Review on the Regional Effects of Gastrointestinal Luminal Stimulation on Appetite and Energy Intake: (Pre)clinical Observations
Abstract
:1. Introduction
- Are regional differences (duodenum, jejunum, ileum) present in the intestinal luminal modulation of appetite and energy intake?
- Is the “intestinal brake” effect on appetite and energy intake macronutrient specific?
- Is the “intestinal brake” effect that is observed in acute intervention studies maintained during repetitive activation?
- Can the “intestinal brake” effect on appetite and energy intake be activated via non-caloric tastants?
2. Nutrient Sensing in the Gut
3. Gastric Satiation Signals
4. Intestinal Satiation Signals
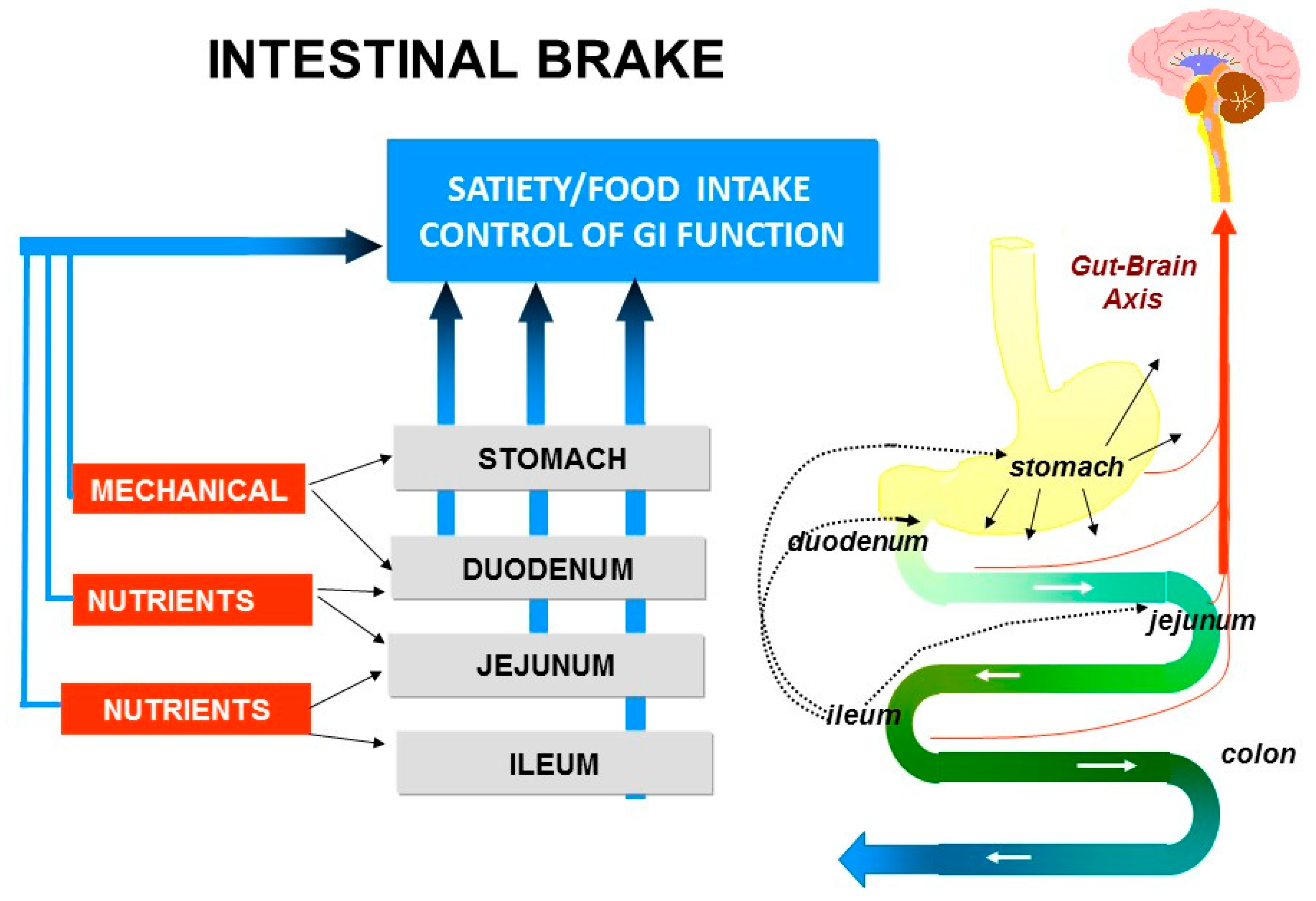
5. Intestinal Brakes
6. Topics in Intestinal Brake, Appetite and Energy Intake
- (1)
- Are regional differences (duodenum, jejunum, ileum) present in the intestinal luminal modulation of appetite and energy intake?
- (2)
- Is the “intestinal brake” effect on appetite and energy macronutrient specific? Are differences present between fat, carbohydrates and proteins?
- (3)
- Is the “intestinal brake” effect observed in acute intervention studies, maintained during repetitive activation?
- (4)
- Can the “intestinal brake” effect on appetite and energy intake be activated via non-caloric tastants?
