Vitamin D Receptor and Vitamin D Binding Protein Gene Polymorphisms Are Associated with Renal Allograft Outcome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Genetic Study
2.3. Statistical Analysis
3. Results
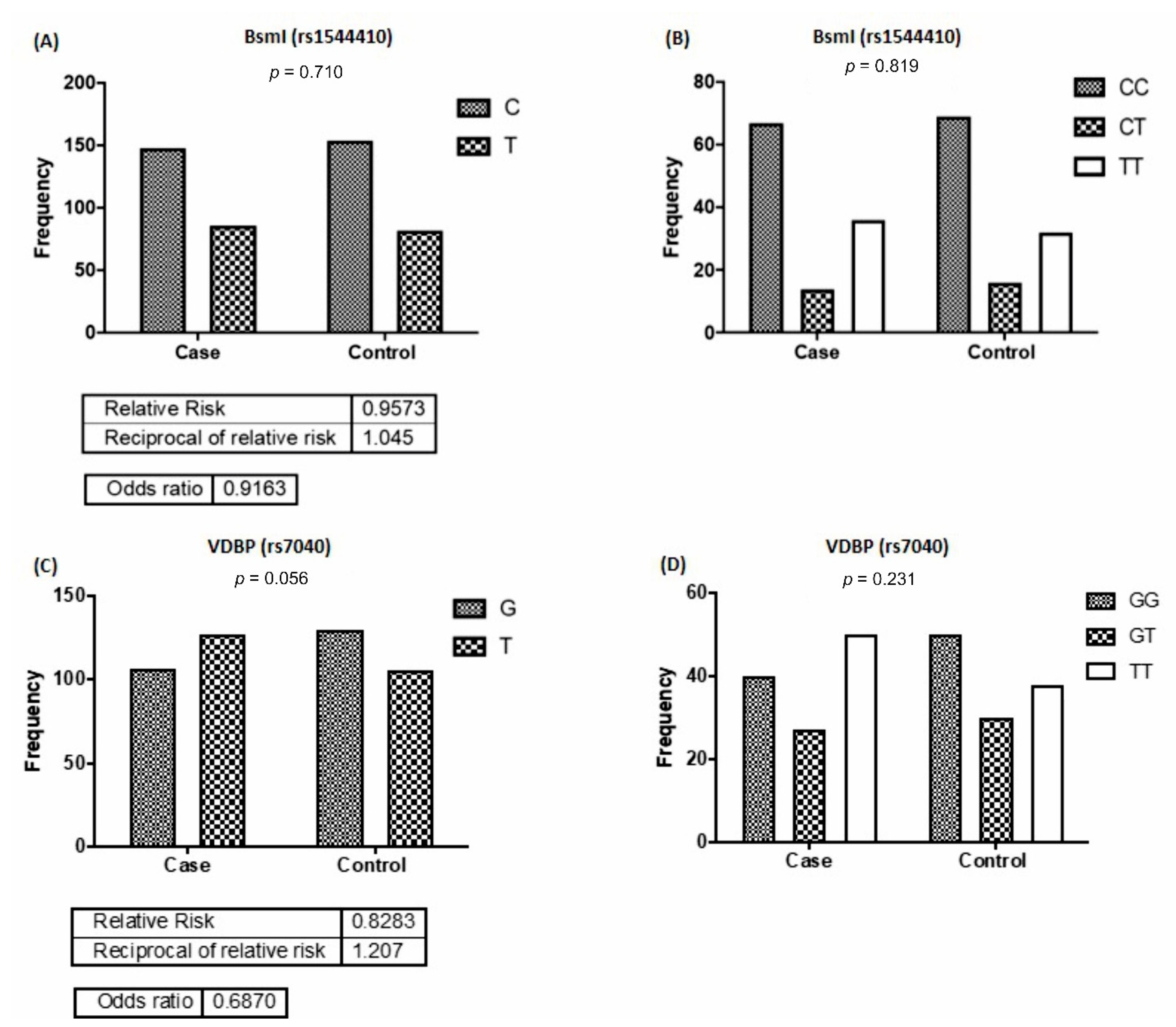
3.1. Frequencies of VDR and VDBP Alleles and Genotypes Distribution
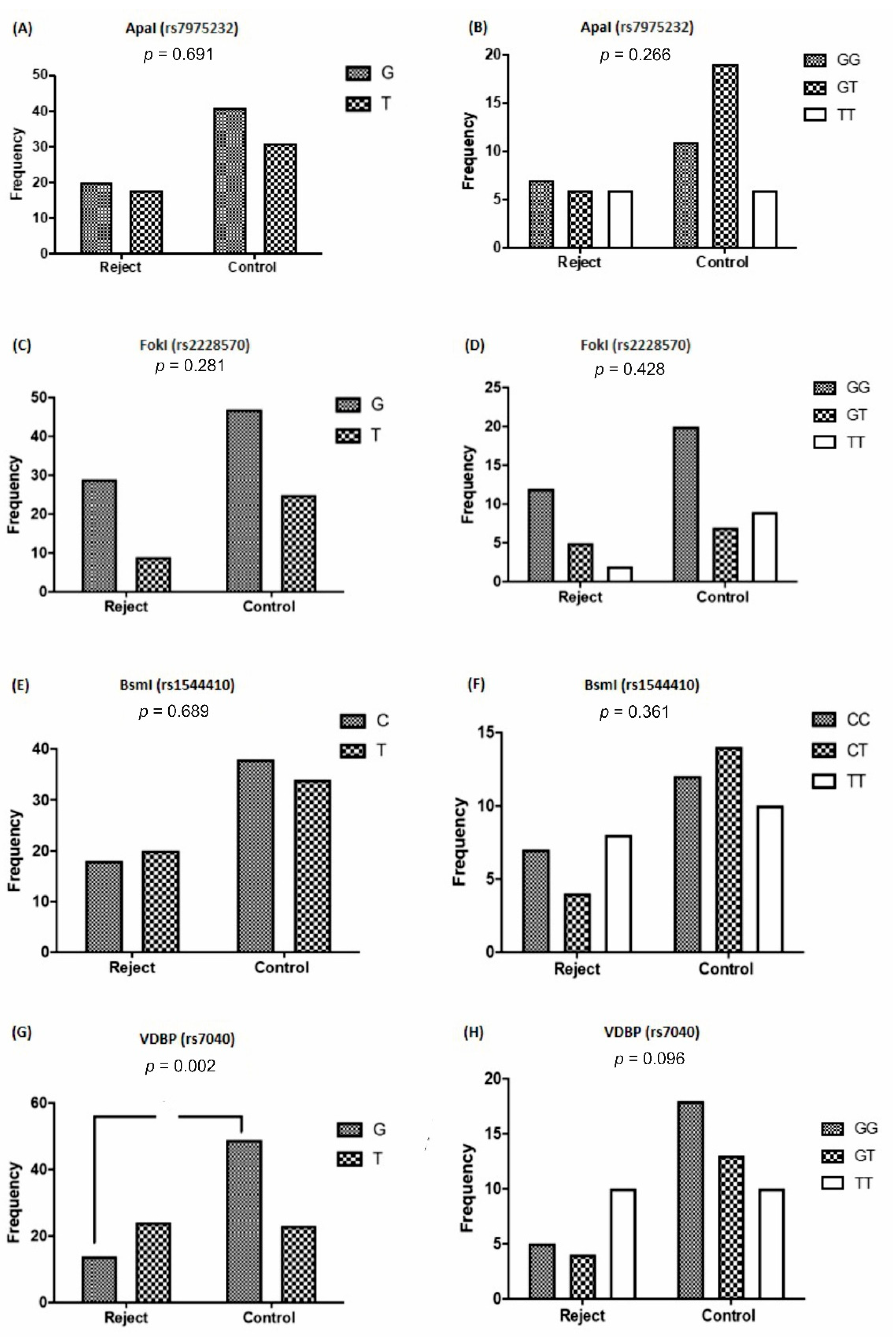
3.2. VDR and VDBP Gene Polymorphisms Based on Allograft Rejection
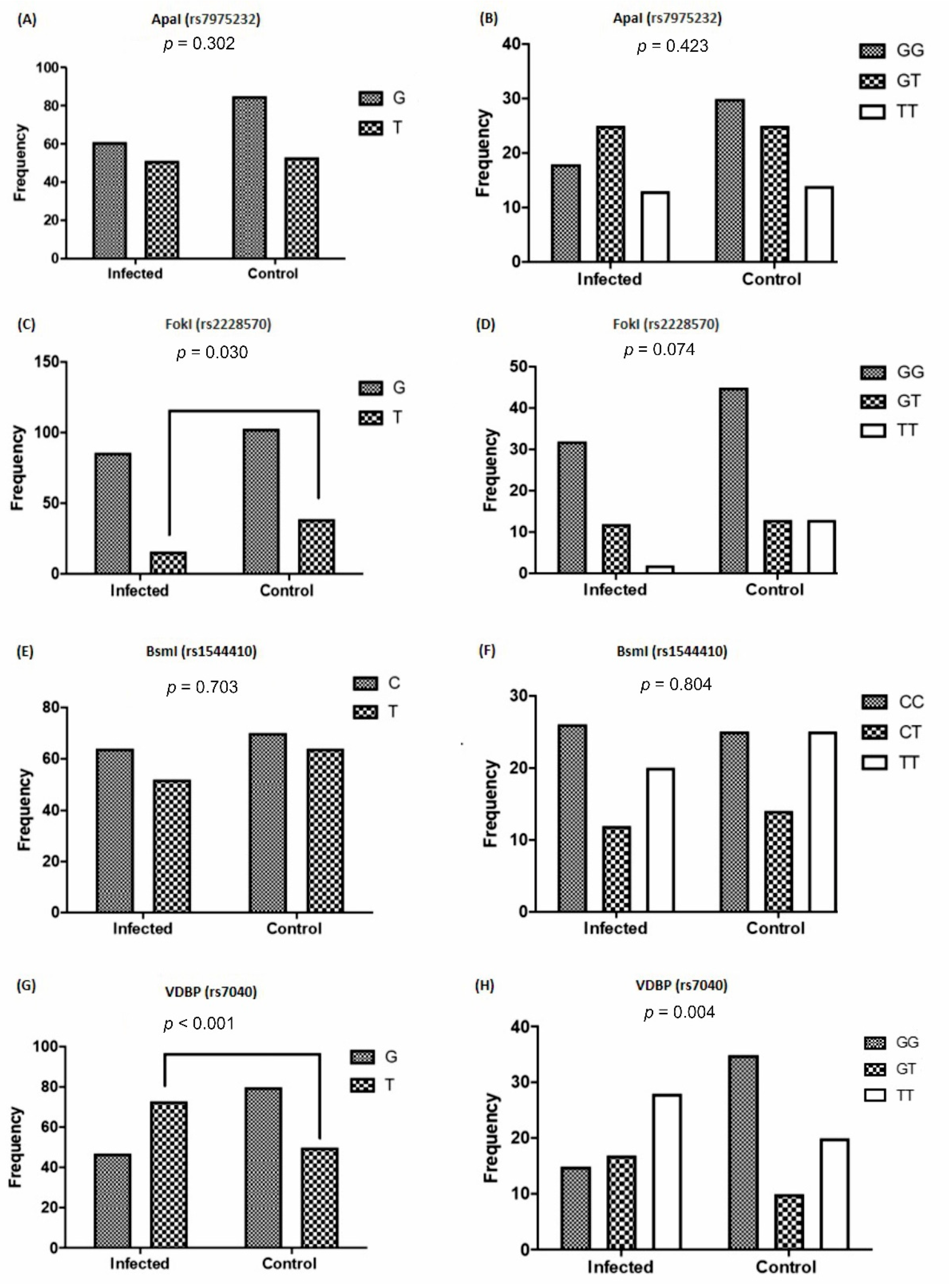
3.3. The Frequency of VDR and VDBP Gene Polymorphisms Based on Viral Infection
3.4. Connection of Vitamin D Levels with VDR and VDBP Gene Polymorphisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rees, M.A.; Dunn, T.B.; Kuhr, C.S.; Marsh, C.L.; Rogers, J.; Rees, S.E.; Cicero, A.; Reece, L.J.; Roth, A.E.; Ekwenna, O.; et al. Kidney exchange to overcome financial barriers to kidney transplantation. Arab. Archaeol. Epigr. 2016, 17, 782–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipworth, L.; Mumma, M.T.; Cavanaugh, K.L.; Edwards, T.L.; Ikizler, T.A.; Tarone, R.E.; McLaughlin, J.K.; Blot, W.J. Incidence and predictors of end stage renal disease among low-income blacks and whites. PLoS ONE 2012, 7, e48407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, A.I.; Rodriguez, R.A.; Bacchetti, P.; Bertenthal, D.; Hernandez, G.T.; O’Hare, A.M. White/black racial differences in risk of end-stage renal disease and death. Am. J. Med. 2009, 122, 672–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haussler, M.R.; Whitfield, G.K.; Haussler, C.A.; Hsieh, J.-C.; Thompson, P.D.; Selznick, S.H.; Dominguez, C.E.; Jurutka, P.W. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J. Bone Miner. Res. 1998, 13, 325–349. