Effects of Vitamin B12 Supplementation on Cognitive Function, Depressive Symptoms, and Fatigue: A Systematic Review, Meta-Analysis, and Meta-Regression
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Criteria for Study Inclusion/Exclusion
2.3. Study Selection, Quality Assessment, and Data Extraction
2.4. Outcomes
2.5. Data Synthesis and Analysis
3. Results
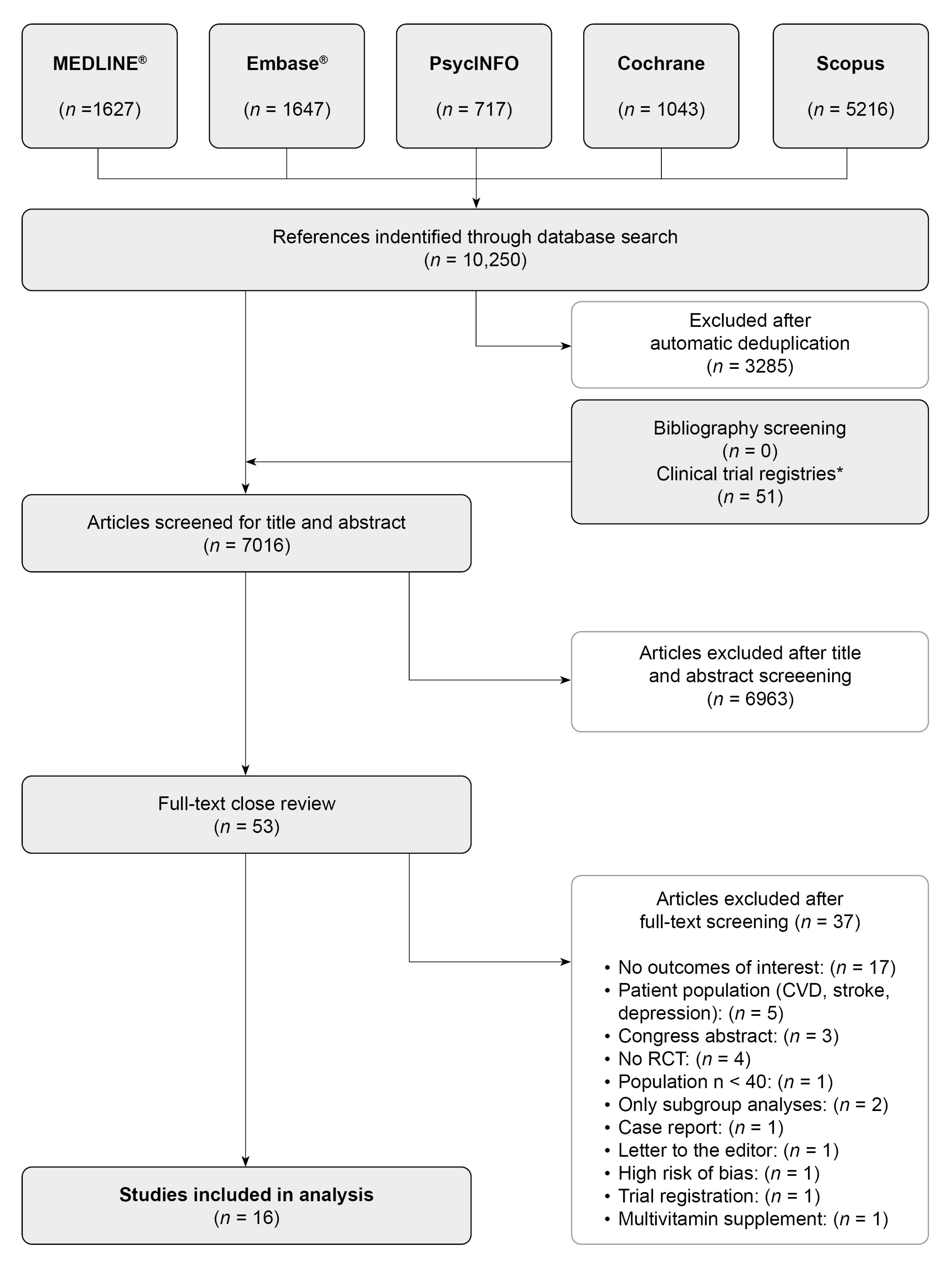
3.1. Study Selection
3.2. Overview and Characteristics of Included Studies
3.3. Quality Assessment of Included Studies and Publication Bias
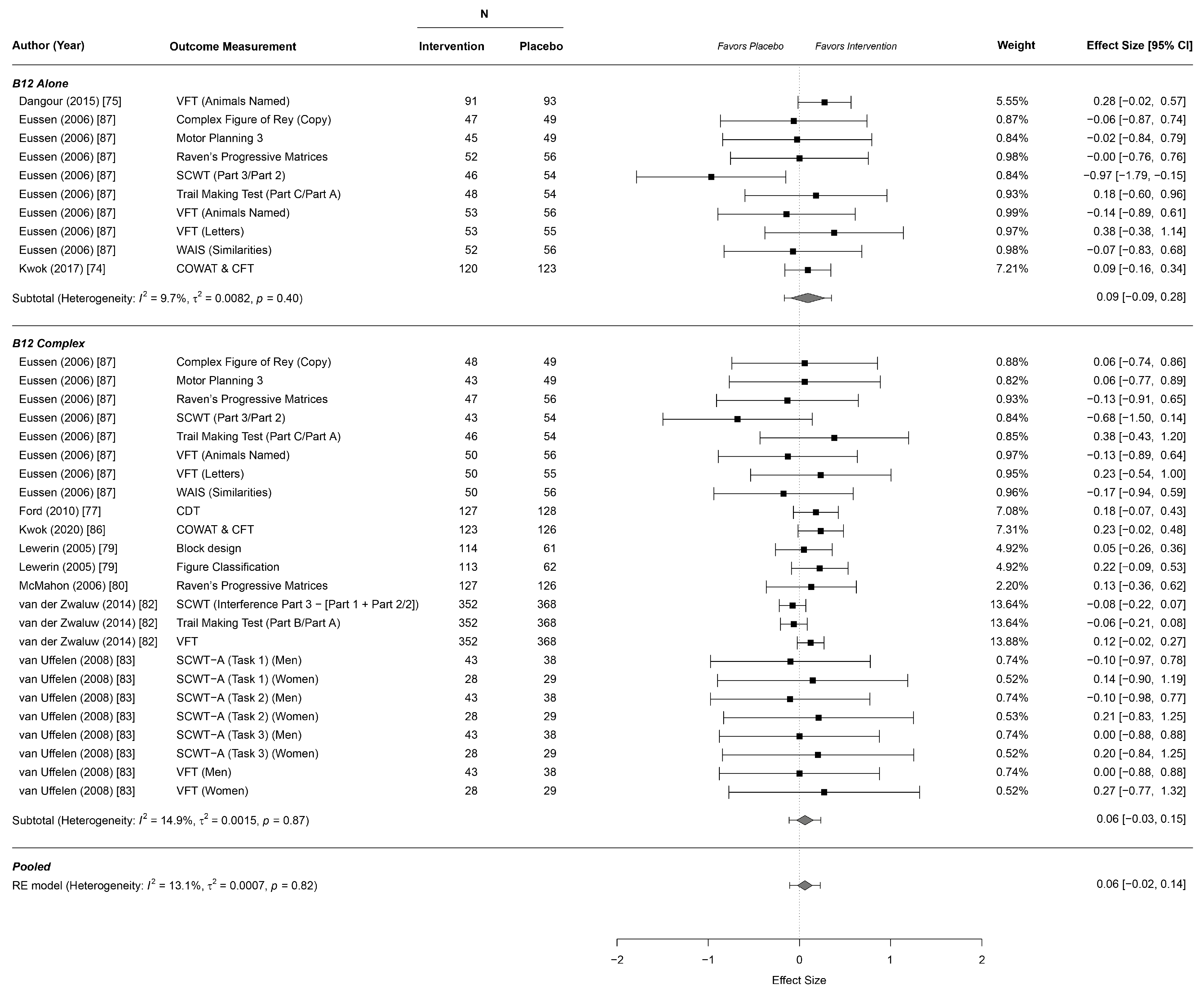
3.4. Effects on Cognitive Function
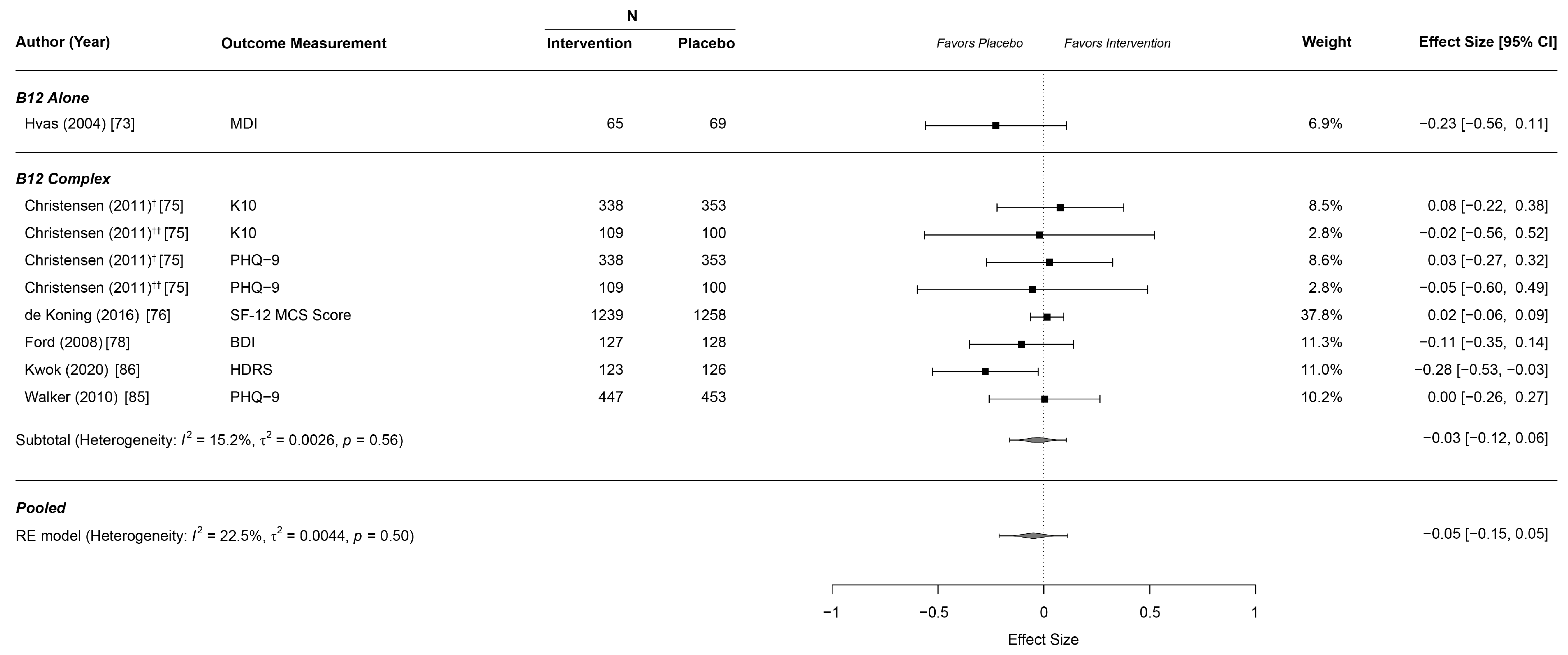
3.5. Effects on Depressive Symptoms
3.6. Effects on Idiopathic Fatigue
3.7. Sensitivity Analyses
4. Discussion
4.1. Main Findings
4.2. Relation to Previously Published Systematic Reviews
4.3. Strengths and Limitations
4.4. Controversies Raised by This Study
4.5. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luck, T.; Riedel-Heller, S.G.; Kaduszkiewicz, H.; Bickel, H.; Jessen, F.; Pentzek, M.; Wiese, B.; Koelsch, H.; Bussche, H.V.D.; Abholz, H.-H.; et al. Mild Cognitive Impairment in General Practice: Age-Specific Prevalence and Correlate Results from the German Study on Ageing, Cognition and Dementia in Primary Care Patients (AgeCoDe). Dement. Geriatr. Cogn. Disord. 2007, 24, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.B.; Lyketsos, C. Mild cognitive impairment: Searching for the prodrome of Alzheimer’s disease. World Psychiatry 2008, 7, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.E.; Studenski, S.A. Qualities of Fatigue and Associated Chronic Conditions Among Older Adults. J. Pain Symptom Manag. 2010, 39, 1033–1042. [Google Scholar] [CrossRef]
- Forlani, C.; Morri, M.; Ferrari, B.; Dalmonte, E.; Menchetti, M.; De Ronchi, D.; Atti, A.R. Prevalence and Gender Differences in Late-Life Depression: A Population-Based Study. Am. J. Geriatr. Psychiatry 2014, 22, 370–380. [Google Scholar] [CrossRef]
- Volkert, J.; Schulz, H.; Härter, M.; Wlodarczyk, O.; Andreas, S. The prevalence of mental disorders in older people in Western countries—A meta-analysis. Ageing Res. Rev. 2013, 12, 339–353. [Google Scholar] [CrossRef]
- Skapinakis, P.; Lewis, G.; Meltzer, H. Clarifying the relationship between unexplained chronic fatigue and psychiatric morbidity: Results from a community survey in Great Britain. Am. J. Psychiatry 2000, 157, 1492–1498. [Google Scholar] [CrossRef]
- Zung, W.W.; Broadhead, W.E.; Roth, M.E. Prevalence of depressive symptoms in primary care. J. Fam. Pr. 1993, 37, 337–344. [Google Scholar]
- Kocalevent, R.-D.; Hinz, A.; Brähler, E. Standardization of the depression screener Patient Health Questionnaire (PHQ-9) in the general population. Gen. Hosp. Psychiatry 2013, 35, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Fukuhara, S.; Green, J. Usefulness of five-item and three-item Mental Health Inventories to screen for depressive symptoms in the general population of Japan. Health Qual. Life Outcomes 2005, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Teasdale, S.B.; Allott, K.; Siskind, D.; Marx, W.; Cotter, J.; Veronese, N.; Schuch, F.; Smith, L.; Solmi, M.; et al. The efficacy and safety of nutrient supplements in the treatment of mental disorders: A meta-review of meta-analyses of randomized controlled trials. World Psychiatry 2019, 18, 308–324. [Google Scholar] [CrossRef]
