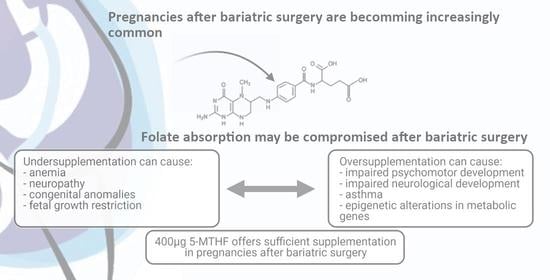
Periconceptional Folate Supplementation in Women after Bariatric Surgery—A Narrative Review
Abstract
:1. Introduction
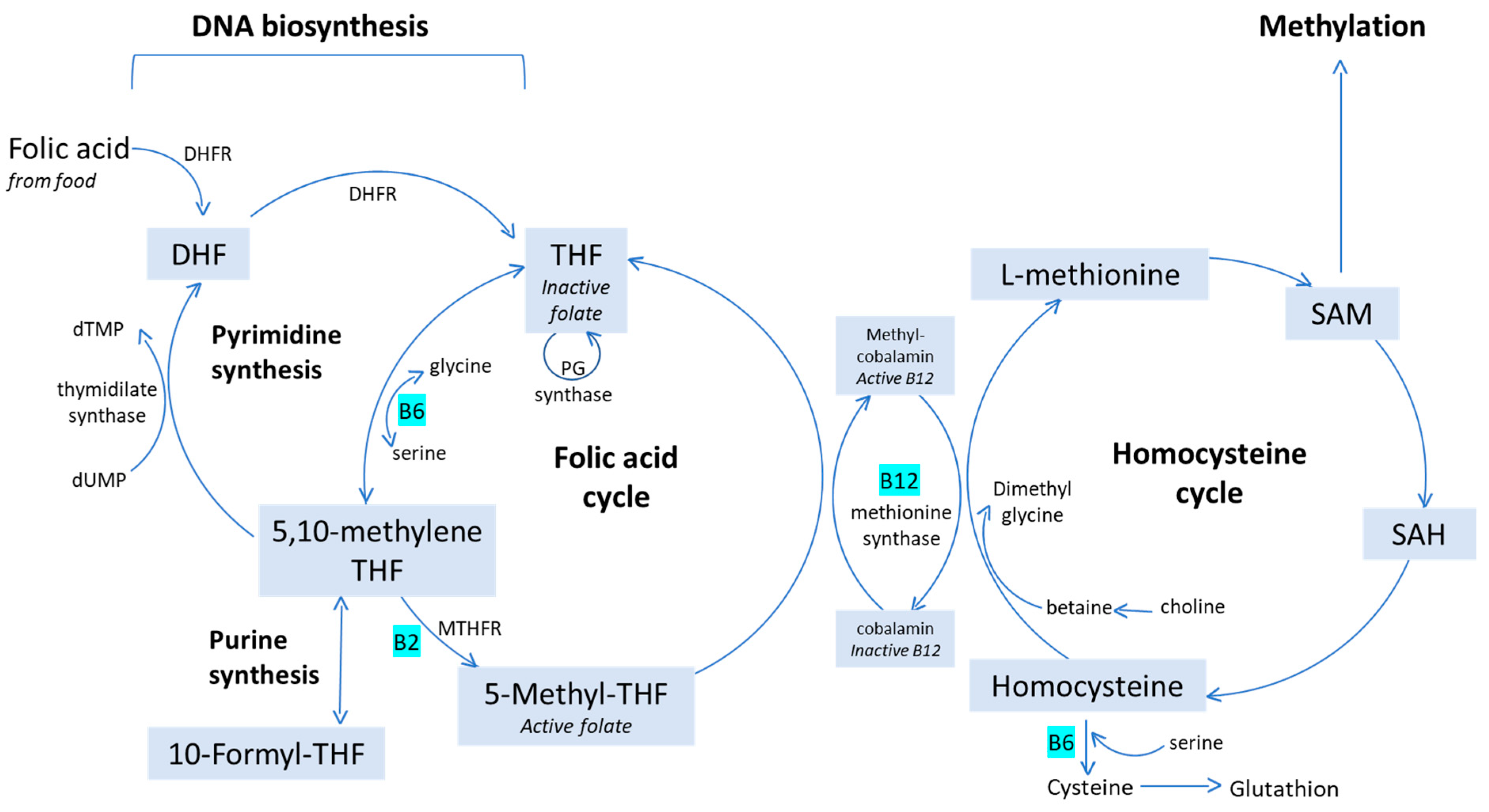
2. Folate: Definition, Uptake and Function
3. Folate-Status in Non-Bariatric Pregnancies
4. Folate Malabsorption after Bariatric Surgery: Pathogenesis and Prevalence
5. Types of Folate Supplementation: Advantages and Disadvantages of the Different Forms
6. Folic Acid Over-Supplementation: Potential Consequences
7. Folate and Fetal Growth
8. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight. 2018. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 13 March 2021).