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and Immune Function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Uitterlinden, A.G.; Fang, Y.; van Meurs, J.B.; Pols, H.A.; van Leeuwen, J.P. Genetics and biology of vitamin D receptor polymorphisms. Gene 2004, 338, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Bikle, D.D. Vitamin D Metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef] [Green Version]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.F.; Shieh, A.; Gottlieb, C.; Yacoubian, V.; Wang, J.; Hewison, M.; Adams, J.S. Vitamin D Binding protein and the biological activity of vitamin D. Front. Endocrinol. 2019, 10, 718. [Google Scholar] [CrossRef]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D binding protein: A historic overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef]
- Lee, J.R.; Dadhania, D.; August, P.; Lee, J.B.; Suthanthiran, M.; Muthukumar, T. Circulating levels of 25-hydroxyvitamin D and acute cellular rejection in kidney allograft recipients. Transplantation 2014, 98, 292–299. [Google Scholar] [CrossRef] [Green Version]
- Thiem, U.; Borchhardt, K. Vitamin D in solid organ transplantation with special emphasis on kidney transplantation. Vitam. Horm. 2011, 86, 429–468. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kang, S.-W.; Yoo, T.-H.; Kim, M.S.; Kim, S.I.; Kim, Y.S.; Choi, K.H. The impact of pretransplant 25-hydroxy vitamin D deficiency on subsequent graft function: An observational study. BMC Nephrol. 2012, 13, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saber, A.; Fotuhi, F.; Rostami, Z.; Einollahi, B.; Nemati, E. Vitamin D levels after kidney transplantation and the risk of cytomegalovirus infection. Nephrourol. Mon. 2015, 7, e29677. [Google Scholar] [CrossRef] [Green Version]