- Carmel, R. Subclinical cobalamin deficiency. Curr. Opin. Gastroenterol. 2012, 28, 151–158. [Google Scholar] [CrossRef]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of B vitamins on the one-carbon transfer pathways. Chem. Interact. 2006, 163, 113–132. [Google Scholar] [CrossRef]
- Morris, M.S. The Role of B Vitamins in Preventing and Treating Cognitive Impairment and Decline. Adv. Nutr. 2012, 3, 801–812. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjorke-Monsen, A.L.; Brito, A.; Gueant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef]
- Mitchell, E.S.; Conus, N.; Kaput, J. B vitamin polymorphisms and behavior: Evidence of associations with neuro-development, depression, schizophrenia, bipolar disorder and cognitive decline. Neurosci. Biobehav. Rev. 2014, 47, 307–320. [Google Scholar] [CrossRef]
- Bottiglieri, T. S-Adenosyl-l-methionine (SAMe): From the bench to the bedside—Molecular basis of a pleiotrophic molecule. Am. J. Clin. Nutr. 2002, 76, 1151S–1157S. [Google Scholar] [CrossRef]
- Tsiminis, G.; Schartner, E.P.; Brooks, J.L.; Hutchinson, M.R. Measuring and tracking vitamin B12: A review of current methods with a focus on optical spectroscopy. Appl. Spectrosc. Rev. 2016, 52, 439–455. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H. Homocysteine, B Vitamins, and Cognitive Impairment. Annu. Rev. Nutr. 2016, 36, 211–239. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.F.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef]
- Vidal, J.S.; Dufouil, C.; Ducros, V.; Tzourio, C. Homocysteine, folate and cognition in a large community-based sample of elderly people—The 3C Dijon Study. Neuroepidemiology 2008, 30, 207–214. [Google Scholar] [CrossRef]
- McCaddon, A.; Davies, G.; Hudson, P.; Tandy, S.; Cattell, H. Total serum homocysteine in senile dementia of Alzheimer type. Int. J. Geriatr. Psychiatry 1998, 13, 235–239. [Google Scholar] [CrossRef]
- Quadri, P.; Fragiacomo, C.; Pezzati, R.; Zanda, E.; Forloni, G.; Tettamanti, M.; Lucca, U. Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am. J. Clin. Nutr. 2004, 80, 114–122. [Google Scholar]
- Quadri, P.; Fragiacomo, C.; Pezzati, R.; Zanda, E.; Tettamanti, M.; Lucca, U. Homocysteine and B vitamins in mild cognitive impairment and dementia. Clin. Chem. Lab. Med. 2005, 43, 1096–1100. [Google Scholar] [CrossRef]
- Kim, J.M.; Stewart, R.; Kim, S.W.; Yang, S.J.; Shin, I.S.; Yoon, J.S. Predictive value of folate, vitamin B12 and homocysteine levels in late-life depression. Br. J. Psychiatry J. Ment. Sci. 2008, 192, 268–274. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Shroff, M.R.; Beydoun, H.A.; Zonderman, A.B. Serum Folate, Vitamin B-12, and Homocysteine and Their Association with Depressive Symptoms among U.S. Adults. Psychosom. Med. 2010, 72, 862–873. [Google Scholar] [CrossRef]
- Allen, L.H. Causes of Vitamin B12and Folate Deficiency. Food Nutr. Bull. 2008, 29, S20–S34. [Google Scholar] [CrossRef]
- Andres, E.; Loukili, N.H.; Noel, E.; Kaltenbach, G.; Abdelgheni, M.B.; Perrin, A.E.; Noblet-Dick, M.; Maloisel, F.; Schlienger, J.-L. Blickle, J.-F.Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ 2004, 171, 251–259. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Qin, L.L.; Song, Y.; Vidal-Alaball, J.; Liu, T.H. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst. Rev. 2018, 3, CD004655. [Google Scholar] [CrossRef]