6.1. Methods
6.2. Topic 1: Site Specific Effects on Food Intake and Satiety: Duodenum-Jejunum-Ileum
6.2.1. Dietary Fat: Site Specific Effects?
6.2.2. Dietary Proteins: Site Specific Effects?
6.2.3. Dietary Carbohydrates: Site Specific Effects?
6.3. Topic 2: Is the Intestinal Brake Effect on Appetite and Energy Intake Macronutrient Specific?
6.4. Topic 3: Is the Acute “Intestinal Brake” Effect Maintained during Repetitive Activation?
6.5. Topic 1–3: Intestinal Brake to Nutrients: Summary of Findings and Perspectives
6.6. Topic 4: Can the “Intestinal Brake” Effect on Appetite and Energy Intake Be Activated via Non-Caloric Tastants?
7. Conclusions
- (1)
- Regional differences exist in the intestinal modulation of appetite and energy intake with a distal to proximal gradient for inhibition of energy intake: ileum and jejunum > duodenum. This distal to proximal gradient effect remains after correction for the caloric load of the nutrients infused.
- (2)
- The “intestinal brake” effect on appetite and energy appears not to be macronutrient specific. At equicaloric amounts, the inhibition on energy intake and appetite is in the same range for fat, protein and carbohydrate.
- (3)
- Data on repetitive ileal brake activation are scarce because of the need for prolonged intestinal intubation. During repetitive activation of the ileal brake for up to 4 days, no adaptation was observed but overall, the inhibitory effect on energy intake was small.
- (4)
- The concept of influencing energy intake by intra-intestinal delivery of non-caloric tastants is intriguing. Thus far, the available data show that, among tastants, bitter compounds appear to be more effective in influencing energy intake. Energy intake, in the acute setting, decreased modestly after post-oral delivery of bitter tastants or a combination of tastants (bitter, sweet and umami). An advantage is that tastants are non-caloric, in contrast to nutrients. Future studies should focus on optimal dosing and delivery of tastants and their mechanisms of action.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Reference | Location | Infusate | Reduction in Energy Intake (EI) of Meal | Net Effect: Reduction EI Meal-EI Infusate | |
|---|---|---|---|---|---|
| Type | Energy Content of Infusate | ||||
| 10 | Jejunum | corn oil | 370 kcal | 1100 kcal | +++ |
| 10 | Ileum | corn oil | 370 kca | 650 kcal | ++ |
| 13 | Ileum | corn oil | 358 kcal | 570 kcal | ++ |
| 19 | Ileum | canola oil | 54 kcal | 60 kcal | = |
| 22 | Duodenum | corn oil | 75 kcal | 55 kcal | − |
| 22 | Duodenum | corn oil | 200 kcal | 150 kcal | − |
| 24 | Duodenum | Intralipid | 200 kcal | 100 kcal | − |
| 26 | Duodenum | Intralipid | 360 kcal | 204 kcal | − (obese: BMI 30–40 kg/m2) |
| 26 | Duodenum | Intralipid | 360 kcal | 214 kcal | − (lean: BMI 19–26 kg/m2) |
| 27 | Duodenum | Intralipid | 343 kcal | 200 kcal | − |
| 29 | Duodenum | Intralipid | 270 kcal | 170 kcal | − |
| 30 | Duodenum | Intralipid | 270 kcal | 250 kcal | = |
| 31 | Jejunum | corn oil | 370 kcal | 200 kcal | − |
| 32 | Ileum | safflower oil | 52 kcal | 120 kcal | + |
| 33 | Duodenum | rapeseed oil | 54 kcal | 14 kcal | − |
| Ileum | rapeseed oil | 54 kcal | 18 kcal | − | |
| 20 | Duodenum | lauric acid | 33 kcal | 714 kcal | +++ |
| 34 | Duodenum | lauric acid | 36 kcal | 270 kcal | ++ |
| 35 | Duodenum | lauric acid | 9 kcal | 52 kcal | + |
| 36 | Duodenum | lauric acid | 24 kcal | 130 kcal | + |
| 37 | Duodenum | LCT | 370 kcal | 325 kcal | − |
| 37 | Duodenum | LCFA | 46 kcal | 370 kcal | ++ |
| Reference | Location | Infusate: | Reduction in Energy Intake (EI) of Meal | Net Effect: Reduction EI Meal-EI Infusate | |
|---|---|---|---|---|---|
| Type | Energy Content of Infusate | ||||
| 29 | Duodenum | whey | 270 kcal | 210 kcal | − |
| 39 | Duodenum | whey | 30 kcal | 46 kcal | + |
| 90 kcal | 160 kcal | + | |||
| 180 kcal | 315 kcal | ++ | |||
| 32 | Ileum | casein | 17 kcal | 60 kcal | + |
| 52 kcal | 130 kcal | + | |||
| 35 | Duodenum | tryptophan | 9 kcal | +37 kcal | − |
| 40Δyoung men, mean age 23 (19–29) yrs | Duodenum | whey | 30 kcal | 147 kcal | ++ |
| 90 kcal | 240 kcal | ++ | |||
| 180 kcal | 419 kcal | ++ | |||
| 40Δolder men, mean age 74 (68–81) yrs | Duodenum | whey | 30 kcal | +60 kcal | − |
| 90 kcal | +55 kcal | − | |||
| 180 kcal | 180 kcal | = | |||
| 41 | Duodenum | casein | 60 kcal | +20 kcal | − |
| Jejunum | 60 kcal | 40 kcal | − | ||
| Ileum | 60 kcal | 84 kcal | + | ||
| 42 | Duodenum | tryptophan | 7 kcal | 60 kcal | + |
| 14 kcal | 220 kcal | ++ | |||
| 43 | Duodenum | leucine | 13 kcal | 59 kcal | + |
| 40 kcal | 170 kcal | ++ | |||
| Reference | Location | Infusate | Reduction in Energy Intake (EI) of Meal | Net Effect: Reduction EI Meal-EI Infusate | |
|---|---|---|---|---|---|
| Type | Energy Content of Infusate | ||||
| 23 | Duodenum | glucose | 120 kcal | +128 kcal | − |
| 240 kcal | +135 kcal | − | |||