- Vu, N.; Sakharkar, P.; Tellez-Corrales, E.; Shah, T.; Hutchinson, I.; Min, D.I. Association of vitamin D binding protein polymorphism with long-term kidney allograft survival in Hispanic kidney transplant recipients. Mol. Biol. Rep. 2013, 40, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Fu, L.; Juras, D.J.; Karmali, M.; Wong, B.Y.L.; Gozdzik, A.; Cole, D.E.C. Common variants of the vitamin D binding protein gene and adverse health outcomes. Crit. Rev. Clin. Lab. Sci. 2013, 50, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Valdivielso, J.M.; Fernandez, E. Vitamin D receptor polymorphisms and diseases. Clin. Chim. Acta 2006, 371, 1–12. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D.R. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Shams, S.S.; Vahed, S.Z.; Soltanzad, F.; Kafil, V.; Barzegari, A.; Atashpaz, S.; Barar, J. Highly effective DNA extraction method from fresh, frozen, dried and clotted blood samples. BioImpacts 2011, 1, 183–187. [Google Scholar]
- Marcen, R.; Ponte, B.; Rodríguez-Mendiola, N.; Fernandez-Rodriguez, A.; Galeano, C.; Villafruela, J.; Teruel, J.; Burgos, F.; Ortuño, J. Vitamin D deficiency in kidney transplant recipients: Risk factors and effects of vitamin D3 supplements. Transplant. Proc. 2009, 41, 2388–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hullett, D.A.; Laeseke, P.F.; Malin, G.; Nessel, R.; Sollinger, H.W.; Becker, B.N. Prevention of chronic allograft nephropathy with vitamin D*. Transpl. Int. 2005, 18, 1175–1186. [Google Scholar] [CrossRef]
- Battaglia, Y.; Cojocaru, E.; Fiorini, F.; Granata, A.; Esposito, P.; Russo, L.; Bortoluzzi, A.; Storari, A.; Russo, D. Vitamin D in kidney transplant recipients. Clin. Nephrol. 2020, 93, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.K.; Mok, M.M.; Yung, S.; Tang, C.S.; Chan, T.M. High prevalence of vitamin D insufficiency in southern chinese renal transplant recipients. Ren. Fail. 2012, 34, 980–984. [Google Scholar] [CrossRef] [Green Version]