- Andres, E.; Kaltenbach, G.; Noel, E.; Noblet-Dick, M.; Perrin, A.E.; Vogel, T.; Schlienger, J.-L.; Berthel, M.; Blickle, J.F. Efficacy of short-term oral cobalamin therapy for the treatment of cobalamin deficiencies related to food-cobalamin malabsorption: A study of 30 patients. Clin. Lab. Haematol. 2003, 25, 161–166. [Google Scholar] [CrossRef]
- Allen, L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009, 89, 693S–696S. [Google Scholar] [CrossRef]
- Bailey, R.L.; Carmel, R.; Green, R.; Pfeiffer, C.M.; Cogswell, M.E.; Osterloh, J.D.; Sempos, C.T.; Yetley, E.A. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am. J. Clin. Nutr. 2011, 94, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, W.K.; Lin, Y.; Dharma, C.; Croxford, R.; Earle, C.C.; Cheung, M.C. Prevalence of Inappropriateness of Parenteral Vitamin B12 Administration in Ontario, Canada. JAMA Intern. Med. 2019, 179, 1434–1436. [Google Scholar] [CrossRef]
- Balk, E.M.; Raman, G.; Tatsioni, A.; Chung, M.; Lau, J.; Rosenberg, I.H. Vitamin B6, B12, and folic acid supplementation and cognitive function: A systematic review of randomized trials. Arch. Intern. Med. 2007, 167, 21–30. [Google Scholar] [CrossRef]
- Behrens, A.; Graessel, E.; Pendergrass, A.; Donath, C. Vitamin B—Can it prevent cognitive decline? A systematic review and meta-analysis. Syst. Rev. 2020, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Bennett, D.; Parish, S.; Lewington, S.; Skeaff, M.; Eussen, S.J.P.M.; Lewerin, C.; Stott, D.J.; Armitage, J.; Hankey, G.J.; et al. Effects of homocysteine lowering with B vitamins on cognitive aging: Meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am. J. Clin. Nutr. 2014, 100, 657–666. [Google Scholar] [CrossRef] [PubMed]
- D’Cunha, N.M.; Georgousopoulou, E.N.; Dadigamuwage, L.; Kellett, J.; Panagiotakos, D.B.; Thomas, J.; McKune, A.J.; Mellor, D.D.; Naumovski, N. Effect of long-term nutraceutical and dietary supplement use on cognition in the elderly: A 10-year systematic review of randomised controlled trials. Br. J. Nutr. 2018, 119, 280–298. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.C.; Holroyd-Leduc, J.M.; Poulin, M.J.; Hogan, D.B. Effect of Nutrients, Dietary Supplements and Vitamins on Cognition: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Can. Geriatr. J. 2015, 18, 231–245. [Google Scholar] [CrossRef]
- Ford, A.H.; Almeida, O.P. Effect of Homocysteine Lowering Treatment on Cognitive Function: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Alzheimer’s Dis. 2012, 29, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.H.; Almeida, O.P. Effect of Vitamin B Supplementation on Cognitive Function in the Elderly: A Systematic Review and Meta-Analysis. Drugs Aging 2019, 36, 419–434. [Google Scholar] [CrossRef]
- Health Quality, O. Vitamin B12 and cognitive function: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2013, 13, 1–45. [Google Scholar]
- Jia, X.; McNeill, G.; Avenell, A. Does taking vitamin, mineral and fatty acid supplements prevent cognitive decline? A systematic review of randomized controlled trials. J. Hum. Nutr. Diet. 2008, 21, 317–336. [Google Scholar] [CrossRef]
- Li, M.M.; Yu, J.T.; Wang, H.F.; Jiang, T.; Wang, J.; Meng, X.F.; Tan, C.-C.; Wang, C.; Tan, L. Efficacy of vitamins B supplementation on mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. Curr. Alzheimer Res. 2014, 11, 844–852. [Google Scholar]
