Immunomodulatory Effects of Dietary Polyphenols
Abstract
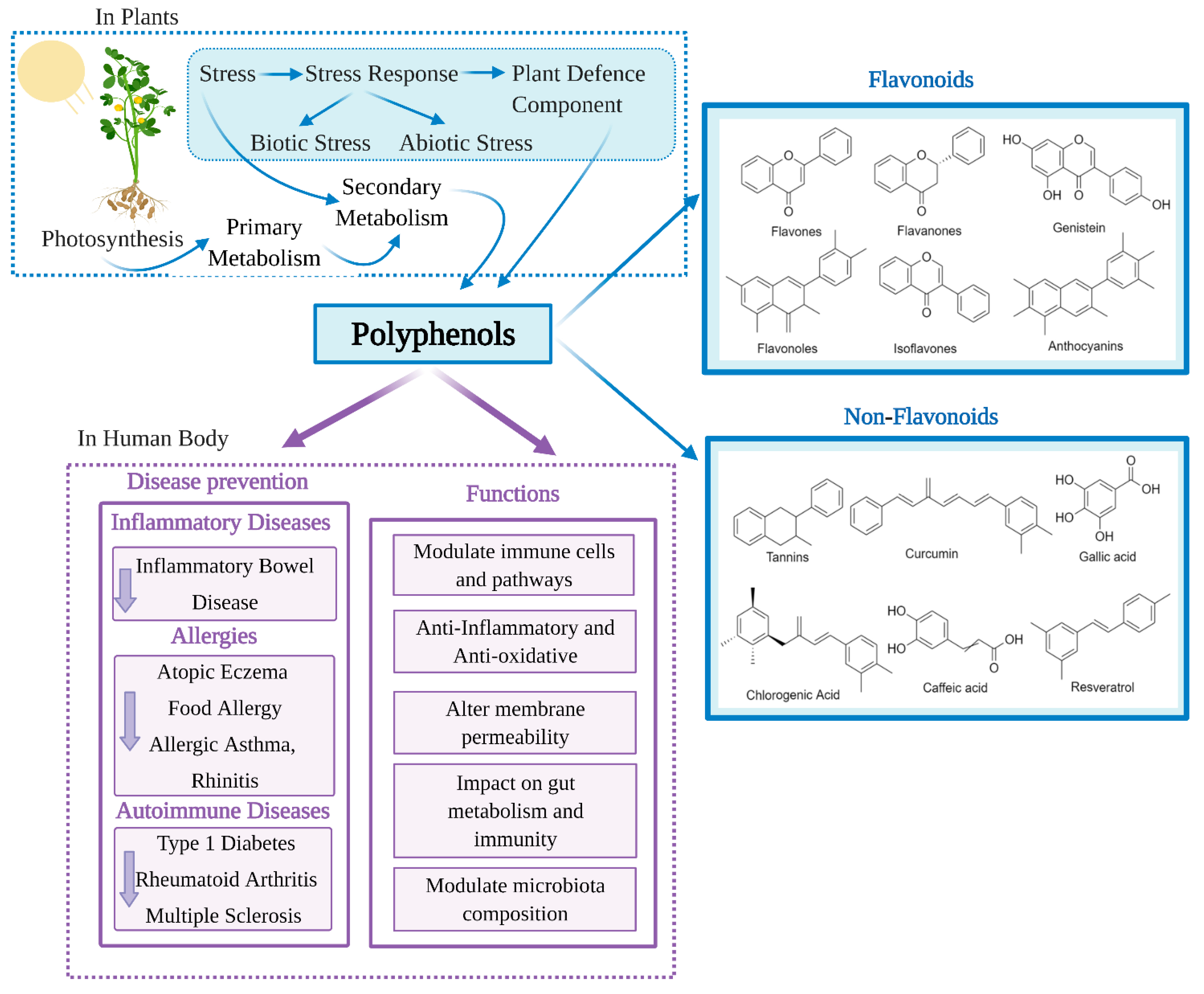
1. Introduction
2. Methods
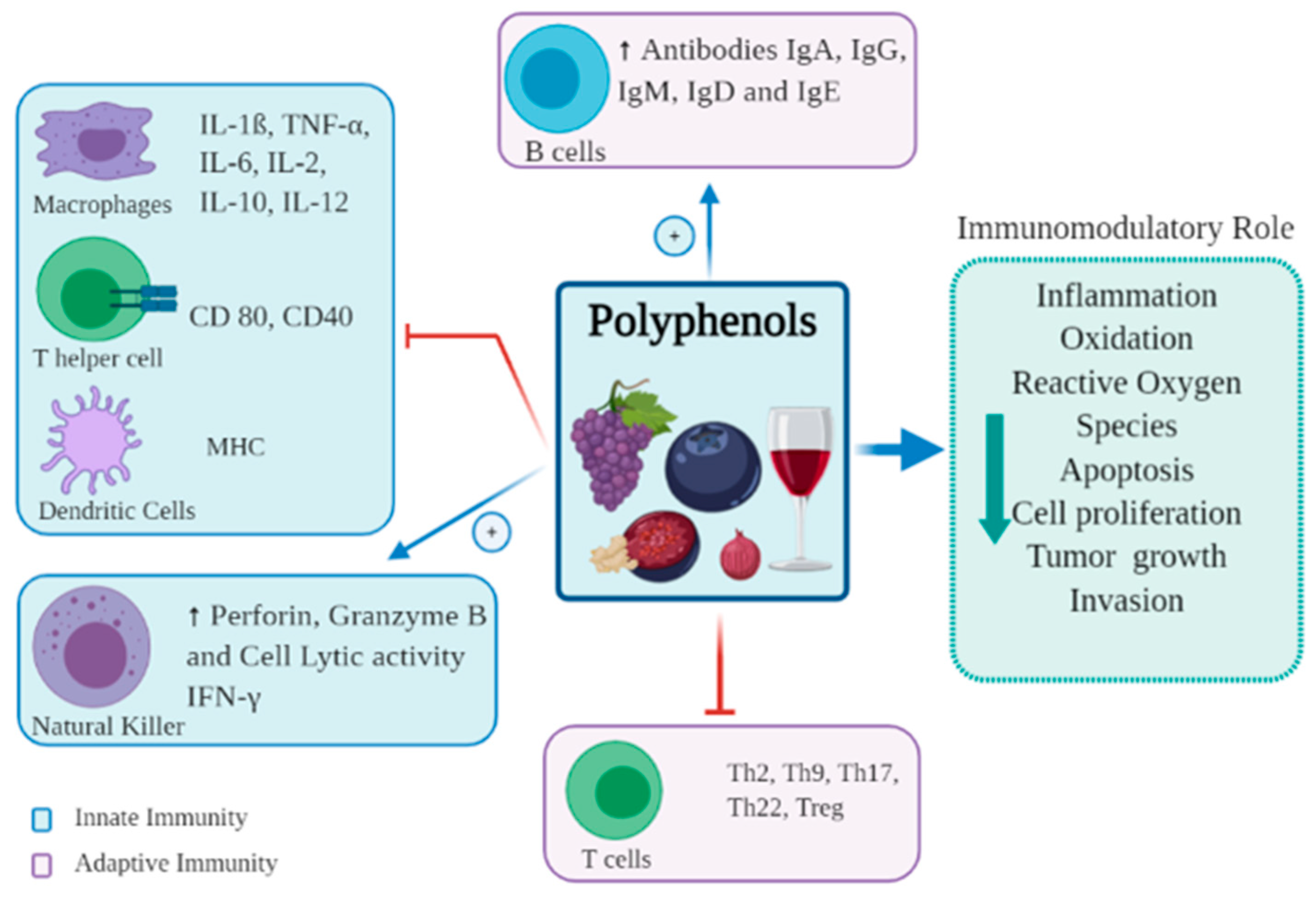
3. Immune Modulation of Polyphenols to Immune Cells
3.1. Effects of Polyphenols on Dendritic Cells
3.2. Effects of Polyphenols on Monocytes and Macrophages
3.3. Effects of Polyphenols on Natural Killer Cells
3.4. Effects of Polyphenols on T and B Cells
3.5. Effects of Polyphenols on T Cell Differentiation
3.6. Effects of Polyphenols in Inflammation
4. Immune Modulation of Polyphenols to Prevent and Control Chronic Diseases
4.1. Polyphenols and Inflammatory Bowel Disease
4.2. Polyphenols and Allergies
4.3. Polyphenols in Atopic Eczema or Dermatitis
4.4. Polyphenols in Allergic Asthma and Rhinitis
5. Immune Modulation of Polyphenols in Autoimmunity
5.1. Polyphenols and Type-1 Diabetes
5.2. Polyphenols and Rheumatoid Arthritis
5.3. Polyphenols and Multiple Sclerosis
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar]
- Chandrasekara, A.; Shahidi, F. Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar]
- Yamagata, K.; Tagami, M.; Yamori, Y. Dietary polyphenols regulate endothelial function and prevent cardiovascular disease. Nutrition 2015, 31, 28–37. [Google Scholar]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar]
- Martin, K.R.; Appel, C.L. Polyphenols as dietary supplements: A double-edged sword. Nutr. Diet. Suppl. 2009, 2, 1–12. [Google Scholar]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar]
- Cassidy, A.; O’Reilly, É.J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.P.; Curhan, G.; Rimm, E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011, 93, 338–347. [Google Scholar]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillén, M.; Lamuela-Raventós, R.M.; Llorach, R.; Andres-Lacueva, C. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013, 32, 200–206. [Google Scholar]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar]
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar]
- Ahtesh, F.B.; Stojanovska, L.; Feehan, J.; de Courten, M.P.; Flavel, M.; Kitchen, B.; Apostolopoulos, V. Polyphenol Rich Sugar Cane Extract Inhibits Bacterial Growth. Prilozi 2020, 41, 49–57. [Google Scholar]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar]