- Bienaimé, F.; Girard, D.; Anglicheau, D.; Canaud, G.; Souberbielle, J.C.; Kreis, H.; Noël, L.H.; Friedlander, G.; Elie, C.; Legendre, C.; et al. Vitamin D status and outcomes after renal transplantation. J. Am. Soc. Nephrol. 2013, 24, 831–841. [Google Scholar] [CrossRef]
- Ye, W.-Z.; Reis, A.F.; Velho, G. Identification of a novel Tru9 I polymorphism in the human vitamin D receptor gene. J. Hum. Genet. 2000, 45, 56–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faraco, J.H.; Morrison, N.A.; Baker, A.; Shine, J.; Frossard, P.M. ApaI dimorphism at the human vitamin D receptor gene locus. Nucleic Acids Res. 1989, 17, 2150. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.; Eccleshall, T.R.; Malloy, P.J.; Villa, M.L.; Marcus, R.; Feldman, D. The presence of a polymorphism at the translation initiation site of the vitamin D receptor gene is associated with low bone mineral density in postmenopausal mexican-American women. J. Bone Miner. Res. 2010, 11, 1850–1855. [Google Scholar] [CrossRef] [PubMed]
- Rubello, M.; Giannini, S.; D’Angelo, A.; Nobile, M.; Carraio, G.; Rigotti, P.; Marchini, F.; Zaninotto, M.; Carbonare, L.D.; Sartori, L.; et al. Secondary hyperparathyroidism is associated with vitamin D receptor polymorphism and bone density after renal transplantation. Biomed. Pharmacother. 2005, 59, 402–407. [Google Scholar] [CrossRef]
- Korucu, B.; Tükün, F.A.; Helvaci, Özant; Yeter, H.H.; Gönen, S.; Güz, G.; Arinsoy, S.T. Vitamin D receptor polymorphisms and bone health after kidney transplantation. Turk. J. Med Sci. 2021. [Google Scholar] [CrossRef]
- Yao, B.; Chen, X.; Shen, F.-X.; Xu, W.; Dong, T.-T.; Chen, L.-Z.; Weng, J.-P. The incidence of posttransplantation diabetes mellitus during follow-up in kidney transplant recipients and relationship to Fok1 vitamin D receptor polymorphism. Transplant. Proc. 2013, 45, 194–196. [Google Scholar] [CrossRef]