- Malouf, R.; Sastre, A.A. Vitamin B12 for cognition. Cochrane Database Syst. Rev. 2003, 2003, CD004326. [Google Scholar] [CrossRef]
- Malouf, R.; Evans, J.G. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst. Rev. 2008, 2008, CD004514. [Google Scholar] [CrossRef] [PubMed]
- McCleery, J.; Abraham, R.P.; Denton, D.A.; Rutjes, A.W.S.; Chong, L.-Y.; Al-Assaf, A.S.; Griffith, D.J.; Rafeeq, S.; Yaman, H.; Malik, M.A.; et al. Vitamin and mineral supple-mentation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2018, 11, CD011905. [Google Scholar]
- Moore, E.; Mander, A.; Ames, D.; Carne, R.; Sanders, K.; Watters, D. Cognitive impairment and vitamin B12: A review. Int. Psychogeriatr. 2012, 24, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, A.W.; Denton, D.A.; Di Nisio, M.; Chong, L.Y.; Abraham, R.P.; Al-Assaf, A.S.; Anderson, J.L.; Malik, M.A.; Vernooij, R.W.M.; Martínez, G.; et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst. Rev. 2018, 12, CD011906. [Google Scholar] [CrossRef]
- Suh, S.W.; Kim, H.S.; Han, J.H.; Bae, J.B.; Oh, D.J.; Han, J.W.; Kim, K.W. Efficacy of Vitamins on Cognitive Function of Non-Demented People: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 22. [Google Scholar] [CrossRef]
- Wald, D.S.; Kasturiratne, A.; Simmonds, M. Effect of folic acid, with or without other B vitamins, on cognitive de-cline: Meta-analysis of randomized trials. Am. J. Med. 2010, 123, 522–527.e2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.-M.; Ye, J.-X.; Mu, J.-S.; Cui, X.-P. Efficacy of Vitamin B Supplementation on Cognition in Elderly Patients with Cognitive-Related Diseases. J. Geriatr. Psychiatry Neurol. 2016, 30, 50–59. [Google Scholar] [CrossRef]
- Almeida, O.P.; Ford, A.H.; Flicker, L. Systematic review and meta-analysis of randomized placebo-controlled trials of folate and vitamin B12 for depression. Int. Psychogeriatr. 2015, 27, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Rohatgi, A. WebPlotDigitizer; (Version 4.2); A. Rohatgi: San Francisco, CA, USA, 2019. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.0.3; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Lüdecke, D. esc: Effect Size Computation for Meta Analysis; (Version 0.5.1); R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 48. [Google Scholar] [CrossRef]
- Borenstein, M.; Higgins, J.P.T. Meta-Analysis and Subgroups. Prev. Sci. 2013, 14, 134–143. [Google Scholar] [CrossRef]
- Konstantopoulos, S. Fixed effects and variance components estimation in three-level meta-analysis. Res. Synth. Methods 2011, 2, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Carmel, R. Efficacy and safety of fortification and supplementation with vitamin B12: Biochemical and physio-logical effects. Food Nutr. Bull. 2008, 29 (Suppl. S2), S177–S187. [Google Scholar] [CrossRef]
- Allen, L.H. Bioavailability of vitamin B12. Int. Z. Vitam. Ernahr. 2010, 80, 330–335. [Google Scholar] [CrossRef]
- Carmel, R. How I treat cobalamin (vitamin B12) deficiency. Blood 2008, 112, 2214–2221. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; A Welch, V.; Higgins, J.P.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Tipton, E. Small sample adjustments for robust variance estimation with meta-regression. Psychol. Methods 2015, 20, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Fisher, Z.; Tipton, E.; Zhipeng, H. robumeta: Robust Variance Meta-Regression; (Version 2.0); R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Benjamin, D.J.; Berger, J.O.; Johannesson, M.; Nosek, B.A.; Wagenmakers, E.J.; Berk, R.; Bollen, K.A.; Brembs, B.; Brown, L.; Camerer, C.; et al. Redefine statistical significance. Nat. Hum. Behav. 2018, 2, 6–10. [Google Scholar] [CrossRef]
