Dietary Selenium Regulates microRNAs in Metabolic Disease: Recent Progress
Abstract
1. Introduction
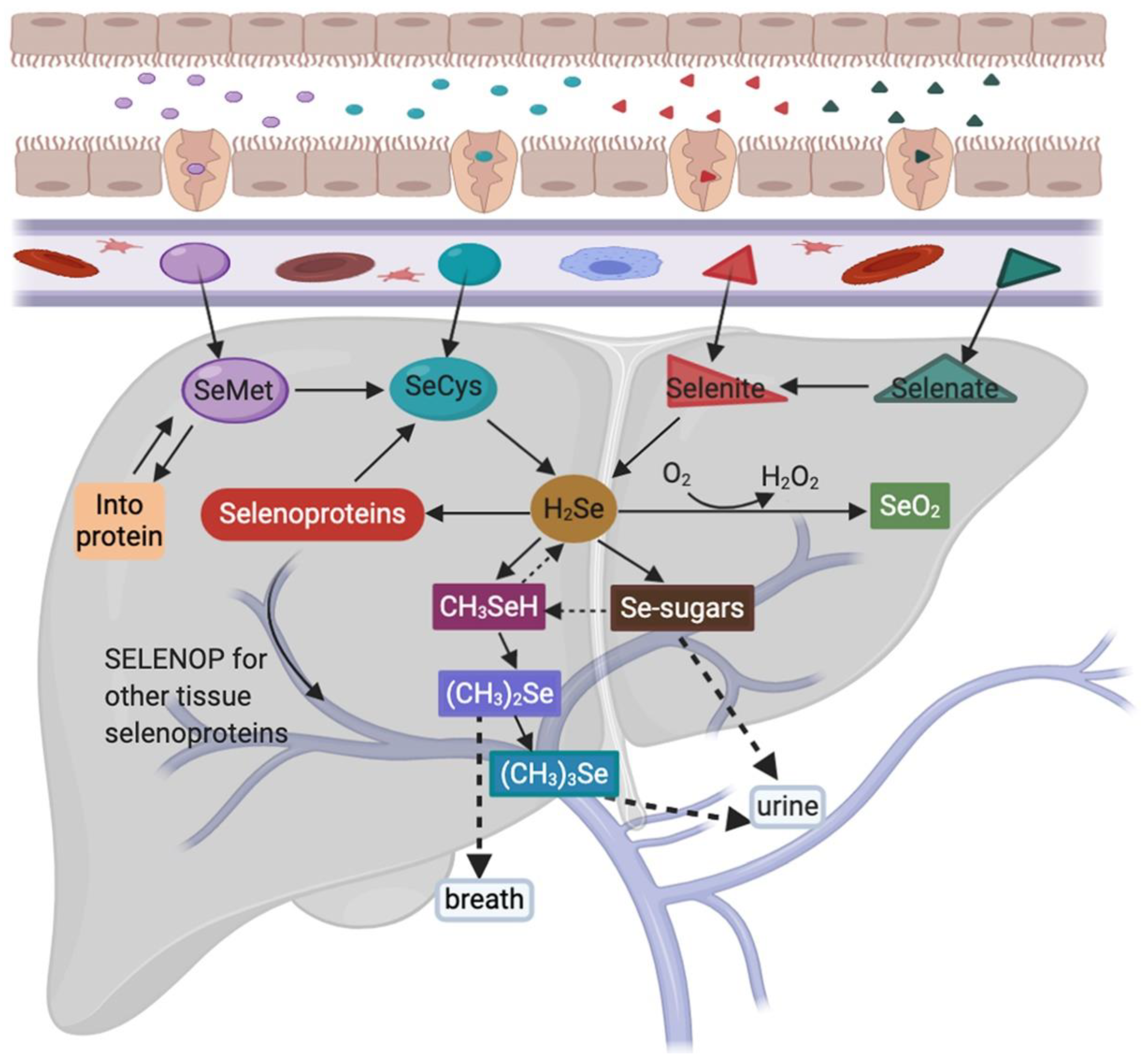
2. Selenium Uptake and Metabolism
3. Selenium Related Pathogenic Mechanisms and Diseases
4. Current Progress in Nutrient Regulation of miRNAs
4.1. miRNAs
4.2. Regulation Mechanisms of Vitamins and Minerals on miRNAs
4.3. Role of Mammalian Target of Rapamycin (mTOR) in Nutrient Regulation of miRNAs
4.4. miRNAs Mediated by Se Status Are Implicated in Disease Development and Progression
| Se Status | Micro RNA | Target | Observed Effect | Note | Reference |
|---|---|---|---|---|---|
| Se deficiency | ↑miR-181a-5P | ↓SBP2 | ↓GPX1, GPX4, and SELENOS levels | In C28/I2 human juvenile chondrocytes and DA rats | [100] |
| ↑Gga-let-7f-3p | ↓SELENOK | ↑Oxidative stress, ERS, and apoptosis | In chicken myoblasts and muscle | [101] | |
| ↑miR-200a-5p | ↓TXNRD2, TXNRD3, SELENON, SELENOT, SELENOF and SELENOP | ↑Glucose metabolism disorder, cardiomyocyte hypertrophy | In chicken cardiomyocytes | [102] | |
| ↓RNF11 | ↑Oxidative stress and myocardial necroptosis | In chicken cardiac tissue and cardiomyocytes | [103] | ||
| ↑miR-138-5p | ↓SELENOM | ↑Apoptosis, oxidative stress, mitochondrial fission | In chicken chondrocytes | [104] | |
| ↑miR-544a | ↓SELENOK | Interferes with SELENOK translation | In HepG2 and HuH-7 human hepatocarcinoma cells | [105] | |
| ↑miR-196-5p | ↓NFκBIA (IκB-α) | ↑LPS-induced oxidative stress and inflammation, respiratory mucosal immune dysfunction | In chicken trachea | [106] | |
| ↑miR-193b-3p | ↓MAML1 | ↑Hepatocyte apoptosis | In the liver tissues and primary hepatocytes from broilers | [107] | |
| ↑miR-33-3p | ↓ADAM10 | ↑Cell cycle arrest and apoptosis | In vivo and in vitro in the chicken kidney | [108] | |
| ↓E4F1 | ↑Oxidative stress, ERS, and apoptosis | In vein endothelial cells from broilers | [109] | ||
| ↑miR-328 | ↓ATP2A2 | ↑Intracellular Ca2+ and cell apoptosis | In H9c2 rat cardiac myoblasts | [110] | |
| ↑miR-215-5p | ↓CTCF | ↑Mitochondrial biosynthesis imbalance, defects in myocardial development | In heart tissue and primary cardiomyocytes from chickens | [111] | |
| ↓PI3K/AKT/TOR | ↑ROS, Myocardial autophagy | In cardiomyocytes of chicken | [112] | ||
| ↑miR-1594 | ↓TNNT2 | ↑Ca2+ | In heart and primary cardiomyocytes from chickens | [113] | |
| ↑miR-2954 | ↓PI3K | ↑Autophagy and apoptosis | In heart and primary cardiomyocytes from chickens | [114] | |
| ↑miR-16-5p | ↓PI3K/AKT | ↑Necroptosis | In tracheal tissues and tracheal epithelial cells of chicken | [115] | |
| ↑miR-128-1-5p | ↓CADM1 | ↑Tight junction structural damage and cell cycle arrested | In vein tissues and vein endothelial cells from broilers | [116] | |
| ↑ miR-374, miR-16, miR-199a-5p, miR-195 and miR-30e ↓ miR-3571, miR-675a and miR-450a | ↑ Wnt/β--catenin | ↑ Cardiac dysfunction | In rat heart | [89] | |
| ↓miR--185 | ↑GPX2, SEPHS2 | ↑Altered expression of 12 miRNA and 50 genes | In Caco-2 human intestinal cells | [88] | |
| ↓miR-29a-3p | ↑TNFR1 | Altered expression of selenoprotein genes, ↑necrotic cells | In the pig brain and IPEC-J2 pig intestinal epithelial cells | [117] | |
| ↓miR-155 | ↑TNFRSF1B | ↑Oxidative stress-induced apoptosis | In splenic cells and spleen of broilers | [118] | |
| ↓miR-146a | ↑MAPKs | ↑ROS-induced inflammation | In the head kidney of carp | [119] | |
| ↓miR-7 | ↓SELENOP | Both are potential biomarkers of HCC | In HCC patients and HepG2 human hepatocarcinoma cells | [120] | |
| Se moderate | ↑miR-146a | ↓TLR2, TLR6, NF-κB and MAPK | ↓S. aureus-infected mastitis | In mammary tissues and mammary epithelial cells from mouse | [93] |
| ↑miR-125a and miR-125b | ↓Bak and caspase-3 | ↓Cd-induced apoptosis | In LLC-PK1 porcine renal epithelial cells | [121] | |
| ↑ miR-29b-3p, miR-30e-5p and miR-19a-3p ↓ miR-199a-5p, miR-130a-3p and miR-191-5p | —— | ↓ Risk of heart failure | In healthy elderly males | [92] | |