- Falleti, E.; Bitetto, D.; Fabris, C.; Cmet, S.; Fornasiere, E.; Cussigh, A.; Fontanini, E.; Avellini, C.; Barbina, G.; Ceriani, E.; et al. Association between vitamin D receptor genetic polymorphisms and acute cellular rejection in liver-transplanted patients. Transpl. Int. 2012, 25, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Azarpira, N.; Sagheb, M.M.; Geramizadeh, B.; Darai, M. Vitamin D receptor genotypes and kidney allograft rejection. Mol. Biol. Rep. 2009, 36, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Lavin, P.; Laing, M.; O’Kelly, P.; Moloney, F.; Gopinathan, D.; Al Aradi, A.; Shields, D.; Murphy, G.; Conlon, P. Improved renal allograft survival with vitamin D receptor polymorphism. Ren. Fail. 2007, 29, 785–789. [Google Scholar] [CrossRef]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D 3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.; Sartory, N.A.; Zahn, N.; Radeke, H.H.; Stein, J.M. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol Is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Ther. 2007, 324, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Rech, M.A.; Fleming, J.N.; Moore, C.L. 25-hydroxyvitamin D deficiency and opportunistic viral infections after kidney transplant. Exp. Clin. Transplant. 2014, 12, 95–100. [Google Scholar]



| Polymorphisms | Position | Description | Sequences (5′-3′) |
|---|---|---|---|
| rs2228570 | chr12:47879112 (GRCh38.p12) | NG_008731.1:g.30920T > G; NG_008731.1:g.30920T > C; NG_008731.1:g.30920T > A | F: 5′-GTGGGTGGCACCAAGGAT-3′ R: GTCTCCACACACCCCACAGAT-3′ |
| rs1544410 | chr12:47846052 (GRCh38.p12) | NG_008731.1:g.63980G > T; NG_008731.1:g.63980G > C; NG_008731.1:g.63980G > A | F: 5′-CTGGGGCCACAGACAGG-3′ R: CCTGCCCGCAAGAAACCTCAA-3′ |