- Oulhaj, A.; Jernerén, F.; Refsum, H.; Smith, A.D.; De Jager, C.A. Omega-3 Fatty Acid Status Enhances the Prevention of Cognitive Decline by B Vitamins in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2016, 50, 547–557. [Google Scholar] [CrossRef]
- De Jager, C.A.; Oulhaj, A.; Jacoby, R.; Refsum, H.; Smith, A.D. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2012, 27, 592–600. [Google Scholar] [CrossRef]
- Ellis, F.R.; Nasser, S. A pilot study of vitamin B12 in the treatment of tiredness. Br. J. Nutr. 1973, 30, 277–283. [Google Scholar] [CrossRef]
- Dangour, A.D.; Allen, E.; Clarke, R.; Elbourne, D.; Fletcher, A.E.; Letley, L.; Richards, M.; Whyte, K.; uauy, R.; Mills, K. Effects of vitamin B-12 supplementation on neurologic and cognitive function in older people: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Hvas, A.-M.; Juul, S.; Lauritzen, L.; Nexø, E.; Ellegaard, J. No effect of vitamin B-12 treatment on cognitive function and depression: A randomized placebo controlled study. J. Affect. Disord. 2004, 81, 269–273. [Google Scholar] [CrossRef]
- Kwok, T.; Lee, J.; Ma, R.C.; Wong, S.Y.; Kung, K.; Lam, A.; Ho, C.; Lee, V.; Harrison, J.; Lam, L. A randomized placebo controlled trial of vitamin B12 supplementation to prevent cognitive decline in older diabetic people with borderline low serum vitamin B12. Clin. Nutr. 2017, 36, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.; Aiken, A.; Batterham, P.J.; Walker, J.; Mackinnon, A.J.; Fenech, M.; Hickie, I.B. No clear potentiation of anti-depressant medication effects by folic acid+vitamin B12 in a large community sample. J. Affect. Disord. 2011, 130, 37–45. [Google Scholar] [CrossRef]
- De Koning, E.J.; van der Zwaluw, N.L.; van Wijngaarden, J.P.; Sohl, E.; Brouwer-Brolsma, E.M.; van Marwijk, H.W.; Enneman, A.W.; Swart, K.M.A.; Van Dijk, S.C.; Ham, A.C.; et al. Effects of Two-Year Vitamin B12 and Folic Acid Supplementation on Depressive Symptoms and Quality of Life in Older Adults with Elevated Homocysteine Concentrations: Additional Results from the B-PROOF Study, an RCT. Nutrients 2016, 8, 748. [Google Scholar] [CrossRef]
- Ford, A.H.; Flicker, L.; Alfonso, H.; Thomas, J.; Clarnette, R.; Martins, R.; Almeida, O.P. Vitamins B12, B6, and folic acid for cognition in older men. Neurology 2010, 75, 1540–1547. [Google Scholar] [CrossRef]
- Ford, A.H.; Flicker, L.; Thomas, J.; Norman, P.; Jamrozik, K.; Almeida, O.P. Vitamins B12, B6, and folic acid for onset of depressive symptoms in older men: Results from a 2-year placebo-controlled randomized trial. J. Clin. Psychiatry 2008, 69, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Lewerin, C.; Matousek, M.; Steen, G.; Johansson, B.; Steen, B.; Nilsson-Ehle, H. Significant correlations of plasma homocysteine and serum methylmalonic acid with movement and cognitive performance in elderly subjects but no improvement from short-term vitamin therapy: A placebo-controlled randomized study. Am. J. Clin. Nutr. 2005, 81, 1155–1162. [Google Scholar] [CrossRef]