- Marchi, J.; Berg, M.V.D.; Dencker, A.; Olander, E.K.; Begley, C. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 2015, 16, 621–638. [Google Scholar] [CrossRef]
- Heslehurst, N.; Simpson, H.; Ells, L.J.; Rankin, J.; Wilkinson, J.; Lang, R.; Brown, T.J.; Summerbell, C.D. The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: A meta-analysis. Obes. Rev. 2008, 9, 635–683. [Google Scholar] [CrossRef] [Green Version]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Vitiello, A.; Zundel, N.; Buchwald, H.; Scopinaro, N. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes. Surg. 2017, 27, 2279–2289. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled interven-tion study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef]
- Jakobsen, G.S.; Småstuen, M.C.; Sandbu, R.; Nordstrand, N.; Hofsø, D.; Lindberg, M.; Hertel, J.K.; Hjelmesæth, J. Association of Bariatric Surgery vs. Medical Obesity Treatment With Long-term Medical Complica-tions and Obesity-Related Comorbidities. JAMA 2018, 319, 291–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santry, H.P.; Gillen, D.L.; Lauderdale, D.S. Trends in Bariatric Surgical Procedures. JAMA 2005, 294, 1909–1917. [Google Scholar] [CrossRef] [Green Version]
- Shawe, J.; Ceulemans, D.; Akhter, Z.; Neff, K.; Hart, K.; Heslehurst, N.; Štotl, I.; Agrawal, S.; Steegers-Theunissen, R.; Taheri, S.; et al. Pregnancy after bariatric surgery: Consensus recommendations for periconception, antenatal and postnatal care. Obes. Rev. 2019, 20, 1507–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhter, Z.; Rankin, J.; Ceulemans, D.; Ngongalah, L.; Ackroyd, R.; Devlieger, R.; Vieira, R.; Heslehurst, N. Pregnancy after bariatric surgery and adverse perinatal outcomes: A systematic review and me-ta-analysis. PLoS Med. 2019, 16, e1002866. [Google Scholar] [CrossRef] [PubMed]
- Jans, G.; Matthys, C.; Bogaerts, A.; Lannoo, M.; Verhaeghe, J.; van der Schueren, B.; Devlieger, R. Maternal micronutrient deficiencies and related adverse neonatal outcomes after bariatric surgery: A systematic review. Adv. Nutr. 2015, 6, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, J.A.; Bell, S.J.; Guan, Y.; Yu, Y.-H. Folic Acid Supplementation and Pregnancy: More than Just Neural Tube Defect Prevention. Rev. Obstet. Gynecol. 2011, 4, 52–59. [Google Scholar]
- Scaglione, F.; Panzavolta, G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica 2013, 44, 480–488. [Google Scholar] [CrossRef]
- McNulty, H.; Pentieva, K. Folate bioavailability. Proc. Nutr. Soc. 2004, 63, 529–536. [Google Scholar] [CrossRef]
- Van der Windt, M.; Schoenmakers, S.; van Rijn, B.; Galjaard, S.; Steegers-Theunissen, R.; van Rossem, L. Epidemiology and (Patho)Physiology of Folic Acid Supplement Use in Obese Women before and during Pregnancy. Nutrients 2021, 13, 331. [Google Scholar] [CrossRef]
- Zhao, R.; Matherly, L.H.; Goldman, I.D. Membrane transporters and folate homeostasis: Intestinal absorption and transport into systemic compartments and tissues. Expert Rev. Mol. Med. 2009, 11, e4. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.L.; Mistry, K.B.; Wang, G.; Zuckerman, B.; Wang, X. Folate Nutrition Status in Mothers of the Boston Birth Cohort, Sample of a US Urban Low-Income Population. Am. J. Public Health 2018, 108, 799–807. [Google Scholar] [CrossRef]