| rs795232 | chr12:47845054 (GRCh38.p12) | NG_008731.1:g.64978G > T | F: 5′-GGCAGTGGTATCACCGGTCAG-3′ R: 5′-CTGTGGGCACGGGGATAGAGA-3′ |
| rs7040 | F: 5′-TTGCCTGATGCCACACCC-3′ R: 5′-GGAACAGCAGTTGGAGGCAAA-3′ |
| Variables | Controls | Cases | p-Value |
|---|---|---|---|
| No. of cases | 100 | 115 | - |
| Demographic characteristics | |||
| Age mean ± SD (years) | 41.3 ± 3.2 | 41.8 ± 5.3 | 0.760 |
| Gender, Male, n (%) | 58 (58%) | 72(62.6%) | 0.810 a |
| Female, n (%) | 32 (32%) | 43(37.4%) | |
| BMI, Kg/m2 (mean ± SD) | 20.92 ± 4.70 | 22.43 ± 3.30 | p = 0.125 |
| Smoking history, n (%) | 25(10) | 15(7) | p = 0.092 a |
| Clinical characteristics | |||
| Urea (mg/dL) | 27.46 ± 1.7 | 58.02 ± 23.7 | <0.001 |
| Serum creatinine (mg/dL) | 1.04 ± 0.1 | 1.53 (1.15) | <0.001 b |
| Serum Calcium (mg/dL) | 8.99 ± 0.75 | 7.16 ± 1.27 | p = 0.041 |
| Phosphate (mg/dL) | 3.8 ± 0.42 | 4.61 ± 0.81 | p = 0.030 |
| Serum 25(OH) D (ng/mL) | 40.02 (14.38) | 16.50 (20.38) | p < 0.001 b |
| GFR (mL/min/1.73 m2) | 84.65 (20.12) | 59.89 (31.32) | <0.001 b |
| PTH (pg/mL) | 43 (15) | 141 (30) | p = 0.024 b |
| Hemoglobin c | 14.71 ± 1.84 | 11.42 ± 5.23 | p = 0.051 |
| Albumin (g/dL) d | 4.31 ± 0.95 | 3.24 ± 1.03 | p = 0.062 |
| Pre-transplant dialysis (months) | - | 25.60 ± 10.45 | |
| Time post-transplantation (months) | - | 65 (40.21) | |
| Underlying ESRD, n (%) | |||
| Glomerulonephritis | - | 48 (41.73) | |
| Interstitial nephropathy | - | 31 (26.95) | |
| Diabetic | - | 14 (12.17) | |
| Hypertension | - | 9 (7.82) | |
| Unknown | - | 5 (4.37) | |
| Polycystic kidney disease | - | 3 (2.60) | |
| Vasculitis | - | 3 (2.60) | |
| Reflux nephropathy | - | 2 (1.73) | |
| Post-transplant complications, n (%) | |||