- McMahon, J.A.; Green, T.J.; Skeaff, C.M.; Knight, R.G.; Mann, J.I.; Williams, S.M. A controlled trial of homocysteine lowering and cognitive performance. N. Engl. J. Med. 2006, 354, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Schlichtiger, U.; Rettig, K.; Kauermann, S. Effects of a vitamin B6, B12, folic acid combination on quality of life and vitality of elderly people. Geriatrie Forschung 1996, 6, 185–196. [Google Scholar]
- Van der Zwaluw, N.L.; Dhonukshe-Rutten, R.A.; van Wijngaarden, J.P.; Brouwer-Brolsma, E.M.; van de Rest, O.; In ’t Veld, P.H.; Enneman, A.W.; van Dijk, S.C.; Ham, A.C.; Swart, K.M.A.; et al. Results of 2-year vitamin B treatment on cognitive performance: Secondary data from an RCT. Neurology 2014, 83, 2158–2166. [Google Scholar] [CrossRef]
- Van Uffelen, J.G.; Chinapaw, M.J.; van Mechelen, W.; Hopman-Rock, M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br. J. Sports Med. 2008, 42, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.G.; Batterham, P.J.; Mackinnon, A.J.; Jorm, A.F.; Hickie, I.; Fenech, M.; Kljakovic, M.; Crispr, D.; Christensen, H. Oral folic acid and vitamin B-12 supplementation to prevent cognitive decline in community-dwelling older adults with depressive symptoms—The Beyond Ageing Project: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.G.; MacKinnon, A.J.; Batterham, P.; Jorm, A.F.; Hickie, I.; McCarthy, A.; Fenech, M.; Christensen, H. Mental health literacy, folic acid and vitamin B12, and physical activity for the prevention of depression in older adults: Randomised controlled trial. Br. J. Psychiatry 2010, 197, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Kwok, T.; Wu, Y.; Lee, J.; Lee, R.; Yung, C.Y.; Choi, G.; Lee, V.; Harrison, J.; Lam, L.; Mok, V. A randomized placebo-controlled trial of using B vitamins to prevent cognitive decline in older mild cognitive impairment patients. Clin. Nutr. 2020, 39, 2399–2405. [Google Scholar] [CrossRef]
- Eussen, S.J.; de Groot, L.C.; Joosten, L.W.; Bloo, R.J.; Clarke, R.; Ueland, P.M.; Schneede, J.; Blom, H.J.; Hoefnagels, W.H.; van Staveren, W.A. Effect of oral vitamin B-12 with or without folic acid on cognitive function in older people with mild vitamin B-12 deficiency: A randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006, 84, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Kim, S.Y.; Sok, S.R. Effects of Multivitamin Supplements on Cognitive Function, Serum Homocysteine Level, and Depression of Korean Older Adults With Mild Cognitive Impairment in Care Facilities. J. Nurs. Scholarsh. 2016, 48, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wu, T.; Zhao, J.; Song, A.; Liu, H.; Xu, W.; Huang, G. Folic acid supplementation improves cognitive function by reducing the levels of peripheral inflammatory cytokines in elderly Chinese subjects with MCI. Sci. Rep. 2016, 6, 37486. [Google Scholar] [CrossRef]
- Rücker, G.; Schwarzer, G.; Carpenter, J.R.; Schumacher, M. Undue reliance on I 2 in assessing heterogeneity may mislead. BMC Med. Res. Methodol. 2008, 8, 79. [Google Scholar] [CrossRef]
- Linde, K.; Atmann, O.; Meissner, K.; Schneider, A.; Meister, R.; Kriston, L.; Werner, C. How often do general practitioners use placebos and non-specific interventions? Systematic review and meta-analysis of surveys. PLoS ONE 2018, 13, e0202211. [Google Scholar] [CrossRef]
- Meyer, H.E.; Willett, W.C.; Fung, T.T.; Holvik, K.; Feskanich, D. Association of High Intakes of Vitamins B6 and B12 From Food and Supplements With Risk of Hip Fracture Among Postmenopausal Women in the Nurses’ Health Study. JAMA Netw. Open 2019, 2, e193591. [Google Scholar] [CrossRef]