- Blumfield, M.L.; Hure, A.J.; Macdonald-Wicks, L.; Smith, R.; Collins, C.E. Micronutrient intakes during pregnancy in developed countries: Systematic review and me-ta-analysis. Nutr. Rev. 2013, 71, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Looman, M.; van den Berg, C.; Geelen, A.; Samlal, R.A.K.; Heijligenberg, R.; Gunnewiek, J.M.T.K.; Balvers, M.G.J.; Leendertz-Eggen, C.L.; Wijnberger, L.D.E.; Feskens, E.J.M.; et al. Supplement Use and Dietary Sources of Folate, Vitamin D, and n-3 Fatty Acids during Preconcep-tion: The GLIMP2 Study. Nutrients 2018, 10, 962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milman, N.; Paszkowski, T.; Cetin, I.; Castelo-Branco, C. Supplementation during pregnancy: Beliefs and science. Gynecol. Endocrinol. 2016, 32, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Pac, S.G.; Fulgoni, V.L.; Reidy, K.C.; Catalano, P.M. Estimation of Total Usual Dietary Intakes of Pregnant Women in the United States. JAMA Netw. Open 2019, 2, e195967. [Google Scholar] [CrossRef] [Green Version]
- Shankar, P.; Boylan, M.; Sriram, K. Micronutrient deficiencies after bariatric surgery. Nutrition 2010, 26, 1031–1037. [Google Scholar] [CrossRef]
- Via, M.A.; Mechanick, J.I. Nutritional and Micronutrient Care of Bariatric Surgery Patients: Current Evidence Up-date. Curr. Obes. Rep. 2017, 6, 286–296. [Google Scholar] [CrossRef]
- Weng, T.-C.; Chang, C.-H.; Dong, Y.-H.; Chang, Y.-C.; Chuang, L.-M. Anaemia and related nutrient deficiencies after Roux-en-Y gastric bypass surgery: A systematic review and meta-analysis. BMJ Open 2015, 5, e006964. [Google Scholar] [CrossRef]
- Lewis, C.-A.; de Jersey, S.; Seymour, M.; Hopkins, G.; Hickman, I.; Osland, E. Iron, Vitamin B12, Folate and Copper Deficiency After Bariatric Surgery and the Impact on Anae-mia: A Systematic Review. Obes. Surg. 2020, 30, 4542–4591. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.; Cnattingius, S.; Näslund, I.; Roos, N.; Lagerros, Y.T.; Granath, F.; Stephansson, O.; Neovius, M. Outcomes of Pregnancy after Bariatric Surgery. N. Engl. J. Med. 2015, 372, 814–824. [Google Scholar] [CrossRef] [Green Version]
- Devlieger, R.; Guelinckx, I.; Jans, G.; Voets, W.; Vanholsbeke, C.; Vansant, G. Micronutrient levels and supplement intake in pregnancy after bariatric surgery: A prospective co-hort study. PLoS ONE 2014, 9, e114192. [Google Scholar] [CrossRef] [Green Version]
- Servy, E.J.; Jacquesson-Fournols, L.; Cohen, M.; Menezo, Y.J.R. MTHFR isoform carriers. 5-MTHF (5-methyl tetrahydrofolate) vs. folic acid: A key to pregnancy out-come: A case series. J. Assist. Reprod. Genet. 2018, 35, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Prinz-Langenohl, R.; Brämswig, S.; Tobolski, O.; Smulders, Y.M.; Smith, D.E.C.; Finglas, P.M.; Pietrzik, K. [6S]-5-methyltetrahydrofolate increases plasma folate more effectively than folic acid in women with the homozygous or wild-type 677C→T polymorphism of methylenetetrahydrofolate reductase. Br. J. Pharmacol. 2009, 158, 2014–2021. [Google Scholar] [CrossRef] [Green Version]
- Bailey, S.W.; Ayling, J.E. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc. Natl. Acad. Sci. USA 2009, 106, 15424–15429. [Google Scholar] [CrossRef] [Green Version]
- Venn, B.J.; Green, T.J.; Moser, R.; McKenzie, J.E.; Skeaff, C.M.; Mann, J. Increases in Blood Folate Indices Are Similar in Women of Childbearing Age Supplemented with [6S]-5-Methyltetrahydrofolate and Folic Acid. J. Nutr. 2002, 132, 3353–3355. [Google Scholar] [CrossRef] [Green Version]
- Hekmatdoost, A.; Vahid, F.; Yari, Z.; Sadeghi, M.; Eini-Zinab, H.; Lakpour, N.; Arefi, S. Methyltetrahydrofolate vs Folic Acid Supplementation in Idiopathic Recurrent Miscarriage with Respect to Methylenetetrahydrofolate Reductase C677T and A1298C Polymorphisms: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0143569. [Google Scholar] [CrossRef] [PubMed]
- Lamers, Y.; Prinz-Langenohl, R.; Brämswig, S.; Pietrzik, K. Red blood cell folate concentrations increase more after supplementation with [6 S]-5-methyltetrahydrofolate than with folic acid in women of childbearing age. Am. J. Clin. Nutr. 2006, 84, 156–161. [Google Scholar] [CrossRef]