| 480 kcal | 119 kcal | − | |||
| 26 | Duodenum | glucose | 342 kcal | 98 kcal (BMI 30–40 kg/m2) | − |
| 342 kcal | 63 kcal (BMI 19–29 kg/m2) | − | |||
| 27 | Duodenum | glucose | 342 kcal | 140 kcal | − |
| 28 | Duodenum | glucose | 348 kcal | 350 kcal | = |
| 32 | Ileum | sucrose | 17 kcal | 95 kcal | + |
| 52 kcal | 187 kcal | ++ | |||
| 45 | Duodenum | glucose | 287 kcal | 402 kcal | + |
| 46 | Duodenum | glucose | 180 kcal | 30 kcal | − |
| fructose | 180 kcal | 200 kcal | + | ||
| 48 | Duodenum | glucose | 56 kcal | 58 kcal | = |
| Ileum | glucose | 56 kcal | 119 kcal | + | |
| 49 | Duodenum | glucose | 288 kcal | 184 kcal | − |
References
- Cummings, D.E.; Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Investig. 2007, 117, 13–23. [Google Scholar] [CrossRef]
- Burdyga, G.; Lal, S.; Varro, A.; Dimaline, R.; Thompson, D.G.; Dockray, G.J. Expression of Cannabinoid CB1 Receptors by Vagal Afferent Neurons Is Inhibited by Cholecystokinin. J. Neurosci. 2004, 24, 2708–2715. [Google Scholar] [CrossRef] [Green Version]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90–94. [Google Scholar] [CrossRef]
- Lauffer, L.M.; Iakoubov, R.; Brubaker, P.L. GPR119 Is Essential for Oleoylethanolamide-Induced Glucagon-Like Peptide-1 Secretion from the Intestinal Enteroendocrine L-Cell. Diabetes 2009, 58, 1058–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Katsuma, S.; Adachi, T.; Koshimizu, T.-A.; Hirasawa, A.; Tsujimoto, G. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedeberg’s Arch. Pharmacol. 2007, 377, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Depoortere, I. Taste receptors of the gut: Emerging roles in health and disease. Gut 2014, 63, 179–190. [Google Scholar] [CrossRef]
- Phillips, R.J.; Powley, T.L. Gastric volume rather than nutrient content inhibits food intake. Am. J. Physiol. 1996, 271, R766–R769. [Google Scholar] [CrossRef] [PubMed]
- Powley, T.L.; Phillips, R.J. Gastric satiation is volumetric, intestinal satiation is nutritive. Physiol. Behav. 2004, 82, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.; Young, R.C.; Smith, G.P. Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature 1973, 245, 323–325. [Google Scholar] [CrossRef]
- Welch, I.M.; Sepple, C.P.; Read, N.W. Comparisons of the effects on satiety and eating behaviour of infusion of lipid into the different regions of the small intestine. Gut 1988, 29, 306–311. [Google Scholar] [CrossRef]
- Read, N.W.; McFarlane, A.; Kinsman, R.I.; Bates, T.E.; Blackhall, N.W.; Farrar, G.B.; Hall, J.C.; Moss, G.; Morris, A.P.; O’Neill, B.; et al. Effect of infusion of nutrient solutions into the ileum on gastrointestinal transit and plasma levels of neurotensin and enteroglucagon. Gastroenterology 1984, 86, 274–280. [Google Scholar] [CrossRef]
- Spiller, R.C.; Trotman, I.F.; Higgins, B.E.; Ghatei, M.A.; Grimble, G.K.; Lee, Y.C.; Bloom, S.R.; Misiewicz, J.J.; Silk, D.B. The ileal brake inhibition of jejunal motility after ileal fat perfusion in man. Gut 1984, 25, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Welch, I.; Saunders, K.; Read, N. Effect of ileal and intravenous infusions of fat emulsions on feeding and satiety in human volunteers. Gastroenterology 1985, 89, 1293–1297. [Google Scholar] [CrossRef]
- Layer, P.; Peschel, S.; Schlesinger, T.; Goebell, H. Human pancreatic secretion and intestinal motility: Effects of ileal nutrient perfusion. Am. J. Physiol. Liver Physiol. 1990, 258, G196–G201. [Google Scholar] [CrossRef]
- Koopmans, H.S.; Sclafani, A. Control of body weight by lower gut signals. Int. J. Obes. 1981, 5, 491–495. [Google Scholar] [PubMed]
- Strader, A.D.; Vahl, T.P.; Jandacek, R.J.; Woods, S.C.; D’Alessio, D.A.; Seeley, R.J. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am. J. Physiol. Metab. 2005, 288, E447–E453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilichiewicz, A.N.; Papadopoulos, P.; Brennan, I.M.; Little, T.J.; Meyer, J.H.; Wishart, J.M.; Horowitz, M.; Feinle-Bisset, C. Load-dependent effects of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am. J. Physiol. Integr. Comp. Physiol. 2007, 293, R2170–R2178. [Google Scholar] [CrossRef] [Green Version]
- Pilichiewicz, A.N.; Little, T.J.; Brennan, I.M.; Meyer, J.H.; Wishart, J.M.; Otto, B.; Horowitz, M.; Feinle-Bisset, C. Effects of load, and duration, of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am. J. Physiol. Integr. Comp. Physiol. 2006, 290, R668–R677. [Google Scholar] [CrossRef] [Green Version]
- Maljaars, P.W.; Masclee, A.A.M. Both intestinal site and timing of fat delivery affect appetite in humans. In Intestinal Fat and Eating Behavior: Role of the Ileal Brake; Datawyse/Universitaire PersMaastricht: Maastricht, The Netherland, 2010; pp. 109–126. [Google Scholar]
- Castiglione, E.K.; Read, N.W.; French, S.J. Food Intake Responses to Upper Gastrointestinal Lipid Infusions in Humans. Physiol. Behav. 1998, 64, 141–145. [Google Scholar] [CrossRef]