- Ebbing, M.; Bønaa, K.H.; Nygård, O.; Arnesen, E.; Ueland, P.M.; Nordrehaug, J.E.; Rasmussen, K.; Njølstad, I.; Refsum, H.; Nilsen, D.W.; et al. Cancer Incidence and Mortality After Treatment With Folic Acid and Vitamin B12. JAMA 2009, 302, 2119–2126. [Google Scholar] [CrossRef]
- Fanidi, A.; Carreras-Torres, R.; LaRose, T.L.; Yuan, J.; Stevens, V.L.; Weinstein, S.J.; Albanes, D.; Prentice, R.; Ms, M.P.; Cai, Q.; et al. Is high vitamin B12 status a cause of lung cancer? Int. J. Cancer 2019, 145, 1499–1503. [Google Scholar] [CrossRef]
- Flores-Guerrero, J.L.; Minovic, I.; Groothof, D.; Gruppen, E.G.; Riphagen, I.J.; Kootstra-Ros, J.; Muller Kobold, A.; Hak, E.; Navis, G.; Gansevoort, R.T.; et al. Association of Plasma Concentration of Vitamin B12 With All-Cause Mortality in the General Population in the Netherlands. JAMA Netw. Open 2020, 3, e1919274. [Google Scholar] [CrossRef]

| Author, Year | Participants, n | Female, n (%) | Mean Age (SD), Years | Population Characteristics | Vitamin B12 Serum Level (SD), pmol/L (I: Intervention P: Placebo) | Vitamin B12 (DDD) Administration, mcg | Vitamin B9 Administration, mcg | Vitamin B6 Administration, mg | Intake Frequency, Route of Administration | Treatment Duration, Follow-Up Duration, Weeks | Outcome Domain |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B12 alone | |||||||||||
| Dangour, 2015 [72] | 201 | 107 (53.2) | 80.0 (3.7) | No MCI | I: 222.9 (197.4–268.9) a P: 228.0 (194.7–271.0) a | 1000 (1000) | n.a. | n.a. | daily, oral | 52, 52 | Cognitive |
| Eussen, 2006 [87] | 195 | 149 (76.4) | 82.3 (5.0) | No MCI to MCI | I: 186.0 (56.0) P: 188.0 (56.0) | 1000 (1000) | n.a. | n.a. | daily, oral | 24, 24 | Cognitive |
| Hvas, 2004 [73] | 140 | 98 (7.0) | n.a. (n.a.) | No MCI to MCI | I: 278.0 (143.0–1348.0) b P: 254.0 (137.0–724.0) b | 1000 (143) | n.a. | n.a. | weekly, intramuscular | 4, 12 | Cognitive, depression |
| Kwok, 2017 [74] | 271 | 113 (41.7) | 75.3 (4.2) | No MCI to MCI | I: 227.5 (40.0) P: 235.9 (37.9) | 2 × 500 (1000) | n.a. | n.a. | daily, oral | 117.5, 117.5 | Cognitive |
| B complex | |||||||||||
| Christensen, 2011 [75] | 900 | 542 (60.2) | 65.9 (4.4) | No MCI | I: 305.0 (151.0) P: 285.0 (106.0) I2 c: n.r. P2 c: n.r. | 100 (100) | 400 | n.a. | daily, oral | 104, 104 | Depression |
| de Koning, 2016 [76] | 2919 | 1459 (50.0) | 74.1 (n.a.) | No MCI | I: 267.0 (213.0–341.0) a P: 266.0 (204.0–343.0) a | 500 (500) | 400 | n.a. | daily, oral | 104, 104 | Depression |
| Eussen, 2006 [87] | 195 | 100 (51.3) | 82.5 (6.0) | No MCI to MCI | I: 199.0 (50.0) P: 188.0 (56.0) | 1000 (1000) | 400 | n.a. | daily, oral | 24, 24 | Cognitive |
| Ford, 2008 [78] | 299 | 0 (0.0) | 79.0 (2.7) | No MCI | I: n.r. P: n.r. | 400 (400) | 2000 | 25 | daily, oral | 104, 104 | Depression |
| Ford, 2010 [77] | 299 | 0 (0.0) | 79.0 (2.8) | No MCI | I: 256.12 (121.86) P: 253.02 (115.35) | 400 (400) | 2000 | 25 | daily, oral | 104, 104 | Cognitive |
| Kwok, 2020 [86] | 279 | 113 (40.5) | 77.5 (n.a.) | MCI | I: n.r. P: n.r. | 500 (500) | 400 | n.a. | daily, oral | 52, 104 | Cognitive, depression |
| Lewerin, 2005 [79] | 209 | 117 (55.9) | 75.7 (4.7) | No MCI | I: 305.0 (130.0) P: 359.0 (198.0) | 500 (500) | 800 | 3 | daily, oral | 16.92, 16 | Cognitive |
| McMahon, 2006 [80] | 276 | 112 (44.3) | 73.5 (5.8) | No MCI | I: 380.0 (136.0) P: 385.0 (138.0) | 500 (500) | 1000 | 10 | daily, oral | 104, 104 | Cognitive |