- Wolowczuk, I.; Verwaerde, C.; Viltart, O.; Delanoye, A.; Delacre, M.; Pot, B.; Grangette, C. Feeding our immune system: Impact on metabolism. Clin. Dev. Immunol. 2008, 2008, 639803. [Google Scholar]
- Medzhitov, R.; Janeway, C. Innate immunity. N. Engl. J. Med. 2000, 343, 338–344. [Google Scholar]
- Beutler, B. Innate immunity: An overview. Mol. Immunol. 2004, 40, 845–859. [Google Scholar]
- Clark, R.; Kupper, T. Old meets new: The interaction between innate and adaptive immunity. J. Investig. Dermatol. 2005, 125, 629–637. [Google Scholar]
- Hachimura, S.; Totsuka, M.; Hosono, A. Immunomodulation by food: Impact on gut immunity and immune cell function. Biosci. Biotechnol. Biochem. 2018, 82, 584–599. [Google Scholar]
- Buckwalter, M.R.; Albert, M.L. Orchestration of the immune response by dendritic cells. Curr. Biol. 2009, 19, R355–R361. [Google Scholar]
- del Cornò, M.; Scazzocchio, B.; Masella, R.; Gessani, S. Regulation of dendritic cell function by dietary polyphenols. Crit. Rev. Food Sci. Nutr. 2016, 56, 737–747. [Google Scholar]
- Švajger, U.; Obermajer, N.; Jeras, M. Dendritic cells treated with resveratrol during differentiation from monocytes gain substantial tolerogenic properties upon activation. Immunology 2010, 129, 525–535. [Google Scholar]
- Yoneyama, S.; Kawai, K.; Tsuno, N.H.; Okaji, Y.; Asakage, M.; Tsuchiya, T.; Yamada, J.; Sunami, E.; Osada, T.; Kitayama, J. Epigallocatechin gallate affects human dendritic cell differentiation and maturation. J. Allergy Clin. Immunol. 2008, 121, 209–214. [Google Scholar]
- Lee, J.S.; Kim, S.G.; Kim, H.K.; Lee, T.H.; Jeong, Y.I.; Lee, C.M.; Yoon, M.S.; Na, Y.J.; Suh, D.S.; Park, N.C. Silibinin polarizes Th1/Th2 immune responses through the inhibition of immunostimulatory function of dendritic cells. J. Cell. Physiol. 2007, 210, 385–397. [Google Scholar]
- Huang, R.-Y.; Yu, Y.-L.; Cheng, W.-C.; OuYang, C.-N.; Fu, E.; Chu, C.-L. Immunosuppressive effect of quercetin on dendritic cell activation and function. J. Immunol. 2010, 184, 6815–6821. [Google Scholar]