- Chapman, I.M.; Goble, E.A.; Wittert, G.A.; Horowitz, M. Effects of small-intestinal fat and carbohydrate infusions on appetite and food intake in obese and nonobese men. Am. J. Clin. Nutr. 1999, 69, 6–12. [Google Scholar] [CrossRef] [Green Version]
- MacIntosh, C.G.; Horowitz, M.; Verhagen, M.A.; Smout, A.J.; Wishart, J.; Morris, H.; Goble, E.; Morley, J.E.; Chapman, I.M. Effect of Small Intestinal Nutrient Infusion on Appetite, Gastrointestinal Hormone Release, and Gastric Myoelectrical Activity in Young and Older Men. Am. J. Gastroenterol. 2001, 96, 997–1007. [Google Scholar] [CrossRef]
- Cook, C.G.; Andrews, J.M.; Jones, K.L.; Wittert, G.A.; Chapman, I.M.; Morley, J.E.; Horowitz, M. Effects of small intestinal nutrient infusion on appetite and pyloric motility are modified by age. Am. J. Physiol. Content 1997, 273, R755–R761. [Google Scholar] [CrossRef]
- Ryan, A.T.; Luscombe-Marsh, N.D.; Saies, A.A.; Little, T.J.; Standfield, S.; Horowitz, M.; Feinle-Bisset, C. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Am. J. Clin. Nutr. 2013, 98, 300–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seimon, R.V.; Feltrin, K.L.; Meyer, J.H.; Brennan, I.M.; Wishart, J.M.; Horowitz, M.; Feinle-Bisset, C. Effects of varying combinations of intraduodenal lipid and carbohydrate on antropyloroduodenal motility, hormone release, and appetite in healthy males. Am. J. Physiol. Integr. Comp. Physiol. 2009, 296, R912–R920. [Google Scholar] [CrossRef] [Green Version]
- Drewe, J.; Gadient, A.; Rovati, L.C.; Beglinger, C. Role of circulating cholecystokinin in control of fat-induced inhibition of food intake in humans. Gastroenterology 1992, 102, 1654–1659. [Google Scholar]
- Maljaars, P.W.J.; Peters, H.P.F.; Kodde, A.; Geraedts, M.; Troost, F.J.; Haddeman, E.; Masclee, A.A.M. Length and site of the small intestine exposed to fat influences hunger and food intake. Br. J. Nutr. 2011, 106, 1609–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maljaars, P.W.J.; Symersky, T.; Kee, B.C.; Haddeman, E.; Peters, H.P.F.; Masclee, A.A.M. Effect of ileal fat perfusion on satiety and hormone release in healthy volunteers. Int. J. Obes. 2008, 32, 1633–1639. [Google Scholar] [CrossRef] [Green Version]
- Van Avesaat, M.; Troost, F.J.; Ripken, D.; Hendriks, H.F.; Masclee, A.A.M. Ileal brake activation: Macronutrient-specific effects on eating behavior? Int. J. Obes. 2014, 39, 235–243. [Google Scholar] [CrossRef]
- Feltrin, K.L.; Little, T.J.; Meyer, J.H.; Horowitz, M.; Rades, T.; Wishart, J.; Feinle-Bisset, C. Effects of lauric acid on upper gut motility, plasma cholecystokinin and peptide YY, and energy intake are load, but not concentration, dependent in humans. J. Physiol. 2007, 581, 767–777. [Google Scholar] [CrossRef]
- McVeay, C.; Fitzgerald, P.C.E.; Ullrich, S.S.; E Steinert, R.; Horowitz, M.; Feinle-Bisset, C. Effects of intraduodenal administration of lauric acid and L-tryptophan, alone and combined, on gut hormones, pyloric pressures, and energy intake in healthy men. Am. J. Clin. Nutr. 2019, 109, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Maljaars, P.J.; van der Wal, R.J.; Wiersma, T.; Peters, H.P.; Haddeman, E.; Masclee, A.A. The effect of lipid droplet size on satiety and peptide secretion is intestinal site-specific. Clin. Nutr. 2012, 31, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Feltrin, K.L.; Little, T.J.; Meyer, J.H.; Horowitz, M.; Rades, T.; Wishart, J.; Feinle-Bisset, C. Comparative effects of intraduodenal infusions of lauric and oleic acids on antropyloroduodenal motility, plasma cholecystokinin and peptide YY, appetite, and energy intake in healthy men. Am. J. Clin. Nutr. 2008, 87, 1181–1187. [Google Scholar] [CrossRef] [Green Version]
- Matzinger, D.; Degen, L.; Drewe, J.; Meuli, J.; Duebendorfer, R.; Ruckstuhl, N.; D’Amato, M.; Rovati, L.; Beglinger, C. The role of long chain fatty acids in regulating food intake and cholecystokinin release in humans. Gut 2000, 46, 689–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, A.T.; Feinle-Bisset, C.; Kallas, A.; Wishart, J.M.; Clifton, P.M.; Horowitz, M.; Luscombe-Marsh, N.D. Intraduodenal protein modulates antropyloroduodenal motility, hormone release, glycemia, appetite, and energy intake in lean men. Am. J. Clin. Nutr. 2012, 96, 474–482. [Google Scholar] [CrossRef] [Green Version]
- Soenen, S.; Giezenaar, C.; Hutchison, A.T.; Horowitz, M.; Chapman, I.; Luscombe-Marsh, N.D. Effects of intraduodenal protein on appetite, energy intake, and antropyloroduodenal motility in healthy older compared with young men in a randomized trial. Am. J. Clin. Nutr. 2014, 100, 1108–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Avesaat, M.; Ripken, D.; Hendriks, H.F.J.; Masclee, A.A.M.; Troost, F.J. Small intestinal protein infusion in humans: Evidence for a location-specific gradient in intestinal feedback on food intake and GI peptide release. Int. J. Obes. 2016, 41, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Luscombe-Marsh, N.D.; Little, T.J.; Standfield, S.; Otto, B.; Horowitz, M.; Feinle-Bisset, C. Effects of Intraduodenal Infusion of L-Tryptophan on ad Libitum Eating, Antropyloroduodenal Motility, Glycemia, Insulinemia, and Gut Peptide Secretion in Healthy Men. J. Clin. Endocrinol. Metab. 2014, 99, 3275–3284. [Google Scholar] [CrossRef] [Green Version]