| DGF | - | 5 (4.34) | |
| Acute allograft rejection | - | 25 (21.70) | |
| Chronic graft dysfunction e | - | 23(20) | |
| New-onset diabetes | - | 9 (7.8) | |
| CMV, n (%) | - | 20 (17.4) | |
| BK polyomavirus, n (%) | - | 16 (13.9) | |
| CMV + BK polyomavirus, n (%) | - | 19 (16.5) | |
| CMV + Parvovirus | - | 1 (0.87) | |
| Chronic allograft failure f | - | 15 (13.04) | |
| Recipients survival | - | 115 (100) |
| Risk Factors | OR (95%CI) | p Value |
|---|---|---|
| rs1544410 and graft rejection | 0.8053 | 0.6892 |
| rs2228570 and graft survival | 1.714 | 0.2817 |
| rs7975232 and graft survival | 0.8401 | 0.6910 |
| rs7040 and graft survival | 0.2738 | 0.0023 |
| rs1544410 and viral infection | 1.125 | 0.7032 |
| rs2228570 and viral infection | 2.035 | 0.0309 |
| rs7975232 and viral infection | 0.7458 | 0.3021 |
| rs7040 and viral infection | 0.4024 | 0.0006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zununi Vahed, S.; Ahmadian, E.; Foroughi, P.; Mostafavi, S.; Madry, H.; Ardalan, M.; Cucchiarini, M. Vitamin D Receptor and Vitamin D Binding Protein Gene Polymorphisms Are Associated with Renal Allograft Outcome. Nutrients 2021, 13, 1101. https://doi.org/10.3390/nu13041101
Zununi Vahed S, Ahmadian E, Foroughi P, Mostafavi S, Madry H, Ardalan M, Cucchiarini M. Vitamin D Receptor and Vitamin D Binding Protein Gene Polymorphisms Are Associated with Renal Allograft Outcome. Nutrients. 2021; 13(4):1101. https://doi.org/10.3390/nu13041101
Chicago/Turabian StyleZununi Vahed, Sepideh, Elham Ahmadian, Peyman Foroughi, Soroush Mostafavi, Henning Madry, Mohammadreza Ardalan, and Magali Cucchiarini. 2021. "Vitamin D Receptor and Vitamin D Binding Protein Gene Polymorphisms Are Associated with Renal Allograft Outcome" Nutrients 13, no. 4: 1101. https://doi.org/10.3390/nu13041101