| Schlichtiger, 1996 [81] | 213 | 150 (70.4) | 73.3 (5.9) | No MCI | I: n.r. P: n.r. | 1000 (286) | 1100 | 5 | 2/weekly, intramuscular | 4, 8 | Fatigue |
| van der Zwaluw, 2014 [82] | 2919 | 1459 (50.0) | 74.1 (6.5) | No MCI | I1: 267.0 (231.0–341.0) a P1: 266.0 (204.0–343.0) a I2 d: 257.0 (200.0–326.0) a P2 d: 263.0 (200.0–345.0) a | 500 (500) | 400 | n.a. | daily, oral | 104, 104 | Cognitive |
| van Uffelen, 2008 [83] | 179 | 67 (37.4) | 75.17 (n.a.) | MCI | I: n.r. P: n.r. | 400 (500) | 5000 | 50 | daily, oral | 52, 52 | Cognitive |
| Walker, 2010 [85] | 900 | 542 (60.2) | 66.0 (4.3) | No MCI | I: 305.32 (151.05) P: 285.27 (105.77) | 100 (100) | 400 | n.a. | daily, oral | 104, 104 | Depression |
| Walker, 2012 [84] | 900 | 542 (60.2) | 66.0 (4.3) | No MCI | I: 305.32 (151.05) P: 285.27 (105.77) | 100 (100) | 400 | n.a. | daily, oral | 104, 104 | Cognitive |
| Outcome (sub)Domain | Effect Size | 95% CI LB | 95% CI UB | I2 | τ2 | p-Value |
|---|---|---|---|---|---|---|
| Vitamin B12 Alone | ||||||
| Cognitive executive | 0.09 | −0.09 | 0.28 | 9.7% | 0.0082 | 0.40 |
| Cognitive memory | 0.04 | −0.19 | 0.26 | 41.7% | 0.0355 | 0.34 |
| Cognitive global | 0.02 | −0.17 | 0.21 | 6.3% | 0.0018 | 0.56 |
| Cognitive speed | −0.10 | −0.23 | 0.04 | 0% | 0 | 0.84 |
| Depression | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Vitamin B Complex | ||||||
| Cognitive executive | 0.06 | −0.03 | 0.15 | 14.9 | 0.0015 | 0.87 |
| Cognitive memory | 0.03 | −0.01 | 0.07 | 0.0 | 0 | 1.00 |
| Cognitive global | 0.07 | 0.00 | 0.13 | 0.0 | 0 | 0.93 |
| Cognitive speed | −0.08 | −0.25 | 0.10 | 43.4 | 0.0149 | 0.68 |
| Depression | −0.03 | −0.12 | 0.06 | 15.2 | 0.0026 | 0.56 |
| Pooled (Vitamin B12 and Vitamin B Complex) | ||||||
| Cognitive executive | 0.06 | −0.021 | 0.141 | 13.1 | 0.0007 | 0.82 |
| Cognitive memory | 0.028 | −0.011 | 0.067 | 0.0 | 0 | 0.98 |
| Cognitive global | 0.061 | −0.001 | 0.123 | 0.0 | 0 | 0.95 |
| Cognitive speed | −0.081 | −0.175 | 0.013 | 13.9 | 0.0037 | 0.88 |
| Depression | −0.049 | −0.146 | 0.047 | 22.5 | 0.0044 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markun, S.; Gravestock, I.; Jäger, L.; Rosemann, T.; Pichierri, G.; Burgstaller, J.M. Effects of Vitamin B12 Supplementation on Cognitive Function, Depressive Symptoms, and Fatigue: A Systematic Review, Meta-Analysis, and Meta-Regression. Nutrients 2021, 13, 923. https://doi.org/10.3390/nu13030923
Markun S, Gravestock I, Jäger L, Rosemann T, Pichierri G, Burgstaller JM. Effects of Vitamin B12 Supplementation on Cognitive Function, Depressive Symptoms, and Fatigue: A Systematic Review, Meta-Analysis, and Meta-Regression. Nutrients. 2021; 13(3):923. https://doi.org/10.3390/nu13030923
Chicago/Turabian StyleMarkun, Stefan, Isaac Gravestock, Levy Jäger, Thomas Rosemann, Giuseppe Pichierri, and Jakob M. Burgstaller. 2021. "Effects of Vitamin B12 Supplementation on Cognitive Function, Depressive Symptoms, and Fatigue: A Systematic Review, Meta-Analysis, and Meta-Regression" Nutrients 13, no. 3: 923. https://doi.org/10.3390/nu13030923
APA StyleMarkun, S., Gravestock, I., Jäger, L., Rosemann, T., Pichierri, G., & Burgstaller, J. M. (2021). Effects of Vitamin B12 Supplementation on Cognitive Function, Depressive Symptoms, and Fatigue: A Systematic Review, Meta-Analysis, and Meta-Regression. Nutrients, 13(3), 923. https://doi.org/10.3390/nu13030923