- Yoon, M.-S.; Lee, J.S.; Choi, B.-M.; Jeong, Y.-I.; Lee, C.-M.; Park, J.-H.; Moon, Y.; Sung, S.-C.; Lee, S.K.; Chang, Y.H. Apigenin inhibits immunostimulatory function of dendritic cells: Implication of immunotherapeutic adjuvant. Mol. Pharmacol. 2006, 70, 1033–1044. [Google Scholar]
- Gupta, S.C.; Tyagi, A.K.; Deshmukh-Taskar, P.; Hinojosa, M.; Prasad, S.; Aggarwal, B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys. 2014, 559, 91–99. [Google Scholar]
- Liu, S.-H.; Lin, C.-H.; Hung, S.-K.; Chou, J.-H.; Chi, C.-W.; Fu, S.-L. Fisetin inhibits lipopolysaccharide-induced macrophage activation and dendritic cell maturation. J. Agric. Food Chem. 2010, 58, 10831–10839. [Google Scholar]
- Buttari, B.; Profumo, E.; Facchiano, F.; Ozturk, E.I.; Segoni, L.; Saso, L.; Riganò, R. Resveratrol prevents dendritic cell maturation in response to advanced glycation end products. Oxidative Med. Cell. Longev. 2013, 2013, 574029. [Google Scholar]
- Bottazzi, B.; Doni, A.; Garlanda, C.; Mantovani, A. An integrated view of humoral innate immunity: Pentraxins as a paradigm. Annu. Rev. Immunol. 2009, 28, 157–183. [Google Scholar]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204. [Google Scholar]
- Kamada, N.; Hisamatsu, T.; Okamoto, S.; Chinen, H.; Kobayashi, T.; Sato, T.; Sakuraba, A.; Kitazume, M.T.; Sugita, A.; Koganei, K. Unique CD14+ intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-γ axis. J. Clin. Investig. 2008, 118, 2269–2280. [Google Scholar]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar]
- Dugo, L.; Belluomo, M.G.; Fanali, C.; Russo, M.; Cacciola, F.; Maccarrone, M.; Sardanelli, A.M. Effect of cocoa polyphenolic extract on macrophage polarization from proinflammatory M1 to anti-inflammatory M2 state. Oxidative Med. Cell. Longev. 2017, 2017, 6293740. [Google Scholar]
- Park, H.-R.; Hwang, D.; Suh, H.-J.; Yu, K.-W.; Kim, T.Y.; Shin, K.-S. Antitumor and antimetastatic activities of rhamnogalacturonan-II-type polysaccharide isolated from mature leaves of green tea via activation of macrophages and natural killer cells. Int. J. Biol. Macromol. 2017, 99, 179–186. [Google Scholar]