| ↓mmu-miR-155 | ↓TNF-α, IL-1β, IL-10, TLR2, NF-κB and MAPKs | ↓ S. aureus-infected mastitis | In mammary tissues and mammary epithelial cells from mouse | [94] | |
| ↓miR-224 | ↑ID1 | ↓Pb-induced oxidative damage and restoring thyroid hormone disequilibrium | In thyroid tissues of male rats | [122] | |
| ↓miR-16-5p | ↑PiK3R1 and IGF1R | ↓Pb-induced neutrophil apoptosis | From chicken peripheral blood | [123] | |
| ↓miR-216a | ↑PI3K/AKT | ↓Cd-triggered necrosis and apoptosis | In the splenic lymphocytes of common carp | [124] | |
| Se excess | ↑miR-122-5p | ↑BMI, SBP and DBP | ↑Risk of MetS | In male adults | [95] |
| ↑miR-454-3p and miR-584-5p ↓miR-375 | A link between Se intake, vitamin D metabolism, and calcium homeostasis | ↑miR-375 as a potential biomarker of MetS | In obese women | [96] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GPX1 | glutathione peroxidase-1 |
| GPX3 | glutathione peroxidase-3 |
| GPX4 | glutathione peroxidase-4 |
| HCC | hepatocellular carcinoma |
| miRNA | microRNA |
| Se | selenium |
| SeMet | selenomethionine |
| SeCys | selenocysteine |
| H2Se | selenide |
| HSePO32− | selenophosphate |
| SELENOP | selenoprotein P |
| oncomiRs | miRNAs acting as tumour suppressors or oncogenes |
| dNTPs | deoxyribonucleoside triphosphates |
| pri-miRNA | primary miRNA transcript |
| DGCR8 | DiGeorge syndrome critical region 8 |
| pre-miRNA | miRNA precursor |
| mRNA | messenger RNA |
| DNMT | DNA methyltransferase |
| HATs | histone acetylase |
| HDAC | histone deacetylase |
| mTOR | Mammalian target of rapamycin |
References
- Kieliszek, M.; Błazejak, S. Selenium: Significance, and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef]
- Bodnar, M.; Szczyglowska, M.; Konieczka, P.; Namiesnik, J. Methods of selenium supplementation: Bioavailability and determination of selenium compounds. Crit. Rev. Food Sci. Nutr. 2016, 56, 36–55. [Google Scholar] [CrossRef]
- Yim, S.H.; Clish, C.B.; Gladyshev, V.N. Selenium deficiency is associated with pro-longevity mechanisms. Cell Rep. 2019, 27, 2785–2797.e3. [Google Scholar] [CrossRef]
- Bitterli, C.; Bañuelos, G.S.; Schulin, R. Use of transfer factors to characterize uptake of selenium by plants. J. Geochem. Explor. 2010, 107, 206–216. [Google Scholar] [CrossRef]
- Liang, Z.Z.; Zhu, R.M.; Li, Y.L.; Jiang, H.M.; Li, R.B.; Wang, Q.; Tang, L.Y.; Ren, Z.F. Differential epigenetic profiles induced by sodium selenite in breast cancer cells. J. Trace Elem. Med. Biol. 2021, 64, 126677. [Google Scholar] [CrossRef]
- Li, Y.; He, M.; Li, J.; Yao, Y.; Zhu, L.; Wu, B. Regulatory protein genes and microRNAs in response to selenium stimuli in Pueraria lobata (Willd.) Ohwi. Metallomics 2021, 13, mfaa004. [Google Scholar] [CrossRef] [PubMed]
- Zichan, H.; Linfei, J.; Jinliang, W.; Zhiqiang, S.; Yimei, C.; Shu, L. MicroRNA-294 regulates apoptosis of the porcine cerebellum caused by selenium deficiency via targeting iNOS. Biol. Trace Elem. Res. 2021. [Google Scholar] [CrossRef]
- Zhirong, Z.; Qiaojian, Z.; Chunjing, X.; Shengchen, W.; Jiahe, L.; Zhaoyi, L.; Shu, L. Methionine selenium antagonizes LPS-induced necroptosis in the chicken liver via the miR-155/TRAF3/MAPK axis. J. Cell. Physiol. 2020, 236, 4024–4035. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Jung, S.; Keller, S.; Gregory, R.I.; Diederichs, S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009, 11, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Vishnoi, A.; Rani, S. MiRNA biogenesis and regulation of diseases: An overview. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2017; Volume 1509, pp. 1–10. [Google Scholar]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–221. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Shi, K.; Yang, S.; Liu, J.; Zhou, Q.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Yuan, W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol. Cancer 2018, 17, 147. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of microRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Lammi, M.J.; Qu, C. Selenium-Related transcriptional regulation of gene expression. Int. J. Mol. Sci. 2018, 19, 2665. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lv, Y.; Wang, Y.; Du, P.; Tan, W.; Lammi, M.J.; Guo, X. Network Analysis of Se-and Zn-related Proteins in the Serum Proteomics Expression Profile of the Endemic Dilated Cardiomyopathy Keshan Disease. Biol. Trace Elem. Res. 2018, 183, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yan, R.; Wang, B.; Du, P.; Tan, W.; Lammi, M.J.; Guo, X. Prediction of co-expression genes and integrative analysis of gene microarray and proteomics profile of Keshan disease. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Ning, Y.; Wang, X.; Zhang, P.; Anatoly, S.V.; Prakash, N.T.; Li, C.; Zhou, R.; Lammi, M.; Zhang, F.; Guo, X. Imbalance of dietary nutrients and the associated differentially expressed genes and pathways may play important roles in juvenile Kashin-Beck disease. J. Trace Elem. Med. Biol. 2018, 50, 441–460. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, A.; Wen, Y.; Xiao, X.; Hao, J.; Zhang, F.; Guo, X. Comparison of microRNA expression profiles of Kashin-Beck disease, osteoarthritis and rheumatoid arthritis. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium metabolism, regulation, and sex differences in mammals. In Molecular and Integrative Toxicology; Springer: Cham, Switzerland, 2018; pp. 89–107. [Google Scholar]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef]
- Ha, H.Y.; Alfulaij, N.; Berry, M.J.; Seale, L.A. From selenium absorption to selenoprotein degradation. Biol. Trace Elem. Res. 2019, 192, 26–37. [Google Scholar] [CrossRef]
- Wrobel, J.K.; Power, R.; Toborek, M. Biological activity of selenium: Revisited. IUBMB Life 2016, 68, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Infante, H.G.; Sargent, M. Food-Chain selenium and human health: Spotlight on speciation. Br. J. Nutr. 2008, 100, 238–253. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Regulation of selenium metabolism and transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, selenoproteins and viral infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef]
- Sonet, J.; Mosca, M.; Bierla, K.; Modzelewska, K.; Flis-Borsuk, A.; Suchocki, P.; Ksiazek, I.; Anuszewska, E.; Bulteau, A.L.; Szpunar, J.; et al. Selenized plant oil is an efficient source of selenium for selenoprotein biosynthesis in human cell lines. Nutrients 2019, 11, 1524. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Sabbagh, M.; Van Hoewyk, D. Malformed selenoproteins are removed by the ubiquitin-proteasome pathway in stanleya pinnata. Plant Cell Physiol. 2012, 53, 555–564. [Google Scholar] [CrossRef]
- Suzuki, K.T. Metabolomics of selenium: Se metabolites based on speciation studies. J. Health Sci. 2005, 51, 107–114. [Google Scholar] [CrossRef]
- Berry, M.J.; Banu, L.; Chen, Y.; Mandel, S.J.; Kieffer, J.D.; Harney, J.W.; Larsen, P.R. Recognition of UGA as a selenocysteine codon in Type I deiodinase requires sequences in the 3′ untranslated region. Nature 1991, 353, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.T.; Doi, C.; Suzuki, N. Metabolism of 76Se-methylselenocysteine compared with that of 77Se-selenomethionine and 82Se-selenite. Toxicol. Appl. Pharmacol. 2006, 217, 185–195. [Google Scholar] [CrossRef]
- Combs, G.F. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef] [PubMed]
- Juresa, D.; Blanusa, M.; Francesconi, K.A.; Kienzl, N.; Kuehnelt, D. Biological availability of selenosugars in rats. Chem. Biol. Interact. 2007, 168, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I.; Zaporowska, H. Selenium interactions and toxicity: A review. Cell Biol. Toxicol. 2012, 28, 31–46. [Google Scholar] [CrossRef]
