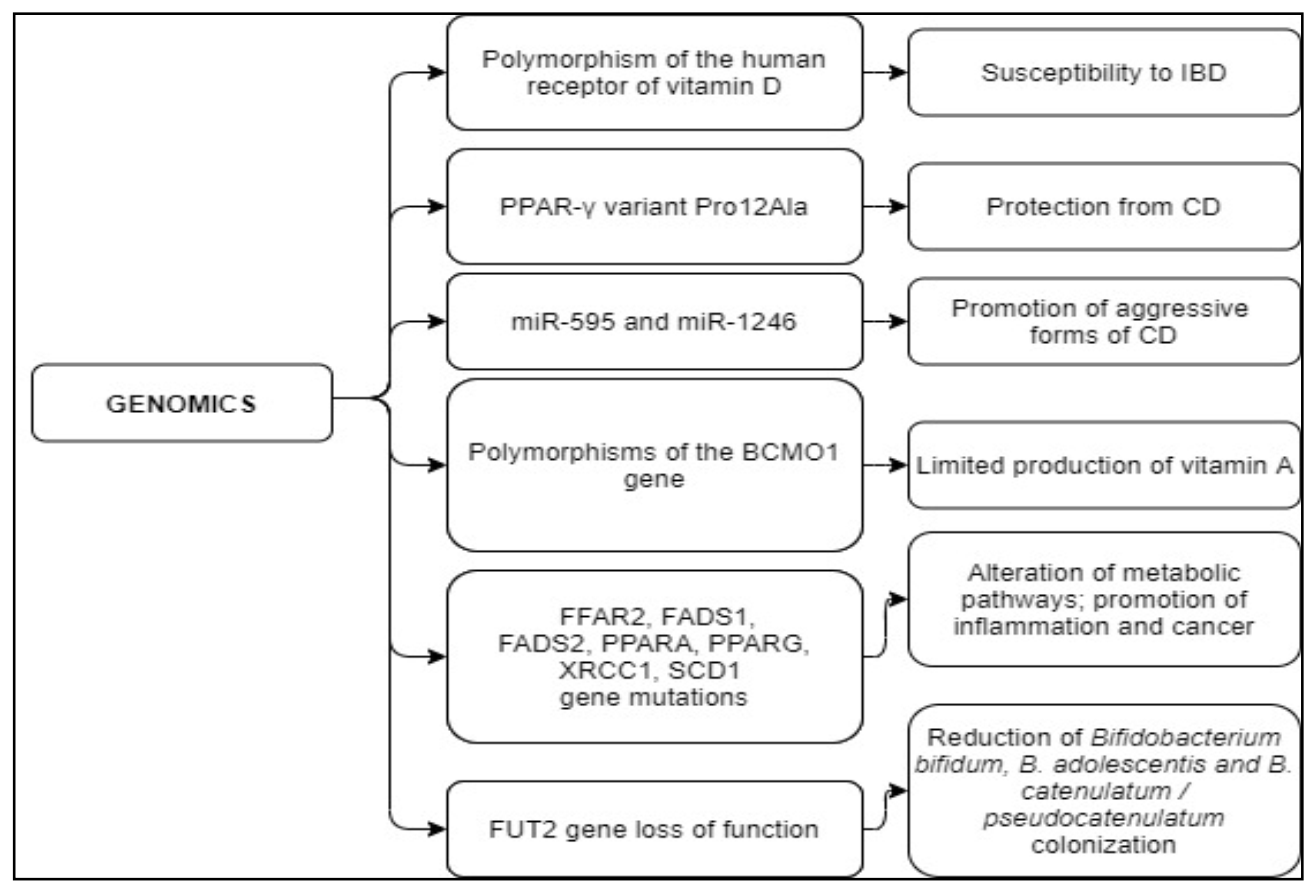
| Polymorphisms of the human receptor of vitamin D | The vitamin D receptor form is different from the classical one [121,122,123] | Polymorphism increasing susceptibility to IBD [121,122,123] |
| PPAR-γ variant Pro12Ala | Regulation of the immune response [134,135] | Variant protecting from CD [134,135] |
| miR-595 and miR-1246 | Small non-coding RNA molecule promote RNA silencing and post-transcriptional regulation of gene expression [139,140,141] | High levels of circulating miR-595 and miR-1246 are associated with a more aggressive form of the disease [141] |
| Polymorphisms of the gene BCMO1 (R267S: rs12934922 or A379V: rs7501331) | The conversion from beta-carotene to retinol does not occur [136,137,138] | Limited vitamin A production [136,137,138] |
| FFAR2, FADS1, FADS2, PPARA, PPARG, XRCC1, SCD1 gene mutations | Act on serum levels of LC-PUFA-omega-3 and omega-6 fatty acids [138] | Affect metabolic pathways and inflammation; increase cancer risk [138] |
| FUT2 gene loss of function | FUT2 function is lost [150] | Significant reduction of Bifidobacterium bifidum, B. adolescentis, and B. catenulatum/pseudocatenulatum colonization [150] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caio, G.; Lungaro, L.; Caputo, F.; Zoli, E.; Giancola, F.; Chiarioni, G.; De Giorgio, R.; Zoli, G. Nutritional Treatment in Crohn’s Disease. Nutrients 2021, 13, 1628. https://doi.org/10.3390/nu13051628
Caio G, Lungaro L, Caputo F, Zoli E, Giancola F, Chiarioni G, De Giorgio R, Zoli G. Nutritional Treatment in Crohn’s Disease. Nutrients. 2021; 13(5):1628. https://doi.org/10.3390/nu13051628
Chicago/Turabian StyleCaio, Giacomo, Lisa Lungaro, Fabio Caputo, Eleonora Zoli, Fiorella Giancola, Giuseppe Chiarioni, Roberto De Giorgio, and Giorgio Zoli. 2021. "Nutritional Treatment in Crohn’s Disease" Nutrients 13, no. 5: 1628. https://doi.org/10.3390/nu13051628
APA StyleCaio, G., Lungaro, L., Caputo, F., Zoli, E., Giancola, F., Chiarioni, G., De Giorgio, R., & Zoli, G. (2021). Nutritional Treatment in Crohn’s Disease. Nutrients, 13(5), 1628. https://doi.org/10.3390/nu13051628