- Berti, C.; Fekete, K.; Dullemeijer, C.; Trovato, M.; Souverein, O.W.; Cavelaars, A.A.; Dhonukshe-Rutten, R.; Massari, M.; Decsi, T.; Veer, P.V.; et al. Folate Intake and Markers of Folate Status in Women of Reproductive Age, Pregnant and Lactating Women: A Meta-Analysis. J. Nutr. Metab. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.A.; Sherwood, K.L.; Pawlosky, R.; Ito, S.; O’Connor, D.L. [6S]-5-Methyltetrahydrofolate is at least as effective as folic acid in preventing a decline in blood folate concentrations during lactation. Am. J. Clin. Nutr. 2006, 83, 842–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeid, R.; Holzgreve, W.; Pietrzik, K. Is 5-methyltetrahydrofolate an alternative to folic acid for the prevention of neural tube defects? J. Périnat. Med. 2013, 41, 469–483. [Google Scholar] [CrossRef]
- Varela-Moreiras, G.; Murphy, M.M.; Scott, J.M. Cobalamin, folic acid, and homocysteine. Nutr. Rev. 2009, 67, S69–S72. [Google Scholar] [CrossRef] [PubMed]
- Pietrzik, K.; Bailey, L.B.; Shane, B. Folic Acid and L-5-Methyltetrahydrofolate. Clin. Pharmacokinet. 2010, 49, 535–548. [Google Scholar] [CrossRef]
- Raghavan, R.; Riley, A.W.; Volk, H.; Caruso, D.; Hironaka, L.; Sices, L.; Hong, X.; Wang, G.; Ji, Y.; Brucato, M.; et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12Levels and Autism Spectrum Disor-der Risk in Offspring. Paediatr. Perinat. Epidemiol. 2018, 32, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Wiens, D.; DeSoto, M.C. Is High Folic Acid Intake a Risk Factor for Autism?—A Review. Brain Sci. 2017, 7, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, M.E.; Westmark, C.J. Folic Acid Fortification and Neural Tube Defect Risk: Analysis of the Food Fortifica-tion Initiative Dataset. Nutrients 2020, 12, 247. [Google Scholar] [CrossRef] [Green Version]
- Parr, C.L.; Magnus, M.C.; Karlstad, Ø.; Haugen, M.; Refsum, H.; Ueland, P.M.; McCann, A.; Nafstad, P.; Håberg, S.E.; Nystad, W.; et al. Maternal Folate Intake during Pregnancy and Childhood Asthma in a Population-based Cohort. Am. J. Respir. Crit. Care Med. 2017, 195, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Pauwels, S.; Ghosh, M.; Duca, R.C.; Bekaert, B.; Freson, K.; Huybrechts, I.; Langie, S.A.S.; Koppen, G.; Devlieger, R.; Godderis, L. Maternal intake of methyl-group donors affects DNA methylation of metabolic genes in infants. Clin. Epigenetics 2017, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwong, W.; Tomlinson, G.; Feig, D.S. Maternal and neonatal outcomes after bariatric surgery; a systematic review and meta-analysis: Do the benefits outweigh the risks? Am. J. Obstet. Gynecol. 2018, 218, 573–580. [Google Scholar] [CrossRef]
- Furness, D.; Fenech, M.; Dekker, G.A.; Khong, T.Y.; Roberts, C.; Hague, W.M. Folate, Vitamin B12, Vitamin B6 and homocysteine: Impact on pregnancy outcome. Matern. Child Nutr. 2013, 9, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, B.; Zaman, S.; Malik, A.; Martin, H.; Ekström, A.M.; Amu, S.; Holmgren, A.; Norman, M. Folate, vitamin B12, and homocysteine levels in South Asian women with growth-retarded fetuses. Acta Obstet. Gynecol. Scand. 2005, 84, 1055–1061. [Google Scholar] [CrossRef]
- Jankovic-Karasoulos, T.; Furness, D.L.; Leemaqz, S.Y.; Dekker, G.A.; Grzeskowiak, L.E.; Grieger, J.A.; Andraweera, P.H.; McCullough, D.; McAninch, D.; McCowan, L.M.; et al. Maternal folate, one-carbon metabolism and pregnancy outcomes. Matern. Child Nutr. 2021, 17, e13064. [Google Scholar] [CrossRef]