- Boylan, L.M.; Spallholz, J.E. Selenium. In Sports Nutrition: Vitamins and Trace Elements, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 275–286. ISBN 9781420037913. [Google Scholar]
- Kieliszek, M.; Lipinski, B.; Błażejak, S. Application of sodium selenite in the prevention and treatment of cancers. Cells 2017, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Allan, C.B.; Lacourciere, G.M.; Stadtman, T.C. Responsiveness of selenoproteins to dietary selenium. Annu. Rev. Nutr. 1999, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Chen, J.; Berry, M.J. Selenium and selenoproteins in the brain and brain diseases. J. Neurochem. 2003, 86, 1–12. [Google Scholar] [CrossRef]
- Huang, J.Q.; Ren, F.Z.; Jiang, Y.Y.; Lei, X.G. Characterization of selenoprotein M and its response to selenium deficiency in chicken brain. Biol. Trace Elem. Res. 2016, 170, 449–458. [Google Scholar] [CrossRef]
- Ullah, H.; Liu, G.; Yousaf, B.; Ali, M.U.; Abbas, Q.; Munir, M.A.M.; Mian, M.M. Developmental selenium exposure and health risk in daily foodstuffs: A systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 2018, 149, 291–306. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błazejak, S. Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef]
- Rayman, M.P.; Winther, K.H.; Pastor-Barriuso, R.; Cold, F.; Thvilum, M.; Stranges, S.; Guallar, E.; Cold, S. Effect of long-term selenium supplementation on mortality: Results from a multiple-dose, randomised controlled trial. Free Radic. Biol. Med. 2018, 127, 46–54. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, W.; Chen, L.L.; Huang, J.Q.; Lei, X.G. Selenoprotein V protects against endoplasmic reticulum stress and oxidative injury induced by pro-oxidants. Free Radic. Biol. Med. 2020, 160, 670–679. [Google Scholar] [CrossRef]
- Chen, L.L.; Huang, J.Q.; Xiao, Y.; Wu, Y.Y.; Ren, F.Z.; Lei, X.G. Knockout of selenoprotein V affects regulation of selenoprotein expression by dietary selenium and fat Intakes in mice. J. Nutr. 2020, 150, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Ren, F.Z.; Jiang, Y.Y.; Xiao, C.; Lei, X.G. Selenoproteins protect against avian nutritional muscular dystrophy by metabolizing peroxides and regulating redox/apoptotic signaling. Free Radic. Biol. Med. 2015, 83, 129–138. [Google Scholar] [CrossRef]
- Bösl, M.R.; Takaku, K.; Oshima, M.; Nishimura, S.; Taketo, M.M. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp). Proc. Natl. Acad. Sci. USA 1997, 94, 5531–5534. [Google Scholar] [CrossRef]
- Han, S.J.; Lee, B.C.; Yim, S.H.; Gladyshev, V.N.; Lee, S.R. Characterization of mammalian selenoprotein O: A redox-active mitochondrial protein. PLoS ONE 2014, 9, e95518. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Rayman, M.P. Multiple nutritional factors and the risk of Hashimoto’s thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Short, S.P.; Pilat, J.M.; Williams, C.S. Roles for selenium and selenoprotein P in the development, progression, and prevention of intestinal disease. Free Radic. Biol. Med. 2018, 127, 26–35. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, R.P.; Cheng, W.H.; Zhu, J.H. Prioritized brain selenium retention and selenoprotein expression: Nutritional insights into Parkinson’s disease. Mech. Ageing Dev. 2019, 180, 89–96. [Google Scholar] [CrossRef]
- Fan, T.W.M.; Teh, S.J.; Hinton, D.E.; Higashi, R.M. Selenium toxicity: Cause and effects in aquatic birds. Aquat. Toxicol. 2002, 57, 27–37. [Google Scholar] [CrossRef]