- Steinert, E.R.; Landrock, M.F.; Ullrich, S.S.; Standfield, S.; Otto, B.; Horowitz, M.; Feinle-Bisset, C. Effects of intraduodenal infusion of the branched-chain amino acid leucine on ad libitum eating, gut motor and hormone functions, and glycemia in healthy men. Am. J. Clin. Nutr. 2015, 102, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Pilichiewicz, A.N.; Chaikomin, R.; Brennan, I.M.; Wishart, J.M.; Rayner, C.K.; Jones, K.L.; Smout, A.J.P.M.; Horowitz, M.; Feinle-Bisset, C. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am. J. Physiol. Metab. 2007, 293, E743–E753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayner, C.K.; Park, H.S.; Wishart, J.M.; Kong, M.-F.; Doran, S.M.; Horowitz, M. Effects of intraduodenal glucose and fructose on antropyloric motility and appetite in healthy humans. Am. J. Physiol. Integr. Comp. Physiol. 2000, 278, R360–R366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaikomin, R.; Wu, K.-L.; Doran, S.; Meyer, J.H.; Jones, K.L.; Feinle-Bisset, C.; Horowitz, M.; Rayner, C.K. Effects of mid-jejunal compared to duodenal glucose infusion on peptide hormone release and appetite in healthy men. Regul. Pept. 2008, 150, 38–42. [Google Scholar] [CrossRef]
- Lavin, J.H.; Wittert, G.A.; Andrews, J.; Yeap, B.; Wishart, J.M.; Morris, H.A.; Morley, J.E.; Horowitz, M.; Read, N.W. Interaction of insulin, glucagon-like peptide 1, gastric inhibitory polypeptide, and appetite in response to intraduodenal carbohydrate. Am. J. Clin. Nutr. 1998, 68, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Poppitt, S.D.; Shin, H.S.; McGill, A.-T.; Budgett, S.C.; Lo, K.; Pahl, M.; Duxfield, J.; Lane, M.; Ingram, J.R. Duodenal and ileal glucose infusions differentially alter gastrointestinal peptides, appetite response, and food intake: A tube feeding study. Am. J. Clin. Nutr. 2017, 106, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Lavin, J.H.; Wittert, G.; Sun, W.M.; Horowitz, M.; Morley, J.E.; Read, N.W. Appetite regulation by carbohydrate: Role of blood glucose and gastrointestinal hormones. Am. J. Physiol. Metab. 1996, 271, E209–E214. [Google Scholar] [CrossRef]
- Feltrin, K.L.; Little, T.J.; Meyer, J.H.; Horowitz, M.; Smout, A.J.P.M.; Wishart, J.; Pilichiewicz, A.N.; Rades, T.; Chapman, I.M.; Feinle-Bisset, C. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am. J. Physiol. Integr. Comp. Physiol. 2004, 287, R524–R533. [Google Scholar] [CrossRef] [Green Version]
- Maljaars, J.; Romeyn, E.A.; Haddeman, E.; Peters, H.P.F.; Masclee, A.A.M. Effect of fat saturation on satiety, hormone release, and food intake. Am. J. Clin. Nutr. 2009, 89, 1019–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feinle, C.; O’Donovan, D.; Doran, S.; Andrews, J.M.; Wishart, J.; Chapman, I.; Horowitz, M. Effects of fat digestion on appetite, APD motility, and gut hormones in response to duodenal fat infusion in humans. Am. J. Physiol. Liver Physiol. 2003, 284, G798–G807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, G.H.; Tecimer, S.N.; Shah, D.; Zafar, T.A. Protein Source, Quantity, and Time of Consumption Determine the Effect of Proteins on Short-Term Food Intake in Young Men. J. Nutr. 2004, 134, 3011–3015. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.C.; Doty, J.E.; Reedy, T.J.; Meyer, J.H. Inhibition of gastric emptying by acids depends on pH, titratable acidity, and length of intestine exposed to acid. Am. J. Physiol. Liver Physiol. 1990, 259, G1025–G1030. [Google Scholar] [CrossRef]
- Meyer, J.H.; Tabrizi, Y.; Dimaso, N.; Hlinka, M.; Raybould, H.E. Length of intestinal contact on nutrient-driven satiety. Am. J. Physiol. Integr. Comp. Physiol. 1998, 275, R1308–R1319. [Google Scholar] [CrossRef] [PubMed]
- Lutz, T.A.; Bueter, M. The physiology underlying Roux-en-Y gastric bypass: A status report. Am. J. Physiol. Integr. Comp. Physiol. 2014, 307, R1275–R1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Avesaat, M.; Troost, F.T.; Ripken, D.; Hendriks, H.F.J.; Masclee, A.A.M. Repeated ileal brake activation: Sustained re-sponses on eating behavior, gastrointestinal motility and peptide release? In Doctoral Thesis: Nutrient sensing in the gut: Appe-Tite Regulation in Health and Obesity; Datawyse/Universitaire PersMaastricht: Maastricht, The Netherlands; pp. 150–164. Available online: https://cris.maastrichtuniversity.nl/en/publications/nutrient-sensing-in-the-gut-appetite-regulation-in-health-and-obe (accessed on 8 April 2020).