- Bulloch, R.E.; Wall, C.R.; McCowan, L.M.E.; Taylor, R.S.; Roberts, C.T.; Thompson, J.M.D. The Effect of Interactions between Folic Acid Supplementation and One Carbon Metabolism Gene Variants on Small-for-Gestational-Age Births in the Screening for Pregnancy Endpoints (SCOPE) Cohort Study. Nutrients 2020, 12, 1677. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Gupta, M.B.; Grattton, R.; Powell, T.L.; Jansson, T. Down-regulation of placental folate transporters in intrauterine growth restriction. J. Nutr. Biochem. 2018, 59, 136–141. [Google Scholar] [CrossRef]
- Peake, J.N.; Copp, A.J.; Shawe, J. Knowledge and periconceptional use of folic acid for the prevention of neural tube defects in ethnic communities in the United Kingdom: Systematic review and meta-analysis. Birth Defects Res. Part A Clin. Mol. Teratol. 2013, 97, 444–451. [Google Scholar] [CrossRef] [Green Version]
- Gaskins, A.J.; Rich-Edwards, J.W.; Hauser, R.; Williams, P.L.; Gillman, M.W.; Ginsburg, E.S.; Missmer, S.A.; Chavarro, J.E. Maternal Prepregnancy Folate Intake and Risk of Spontaneous Abortion and Stillbirth. Obstet. Gynecol. 2014, 124, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Gaskins, A.J.; Afeiche, M.C.; Wright, D.L.; Toth, T.L.; Williams, P.L.; Gillman, M.W.; Hauser, R.; Chavarro, J.E. Dietary Folate and Reproductive Success Among Women Undergoing Assisted Reproduction. Obstet. Gynecol. 2014, 124, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Valera-Gran, D.; De La Hera, M.G.; Navarrete-Muñoz, E.M.; Fernandez-Somoano, A.; Tardón, A.; Julvez, J.; Forns, J.; Lertxundi, N.; Ibarluzea, J.M.; Murcia, M.; et al. Folic Acid Supplements During Pregnancy and Child Psychomotor Development After the First Year of Life. JAMA Pediatr. 2014, 168, e142611. [Google Scholar] [CrossRef] [Green Version]
- Harreiter, J.; Schindler, K.; Bancher-Todesca, D.; Göbl, C.; Langer, F.; Prager, G.; Gessl, A.; Leutner, M.; Ludvik, B.; Luger, A.; et al. Management of Pregnant Women after Bariatric Surgery. J. Obes. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jans, G. Maternal Obesity and Bariatric Surgery—A Nutritional and Dietary Point of View. Ph.D. Thesis, Katholieke Universiteit Leuven, Leuven, Belgium, November 2016. [Google Scholar]
- Xanthakos, S.A.; Khoury, J.C.; Inge, T.H.; Jenkins, T.M.; Modi, A.C.; Michalsky, M.P.; Chen, M.K.; Courcoulas, A.P.; Harmon, C.M.; Brandt, M.L.; et al. Nutritional Risks in Adolescents After Bariatric Surgery. Clin. Gastroenterol. Hepatol. 2020, 18, 1070–1081.e5. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures 2019 update: Cosponsored by American Association of Clinical En-docrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg. Obes. Relat. Dis. 2020, 16, 175–247. [Google Scholar] [PubMed]
- Crider, K.S.; Devine, O.; Qi, Y.P.; Yeung, L.F.; Sekkarie, A.; Zaganjor, I.; Wong, E.; Rose, C.E.; Berry, R.J. Systematic Review and Bayesian Meta-analysis of the Dose-response Relationship between Folic Acid Intake and Changes in Blood Folate Concentrations. Nutrients 2019, 11, 71. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vynckier, A.-K.; Ceulemans, D.; Vanheule, G.; De Mulder, P.; Van Den Driessche, M.; Devlieger, R. Periconceptional Folate Supplementation in Women after Bariatric Surgery—A Narrative Review. Nutrients 2021, 13, 1557. https://doi.org/10.3390/nu13051557
Vynckier A-K, Ceulemans D, Vanheule G, De Mulder P, Van Den Driessche M, Devlieger R. Periconceptional Folate Supplementation in Women after Bariatric Surgery—A Narrative Review. Nutrients. 2021; 13(5):1557. https://doi.org/10.3390/nu13051557
Chicago/Turabian StyleVynckier, An-Katrien, Dries Ceulemans, Greet Vanheule, Paulien De Mulder, Mieke Van Den Driessche, and Roland Devlieger. 2021. "Periconceptional Folate Supplementation in Women after Bariatric Surgery—A Narrative Review" Nutrients 13, no. 5: 1557. https://doi.org/10.3390/nu13051557