- Wang, N.; Tan, H.Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of micronutrient selenium in metabolic diseases: Its role as an antioxidant. Oxidative Med. Cell. Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.M. Is selenium a global contaminant of potential concern? Integr. Environ. Assess. Manag. 2009, 5, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; Davis, C.D. MicroRNA, nutrition, and cancer prevention. Adv. Nutr. 2011, 2, 472–485. [Google Scholar] [CrossRef]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Quintanilha, B.J.; Reis, B.Z.; Silva Duarte, G.B.; Cozzolino, S.M.F.; Rogero, M.M. Nutrimiromics: Role of micrornas and nutrition in modulating inflammation and chronic diseases. Nutrients 2017, 9, 1168. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Lee, C.T.; Risom, T.; Strauss, W.M. Evolutionary conservation of microRNA regulatory circuits: An examination of microRNA gene complexity and conserved microRNA-target interactions through metazoan phylogeny. DNA Cell Biol. 2007, 26, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.; Brennecke, J.; Bushati, N.; Russell, R.B.; Cohen, S.M. Animal microRNAs confer robustness to gene expression and have a significant impact on 3′UTR evolution. Cell 2005, 123, 1133–1146. [Google Scholar] [CrossRef]
- Selbach, M.; Schwanhäusser, B.; Thierfelder, N.; Fang, Z.; Khanin, R.; Rajewsky, N. Widespread changes in protein synthesis induced by microRNAs. Nature 2008, 455, 58–63. [Google Scholar] [CrossRef]
- Brennecke, J.; Stark, A.; Russell, R.B.; Cohen, S.M. Principles of microRNA-target recognition. PLoS Biol. 2005, 3, e85. [Google Scholar] [CrossRef] [PubMed]
- Lynn, F.C. Meta-Regulation: microRNA regulation of glucose and lipid metabolism. Trends Endocrinol. Metab. 2009, 20, 452–459. [Google Scholar] [CrossRef]
- Bishop, K.S.; Ferguson, L.R. The interaction between epigenetics, nutrition and the development of cancer. Nutrients 2015, 7, 922–947. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhou, B.; Ross, S.A.; Zempleni, J. Nutrition, microRNAs, and human health. Adv. Nutr. 2017, 8, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, B.; Karlic, H.; Varga, F.; Fabianowska-Majewska, K.; Haslberger, A.G. Epigenetic mechanisms in anti-cancer actions of bioactive food components—The implications in cancer prevention. Br. J. Pharmacol. 2012, 167, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Beckett, E.L.; Yates, Z.; Veysey, M.; Duesing, K.; Lucock, M. The role of vitamins and minerals in modulating the expression of microRNA. Nutr. Res. Rev. 2014, 27, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Xue, Z.; Zhang, H.; Li, Y.; Wang, Y. Local and global effects of Mg2+ on Ago and miRNA-target interactions. J. Mol. Model. 2012, 18, 3769–3781. [Google Scholar] [CrossRef] [PubMed]
- Barr, I.; Smith, A.T.; Chen, Y.; Senturia, R.; Burstyn, J.N.; Guo, F. Ferric, not ferrous, heme activates RNA-binding protein DGCR8 for primary microRNA processing. Proc. Natl. Acad. Sci. USA 2012, 109, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Pogue, A.I.; Percy, M.E.; Cui, J.G.; Li, Y.Y.; Bhattacharjee, S.; Hill, J.M.; Kruck, T.P.A.; Zhao, Y.; Lukiw, W.J. Up-Regulation of NF-kB-sensitive miRNA-125b and miRNA-146a in metal sulfate-stressed human astroglial (HAG) primary cell cultures. J. Inorg. Biochem. 2011, 105, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Gabory, A.; Attig, L.; Junien, C. Sexual dimorphism in environmental epigenetic programming. Mol. Cell. Endocrinol. 2009, 304, 8–18. [Google Scholar] [CrossRef]
- Biswas, S.; Rao, C.M. Epigenetics in cancer: Fundamentals and beyond. Pharmacol. Ther. 2017, 173, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Hulf, T.; Sibbritt, T.; Wiklund, E.D.; Bert, S.; Strbenac, D.; Statham, A.L.; Robinson, M.D.; Clark, S.J. Discovery pipeline for epigenetically deregulated miRNAs in cancer: Integration of primary miRNA transcription. BMC Genom. 2011, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Rubio, B.; Mora, C.; Pintado, C.; Mazuecos, L.; Fernández, A.; López, V.; Andrés, A.; Gallardo, N. The nutrient sensing pathways FoxO1/3 and mTOR in the heart are coordinately regulated by central leptin through PPARβ/δ. Implications in cardiac remodeling. Metabolism 2021, 115, 154453. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Buel, G.R.; Blenis, J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol. Cells 2013, 35, 463–473. [Google Scholar] [CrossRef]