- Burns, A.; Livingstone, M.; Welch, R.; Dunne, A.; Reid, C.; Rowland, I. The effects of yoghurt containing a novel fat emulsion on energy and macronutrient intakes in non-overweight, overweight and obese subjects. Int. J. Obes. 2001, 25, 1487–1496. [Google Scholar] [CrossRef] [Green Version]
- Poppitt, S.D.; Han, S.; Strik, C.M.; Kindleysides, S.; Chan, Y.-K. Investigating acute satiation and meal termination effects of a commercial lipid emulsion: A breakfast meal study. Physiol. Behav. 2015, 152, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Corstens, M.N.; Troost, F.J.; Alleleyn, A.M.; Klaassen, T.; Berton-Carabin, C.C.; Schroën, K.; Masclee, A.A. Encapsulation of lipids as emulsion-alginate beads reduces food intake: A randomized placebo-controlled cross-over human trial in overweight adults. Nutr. Res. 2019, 63, 86–94. [Google Scholar] [CrossRef]
- Corstens, M.N.; Berton-Carabin, C.C.; de Vries, R.; Troost, F.J.; Masclee, A.A.; Schroën, K. Food-grade micro-encapsulation systems that may induce satiety via delayed lipolysis: A review. Crit. Rev. Food. Sci. Nutr. 2017, 57, 2218–2244. [Google Scholar] [CrossRef] [PubMed]
- Alleleyn, A.M.E.; Van Avesaat, M.; Ripken, D.; Bleiel, S.B.; Keszthelyi, D.; Wilms, E.; Troost, F.J.; Hendriks, H.F.J.; Masclee, A.A.M. The Effect of an Encapsulated Nutrient Mixture on Food Intake and Satiety: A Double-Blind Randomized Cross-Over Proof of Concept Study. Nutrients 2018, 10, 1787. [Google Scholar] [CrossRef] [Green Version]
- Cines, B.M.; Rozin, P. Some aspects of the liking for hot coffee and coffee flavor. Appetite 1982, 3, 23–34. [Google Scholar] [CrossRef]
- Chambers, L.; Mobini, S.; Yeomans, M.R. Caffeine Deprivation State Modulates Expression of Acquired Liking for Caffeine-Paired Flavours. Q. J. Exp. Psychol. 2007, 60, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Van Der Wielen, N.; Van Avesaat, M.; De Wit, N.J.W.; Vogels, J.T.W.E.; Troost, F.; Masclee, A.; Koopmans, S.J.; Van Der Meulen, J.; Boekschoten, M.V.; Müller, M.; et al. Cross-Species Comparison of Genes Related to Nutrient Sensing Mechanisms Expressed along the Intestine. PLoS ONE 2014, 9, e107531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, F.; Liu, X.; Liang, J.; Chen, J.; Chen, F.; Li, F. Bitter taste receptor mTas2r105 is expressed in small intestinal villus and crypts. Biochem. Biophys. Res. Commun. 2015, 463, 934–941. [Google Scholar] [CrossRef]
- Klaassen, T.; Keszthelyi, D.; Troost, F.J.; Bast, A.; Masclee, A.A.M. Effects of gastrointestinal delivery of non-caloric tastants on energy intake: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 1–25. [Google Scholar] [CrossRef]
- Rogers, P.J.; Pleming, H.C.; Blundell, J.E. Aspartame ingested without tasting inhibits hunger and food intake. Physiol. Behav. 1990, 47, 1239–1243. [Google Scholar] [CrossRef]
- Black, R.M.; Leiter, L.A.; Anderson, G. Consuming aspartame with and without taste: Differential effects on appetite and food intake of young adult males. Physiol. Behav. 1993, 53, 459–466. [Google Scholar] [CrossRef]
- Van Avesaat, M.; Troost, F.J.; Ripken, D.; Peters, J.; Hendriks, H.F.; Masclee, A.A. Intraduodenal infusion of a combination of tastants decreases food intake in humans. Am. J. Clin. Nutr. 2015, 102, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Andreozzi, P.; Sarnelli, G.; Pesce, M.; Zito, F.P.; Alessandro, A.D.; Verlezza, V.; Palumbo, I.; Turco, F.; Esposito, K.; Cuomo, R. The Bitter Taste Receptor Agonist Quinine Reduces Calorie Intake and Increases the Postprandial Release of Cholecystokinin in Healthy Subjects. J. Neurogastroenterol. Motil. 2015, 21, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Mennella, I.; Fogliano, V.; Ferracane, R.; Arlorio, M.; Pattarino, F.; Vitaglione, P. Microencapsulated bitter compounds (from Gentiana lutea) reduce daily energy intakes in humans. Br. J. Nutr. 2016, 116, 1841–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, H.P.; Koppenol, W.; Schuring, E.A.; Gouka, R.; Mela, D.J.; Blom, W.A. The effect of two weeks ingestion of a bitter tastant mixture on energy intake in overweight females. Appetite 2016, 107, 268–273. [Google Scholar] [CrossRef]