- Zhang, M.; Xie, Y.; Su, X.; Liu, K.; Zhang, Y.; Pang, W.; Wang, J. Inonotus sanghuang polyphenols attenuate inflammatory response via modulating the crosstalk between macrophages and adipocytes. Front. Immunol. 2019, 10, 286. [Google Scholar]
- Overman, A.; Bumrungpert, A.; Kennedy, A.; Martinez, K.; Chuang, C.C.; West, T.; Dawson, B.; Jia, W.; McIntosh, M. Polyphenol-rich grape powder extract (GPE) attenuates inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. Int. J. Obes. 2010, 34, 800–808. [Google Scholar]
- McLaren, J.E.; Michael, D.R.; Ashlin, T.G.; Ramji, D.P. Cytokines, macrophage lipid metabolism and foam cells: Implications for cardiovascular disease therapy. Prog. Lipid Res. 2011, 50, 331–347. [Google Scholar]
- Moss, J.W.E.; Ramji, D.P. Nutraceutical therapies for atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 513. [Google Scholar]
- Ramji, D.P.; Davies, T.S. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015, 26, 673–685. [Google Scholar]
- Sevov, M.; Elfineh, L.; Cavelier, L.B. Resveratrol regulates the expression of LXR-α in human macrophages. Biochem. Biophys. Res. Commun. 2006, 348, 1047–1054. [Google Scholar]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar]
- Hu, J.-Y.; Zhang, J.; Cui, J.-L.; Liang, X.-Y.; Lu, R.; Du, G.-F.; Xu, X.-Y.; Zhou, G. Increasing CCL5/CCR5 on CD4+ T cells in peripheral blood of oral lichen planus. Cytokine 2013, 62, 141–145. [Google Scholar]
- Kim, Y.H.; Won, Y.-S.; Yang, X.; Kumazoe, M.; Yamashita, S.; Hara, A.; Takagaki, A.; Goto, K.; Nanjo, F.; Tachibana, H. Green tea catechin metabolites exert immunoregulatory effects on CD4+ T cell and natural killer cell activities. J. Agric. Food Chem. 2016, 64, 3591–3597. [Google Scholar]
- Exon, J.H.; Magnuson, B.A.; South, E.H.; Hendrix, K. Dietary quercetin, immune functions and colonic carcinogenesis in rats. Immunopharmacol. Immunotoxicol. 1998, 20, 173–190. [Google Scholar]
- Bub, A.; Watzl, B.; Blockhaus, M.; Briviba, K.; Liegibel, U.; Müller, H.; Pool-Zobel, B.L.; Rechkemmer, G. Fruit juice consumption modulates antioxidative status, immune status and DNA damage. J. Nutr. Biochem. 2003, 14, 90–98. [Google Scholar]