- Ma, X.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ge, Y.; Drnevich, J.; Zhao, Y.; Band, M.; Chen, J. Mammalian target of rapamycin regulates miRNA-1 and follistatin in skeletal myogenesis. J. Cell Biol. 2010, 189, 1157–1169. [Google Scholar] [CrossRef]
- Rao, P.K.; Kumar, R.M.; Farkhondeh, M.; Baskerville, S.; Lodish, H.F. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc. Natl. Acad. Sci. USA 2006, 103, 8721–8726. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Liu, Y.; Chen, C.; Tang, F.; Wu, Q.; Wang, X.; Liu, C.G.; Liu, X.; Liu, R.; Liu, Y.; et al. An mTORC1-Mdm2-Drosha axis for miRNA biogenesis in response to glucose- and amino acid-deprivation. Mol. Cell 2015, 57, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. MTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, B.; He, J.; Chen, D. From nutrient to microRNA: A novel insight into cell signaling involved in skeletal muscle development and disease. Int. J. Biol. Sci. 2016, 12, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- Maciel-Dominguez, A.; Swan, D.; Ford, D.; Hesketh, J. Selenium alters miRNA profile in an intestinal cell line: Evidence that miR-185 regulates expression of GPX2 and SEPSH2. Mol. Nutr. Food Res. 2013, 57, 2195–2205. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, Z.; Yang, G.; Gao, D.; Niu, X. MicroRNA expression profiles in rats with selenium deficiency and the possible role of the Wnt/β-catenin signaling pathway in cardiac dysfunction. Int. J. Mol. Med. 2015, 35, 143–152. [Google Scholar] [CrossRef]
- Matoušková, P.; Hanousková, B.; Skálová, L. MicroRNAs as potential regulators of glutathione peroxidases expression and their role in obesity and related pathologies. Int. J. Mol. Sci. 2018, 19, 1199. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Zhou, J.C.; Wu, Y.Y.; Ren, F.Z.; Lei, X.G. Role of glutathione peroxidase 1 in glucose and lipid metabolism-related diseases. Free Radic. Biol. Med. 2018, 127, 108–115. [Google Scholar] [CrossRef]
- Alehagen, U.; Johansson, P.; Aaseth, J.; Alexander, J.; Wågsäter, D. Significant changes in circulating microRNA by dietary supplementation of selenium and coenzyme Q10 in healthy elderly males. A subgroup analysis of a prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. PLoS ONE 2017, e0174880. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Q.; Guo, Y.; Zhao, Y.; Wang, X.; Zhang, Z.; Deng, G.; Guo, M. Selenium suppresses inflammation by inducing microRNA-146a in Staphylococcus aureus-infected mouse mastitis model. Oncotarget 2017, 8, 110949–110964. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Guo, Y.F.; Li, C.Y.; Qiu, C.W.; Guo, M.Y. Selenium influences mmu-miR-155 to inhibit inflammation in: Staphylococcus aureus-induced mastitis in mice. Food Funct. 2019, 10, 6543–6555. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Q.; Zhang, W.; Chen, Y.; Ren, J.; Gao, A. Associations of blood levels of trace elements and heavy metals with metabolic syndrome in Chinese male adults with microRNA as mediators involved. Environ. Pollut. 2019, 248, 66–73. [Google Scholar] [CrossRef]
- Reis, B.Z.; Duarte, G.B.S.; Vargas-Mendez, E.; Ferreira, L.R.P.; Barbosa, F.; Cercato, C.; Rogero, M.M.; Cozzolino, S.M.F. Brazil nut intake increases circulating miR-454-3p and miR-584-5p in obese women. Nutr. Res. 2019, 67, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, B.; Grune, T. Epigenetic effects of selenium and their implications for health. Epigenetics 2015, 10, 179–190. [Google Scholar] [CrossRef]