- Deloose, E.; Janssen, P.; Corsetti, M.; Biesiekierski, J.; Masuy, I.; Rotondo, A.; Van Oudenhove, L.; Depoortere, I.; Tack, J. Intragastric infusion of denatonium benzoate attenuates interdigestive gastric motility and hunger scores in healthy female volunteers. Am. J. Clin. Nutr. 2017, 105, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Bitarafan, V.; Fitzgerald, P.C.E.; Little, T.J.; Meyerhof, W.; Jones, K.L.; Wu, T.; Horowitz, M.; Feinle-Bisset, C. Intragastric administration of the bitter tastant quinine lowers the glycemic response to a nutrient drink without slowing gastric emptying in healthy men. Am. J. Physiol. Integr. Comp. Physiol. 2020, 318, R263–R273. [Google Scholar] [CrossRef]
- Iven, J.; Biesiekierski, J.R.; Zhao, D.; Deloose, E.; O’Daly, O.G.; Depoortere, I.; Tack, J.; Van Oudenhove, L. Intragastric quinine administration decreases hedonic eating in healthy women through peptide-mediated gut-brain signaling mechanisms. Nutr. Neurosci. 2018, 22, 850–862. [Google Scholar] [CrossRef]
- Bitarafan, V.; Fitzgerald, P.C.E.; Little, T.J.; Meyerhof, W.; Wu, T.; Horowitz, M.; Feinle-Bisset, C. Effects of Intraduodenal Infusion of the Bitter Tastant, Quinine, on Antropyloroduodenal Motility, Plasma Cholecystokinin, and Energy Intake in Healthy Men. J. Neurogastroenterol. Motil. 2019, 25, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, T.; Alleleyn, A.M.E.; Van Avesaat, M.; Troost, F.J.; Keszthelyi, D.; Masclee, A.A.M. Intraintestinal Delivery of Tastants Using a Naso-Duodenal-Ileal Catheter Does Not Influence Food Intake or Satiety. Nutrients 2019, 11, 472. [Google Scholar] [CrossRef] [Green Version]
- Deloose, E.; Corsetti, M.; Van Oudenhove, L.; Depoortere, I.; Tack, J. Intragastric infusion of the bitter tastant quinine suppresses hormone release and antral motility during the fasting state in healthy female volunteers. Neurogastroenterol. Motil. 2017, 30, e13171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tack, J.; Deloose, E.; Ang, D.; Scarpellini, E.; Vanuytsel, T.; Van Oudenhove, L.; Depoortere, I. Motilin-induced gastric contractions signal hunger in man. Gut 2016, 65, 214–224. [Google Scholar] [CrossRef] [PubMed]
| Location | Infusate and Infusion Rate: Kcal per min | Energy Intake | Satiety ↑ ↓ | References | |
|---|---|---|---|---|---|
| % Reduction | ↑ ↓ | ||||
| Fat | |||||
| Duodenum | 1.1 (0.25–1.5) Corn oil, safflower oil, Intralipid | 3% (0–8) | = | =-↑ | [17,18,19] |
| 3.3 (2.0–4.9) corn oil, Intralipid | 21% (10–32) | ↓↓ | ↑ | [10,17,18,20,21,22,23,24,25] | |
| Jejunum | 4.9 corn oil | 12% | ↓↓ | ↑↑ | [26] |
| 4.9 corn oil | 50% | ↓↓↓ | ↑↑ | [10] | |
| Ileum | 0.5–0.6 rapeseed, safflower oil | 18% (15–21) | ↓↓ | ↑ | [19,27,28,29] |
| 1.8–4.9, corn oil, safflower oil | 31% (30–32) | ↓↓↓ | ↑↑ | [10,13,28] | |
| Fatty acids | |||||
| Duodenum | 0.2–0.3 lauric acid | 0–4% | =-↓ | = | [30,31] |
| 0.4–0.75 lauric, LCFA | 10–15% (60%) * | ↓↓ | ↑ | [30,32,33,34] | |
| Jejunum | - | - | - | - | - |
| Ileum | - | - | - | - | - |
| Location | Infusate and Infusion Rate Kcal per min | Energy Intake | Satiety | References | |
|---|---|---|---|---|---|
| % Reduction | ↑ ↓ | ||||
| Protein | |||||
| Duodenum | 0.5–1.5 whey, casein | 8% | ↓ | = | [35,36,37] |
| 3.0 whey, casein | 21% | ↓↓ | = | [24,35,36] | |
| Jejunum | 0.85 casein | 9% | ↓ | = | [37] |
| Ileum | 0.19 casein | 9.9% | ↓ | = | [29] |
| 0.57–0.85 casein | 14–22% | ↓↓ | ↑↑ | [29,37] | |
| Amino acids | |||||
| Duodenum | 0.07–0.15 tryptophan | 5% | ↓ | ↑ | [31,38] |
| 0.2–0.4 tryptophan, leucine | 13% | ↓↓ | = | [38,39] | |
| Jejunum | - | - | - | - | - |
| Ileum | - | - | - | - | - |
| Location | Infusate and Infusion Rate Kcal per min | Energy Intake | Satiety | References | |
|---|---|---|---|---|---|
| % Reduction | ↑ ↓ | ||||
| Duodenum | 0.6–2.0 glucose | 10% (5–13) | ↓ | ↑ | [40,41,42] |