- Joven, J.; Micol, V.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menéndez, J.A.; Aragonès, G.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Camps, J.; Cufí, S.; et al. Polyphenols and the modulation of gene expression pathways: Can we eat our way out of the danger of chronic disease? Crit. Rev. Food Sci. Nutr. 2014, 54, 985–1001. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, E.; Reszka, E. Selenium and epigenetics in cancer: Focus on DNA methylation. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 136, pp. 193–234. [Google Scholar]
- Min, Z.; Guo, Y.; Sun, M.; Hussain, S.; Zhao, Y.; Guo, D.; Huang, H.; Heng, L.; Zhang, F.; Ning, Q.; et al. Selenium-Sensitive miRNA-181a-5p targeting SBP2 regulates selenoproteins expression in cartilage. J. Cell. Mol. Med. 2018, 22, 5888–5898. [Google Scholar] [CrossRef]
- Fan, R.F.; Cao, C.Y.; Chen, M.H.; Shi, Q.X.; Xu, S.W. Gga-let-7f-3p promotes apoptosis in selenium deficiency-induced skeletal muscle by targeting selenoprotein K. Metallomics 2018, 10, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Liu, T.; Cao, C.; Xu, S. MiR-200a-5p augments cardiomyocyte hypertrophy induced by glucose metabolism disorder via the regulation of selenoproteins. J. Cell. Physiol. 2019, 234, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Cao, C.; Yang, J.; Liu, T.; Lei, X.G.; Zhang, Z.; Xu, S. MiR-200a-5p regulates myocardial necroptosis induced by Se deficiency via targeting RNF11. Redox Biol. 2018, 15, 159–169. [Google Scholar] [CrossRef]
- Chi, Q.; Luan, Y.; Zhang, Y.; Hu, X.; Li, S. The regulatory effects of miR-138-5p on selenium deficiency-induced chondrocyte apoptosis are mediated by targeting SelM. Metallomics 2019, 11, 845–857. [Google Scholar] [CrossRef]
- Potenza, N.; Castiello, F.; Panella, M.; Colonna, G.; Ciliberto, G.; Russo, A.; Costantini, S.C. Human miR-544a modulates SELK expression in hepatocarcinoma cell lines. PLoS ONE 2016, 11, e0156908. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, Y.; Wan, C.; Wang, Z.; Cong, Y.; Li, S. MiR-196-5p involvement in selenium deficiency-induced immune damage: Via targeting of NFκBIA in the chicken trachea. Metallomics 2020, 12, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yang, T.; Xu, Z.; Tan, S.; Pan, T.; Wan, N.; Li, S. MicroRNA-193b-3p regulates hepatocyte apoptosis in selenium-deficient broilers by targeting MAML1. J. Inorg. Biochem. 2018, 186, 235–245. [Google Scholar] [CrossRef]
- Wan, N.; Xu, Z.; Chi, Q.; Hu, X.; Pan, T.R.; Liu, T.; Li, S. MicroRNA-33-3p involved in selenium deficiency-induced apoptosis via targeting ADAM10 in the chicken kidney. J. Cell. Physiol. 2019, 234, 13693–13704. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, N.; Pan, T.; Hu, X.; Liu, Q.; Li, S. MicroRNA-33-3p regulates vein endothelial cell apoptosis in selenium-deficient broilers by targeting E4F1. Oxidative Med. Cell. Longev. 2019, 2019, 6274010. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Hu, X.; Ge, T.; Li, M.; Shi, M.; Luo, J.; Lai, H.; Nie, T.; Li, F.; Li, H. MicroRNA-328 is involved in the effect of selenium on hydrogen peroxide-induced injury in H9c2 cells. J. Biochem. Mol. Toxicol. 2017, 31, e21920. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yang, J.; Liu, Q.; Gong, Y.; Zhang, Y.; Zhang, Z. Selenium deficiency inhibits myocardial development and differentiation by targeting the mir-215-5p/CTCF axis in chicken. Metallomics 2019, 11, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yang, J.; Liu, Q.; Gong, Y.; Zhang, Y.; Zheng, Y.; Yu, D.; Zhang, Z. Mir-215-5p induces autophagy by targeting PI3K and activating ROS-mediated MAPK pathways in cardiomyocytes of chicken. J. Inorg. Biochem. 2019, 193, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gong, Y.; Cai, J.; Liu, Q.; Zhang, Z. lnc-3215 suppression leads to calcium overload in selenium deficiency-induced chicken heart lesion via the lnc-3215-miR-1594-TNN2 pathway. Mol. Ther. Nucleic Acids 2019, 18, 1–15. [Google Scholar] [CrossRef]
- Liu, Q.; Cai, J.; Gao, Y.; Yang, J.; Gong, Y.; Zhang, Z. MiR-2954 Inhibits PI3K signaling and induces autophagy and apoptosis in myocardium selenium deficiency. Cell. Physiol. Biochem. 2018, 51, 778–792. [Google Scholar] [CrossRef]
- Wang, L.; Shi, X.; Zheng, S.; Xu, S. Selenium deficiency exacerbates LPS-induced necroptosis by regulating miR-16-5p targeting PI3K in chicken tracheal tissue. Metallomics 2020, 12, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Hu, X.; Liu, T.; Xu, Z.; Wan, N.; Zhang, Y.; Li, S. MiR-128-1-5p regulates tight junction induced by selenium deficiency via targeting cell adhesion molecule 1 in broilers vein endothelial cells. J. Cell. Physiol. 2018, 233, 8802–8814. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, H.; Xu, S. Selenium-Deficient diet induces necroptosis in the pig brain by activating TNFR1: Via mir-29a-3p. Metallomics 2020, 12, 1290–1301. [Google Scholar] [CrossRef]
- Liu, C.; Sun, Z.; Xu, Z.; Liu, T.; Pan, T.; Li, S. Down-Regulation of microRNA-155 promotes selenium deficiency-induced apoptosis by tumor necrosis factor receptor superfamily member 1B in the broiler spleen. Oncotarget 2017, 8, 58513–58525. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.J.; Tang, B.; Liang, H.H.; Yi, L.; Wei, Z.G. Selenium deficiency inhibits micRNA-146a to promote ROS-induced inflammation via regulation of the MAPK pathway in the head kidney of carp. Fish Shellfish Immunol. 2019, 91, 284–292. [Google Scholar] [CrossRef]
- Tarek, M.; Louka, M.L.; Khairy, E.; Ali-Labib, R.; Zaky, D.Z.; Montasser, I.F. Role of microRNA-7 and selenoprotein P in hepatocellular carcinoma. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, D.; Zhou, M.; Shi, H.; Yan, S.; Cai, Y. Regulatory role of miR-125a/b in the suppression by selenium of cadmium-induced apoptosis via the mitochondrial pathway in LLC-PK1 cells. Chem. Biol. Interact. 2016, 243, 35–44. [Google Scholar] [CrossRef]
- Atteia, H.H.; Arafa, M.H.; Prabahar, K. Selenium nanoparticles prevents lead acetate-induced hypothyroidism and oxidative damage of thyroid tissues in male rats through modulation of selenoenzymes and suppression of miR-224. Biomed. Pharmacother. 2018, 99, 486–491. [Google Scholar] [CrossRef]
- Yin, K.; Cui, Y.; Sun, T.; Qi, X.; Zhang, Y.; Lin, H. Antagonistic effect of selenium on lead-induced neutrophil apoptosis in chickens via miR-16-5p targeting of PiK3R1 and IGF1R. Chemosphere 2020, 246, 125794. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, S.; Wang, S.; Liu, Q.; Xu, S. Cadmium-Induced oxidative stress promotes apoptosis and necrosis through the regulation of the miR-216a-PI3K/AKT axis in common carp lymphocytes and antagonized by selenium. Chemosphere 2020, 258, 127341. [Google Scholar] [CrossRef]
- Diwadkar-Navsariwala, V.; Prins, G.S.; Swanson, S.M.; Birch, L.A.; Ray, V.H.; Hedayat, S.; Lantvit, D.L.; Diamond, A.M. Selenoprotein deficiency accelerates prostate carcinogenesis in a transgenic model. Proc. Natl. Acad. Sci. USA 2006, 103, 8179–8184. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Dong, Y.-L.; Li, T.; Xiong, W.; Zhang, X.; Wang, P.-J.; Huang, J.-Q. Dietary Selenium Regulates microRNAs in Metabolic Disease: Recent Progress. Nutrients 2021, 13, 1527. https://doi.org/10.3390/nu13051527
Huang X, Dong Y-L, Li T, Xiong W, Zhang X, Wang P-J, Huang J-Q. Dietary Selenium Regulates microRNAs in Metabolic Disease: Recent Progress. Nutrients. 2021; 13(5):1527. https://doi.org/10.3390/nu13051527
Chicago/Turabian StyleHuang, Xin, Yu-Lan Dong, Tong Li, Wei Xiong, Xu Zhang, Peng-Jie Wang, and Jia-Qiang Huang. 2021. "Dietary Selenium Regulates microRNAs in Metabolic Disease: Recent Progress" Nutrients 13, no. 5: 1527. https://doi.org/10.3390/nu13051527
APA StyleHuang, X., Dong, Y.-L., Li, T., Xiong, W., Zhang, X., Wang, P.-J., & Huang, J.-Q. (2021). Dietary Selenium Regulates microRNAs in Metabolic Disease: Recent Progress. Nutrients, 13(5), 1527. https://doi.org/10.3390/nu13051527