| 2.9–4.0 glucose | 17% (11–26) | ↓↓ | ↑↑ | [21,22,23,40,41,42,43,44,45] | |
| Jejunum | 1.0 glucose | +11% * | ↑↑ | = | [42] |
| Ileum | 0.19 sucrose | 21% | ↓↓ | = | [29] |
| 0.57 sucrose | 32% | ↓↓↓ | = | [29] | |
| 0.66 glucose | 10% | ↓ | = | [44] | |
| Location | Fat | Carbohydrate | Protein | |||
|---|---|---|---|---|---|---|
| kcal/min | EI-Red | kcal/min | EI-Red | kcal/min | EI-Red | |
| Duodenum | 0.25–1.5 | 0–15% | 0.6–2 | 5–13% | 0.5–1.5 | 6–13% |
| 2–5 | 10–32% | 2.86–4 | 11–26% | 3.0 | 21% | |
| Jejunum | 4.9 | 12–50% | 1 | +11% | 0.85 | 9% |
| Ileum | 0.5–0.6 | 15–21% | 0.19–0.66 | 10–32% | 0.19–0.85 | 14–22% |
| 1.8–4.9 | 30–32% | |||||
| Reference | Location | Infusate | Reduction in Energy Intake (EI) of Meal | Net Effect: Reduction EI Meal-EI Infusate | |
|---|---|---|---|---|---|
| Type | Energy Content of Infusate | ||||
| 41 | Duodenum | casein | 60 kcal | +20 kcal | − |
| Jejunum | casein | 60 kcal | 40 kcal | − | |
| Ileum | casein | 60 kcal | 80 kcal | + | |
| 47 | Duodenum | glucose | 90 kcal | +160 kcal | − |
| Jejunum | glucose | 90 kcal | − | ||
| 48 | Duodenum | glucose | 56 kcal | 58 kcal | = |
| Ileum | glucose | 56 kcal | 119 kcal | + | |
| 10 | Jejunum | corn oil | 370 kcal | 1100 kcal | ++ |
| Ileum | corn oil | 370 kcal | 650 kcal | ++ | |
| 33 | Duodenum | rapeseed oil | 54 kcal | 14 kcal | − |
| Ileum | rapeseed oil | 54 kcal | 18 kcal | − | |
| 19 | Duodenum | canola oil | 54 kcal | ileum vs. duo: | |
| Ileum | canola oil | 54 kcal | 76 kcal | + | |
| Location | Infusate | Infusate | Infusate | Infusate | Infusate |
|---|---|---|---|---|---|
| LCT fat | LC fatty acids | Proteins | Amino acids | Carbohydrates | |
| Duodenum | − | + | + | + | − |
| Jejunum | ++ | NA | + | NA | − |
| Ileum | ++ | NA | ++ | NA | ++ |
| Taste | Tastant | Administration | Energy Intake Reduction | Reference | |
|---|---|---|---|---|---|
| kcal | % | ||||
| Sweet | aspartame | gastric capsule | 10% | [64] | |
| aspartame | gastric capsule | 0% | [65] | ||
| rebaudioside A | duodenal tube | 26 kcal | 5% | [66] | |
| Bitter | quinine | acid resistant capsule | [67] | ||
| quinine | duodenal tube | 44 kcal | 9% | [66] | |
| secoiridoids | micro encapsulation | 88 kcal | 11% | [68] | |
| 340 kcal (day) | 22% | ||||
| bitter mixture | gastric capsule | 109 kcal | 7% | [69] | |
| denatonium bezoate | gastric tube | 76 kcal | 9.5% | [70] | |
| quinine 600 mg | gastric tube | 53 kcal | 5% | [71] | |
| quinine 275 mg | gastric tube | +26 kcal | +3% | [71] | |
| quinine | gastric tube | 68 kcal | 9% | [72] | |
| quinine 37.5 mg | duodenal tube | 31 kcal | 3% | [73] | |
| quinine 75 mg | duodenal tube | 59 kcal | 5% | [73] | |
| quinine 225 mg | duodenal tube | 11 kcal | 1% | [73] | |
| Umami | monosodium glutamate | duodenal tube | +5 kcal | +1% | [66] |
| Combination: sweet, bitter and umami | Reb A, quinine and MSG | duodenal tube | 64 kcal | 14% | [66] |
| Reb A, quinine and MSG | duodenal tube | 17 kcal | +2% | [72] | |
| ileal tube | 28 kcal | +4% | [74] | ||
| duodenal+ ileal tube | 31 kcal | +4% | [74] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilbrink, J.; Masclee, G.; Klaassen, T.; van Avesaat, M.; Keszthelyi, D.; Masclee, A. Review on the Regional Effects of Gastrointestinal Luminal Stimulation on Appetite and Energy Intake: (Pre)clinical Observations. Nutrients 2021, 13, 1601. https://doi.org/10.3390/nu13051601
Wilbrink J, Masclee G, Klaassen T, van Avesaat M, Keszthelyi D, Masclee A. Review on the Regional Effects of Gastrointestinal Luminal Stimulation on Appetite and Energy Intake: (Pre)clinical Observations. Nutrients. 2021; 13(5):1601. https://doi.org/10.3390/nu13051601
Chicago/Turabian StyleWilbrink, Jennifer, Gwen Masclee, Tim Klaassen, Mark van Avesaat, Daniel Keszthelyi, and Adrian Masclee. 2021. "Review on the Regional Effects of Gastrointestinal Luminal Stimulation on Appetite and Energy Intake: (Pre)clinical Observations" Nutrients 13, no. 5: 1601. https://doi.org/10.3390/nu13051601