- McAnulty, L.S.; Nieman, D.C.; Dumke, C.L.; Shooter, L.A.; Henson, D.A.; Utter, A.C.; Milne, G.; McAnulty, S.R. Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running. Appl. Physiol. Nutr. Metab. 2011, 36, 976–984. [Google Scholar]
- McAnulty, L.S.; Collier, S.R.; Landram, M.J.; Whittaker, D.S.; Isaacs, S.E.; Klemka, J.M.; Cheek, S.L.; Arms, J.C.; McAnulty, S.R. Six weeks daily ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutr. Res. 2014, 34, 577–584. [Google Scholar]
- Chen, C.-M.; Li, S.-C.; Lin, Y.-L.; Hsu, C.-Y.; Shieh, M.-J.; Liu, J.-F. Consumption of purple sweet potato leaves modulates human immune response: T-lymphocyte functions, lytic activity of natural killer cell and antibody production. World J. Gastroenterol. WJG 2005, 11, 5777. [Google Scholar]
- Hassanain, E.; Silverberg, J.I.; Norowitz, K.B.; Chice, S.; Bluth, M.H.; Brody, N.; Joks, R.; Durkin, H.G.; Smith-Norowitz, T.A. Green tea (Camelia sinensis) suppresses B cell production of IgE without inducing apoptosis. Ann. Clin. Lab. Sci. 2010, 40, 135–143. [Google Scholar]
- Sanbongi, C.; Suzuki, N.; Sakane, T. Polyphenols in chocolate, which have antioxidant activity, modulate immune functions in humansin vitro. Cell. Immunol. 1997, 177, 129–136. [Google Scholar]
- Kuo, C.-L.; Chen, T.-S.; Liou, S.-Y.; Hsieh, C.-C. Immunomodulatory effects of EGCG fraction of green tea extract in innate and adaptive immunity via T regulatory cells in murine model. Immunopharmacol. Immunotoxicol. 2014, 36, 364–370. [Google Scholar]
- Wong, C.P.; Nguyen, L.P.; Noh, S.K.; Bray, T.M.; Bruno, R.S.; Ho, E. Induction of regulatory T cells by green tea polyphenol EGCG. Immunol. Lett. 2011, 139, 7–13. [Google Scholar]
- Gorenec, L.; Lepej, S.Z.; Grgic, I.; Planinic, A.; Bes, J.I.; Vince, A.; Begovac, J. The comparison of Th1, Th2, Th9, Th17 and Th22 cytokine profiles in acute and chronic HIV-1 infection. Microb. Pathog. 2016, 97, 125–130. [Google Scholar]
- Louten, J.; Boniface, K.; de Waal Malefyt, R. Development and function of TH17 cells in health and disease. J. Allergy Clin. Immunol. 2009, 123, 1004–1011. [Google Scholar]
- Cavani, A.; Pennino, D.; Eyerich, K. Th17 and Th22 in Skin Allergy. In New Trends in Allergy and Atopic Eczema; Karger Publishers: Basel, Switzerland, 2012; Volume 96, pp. 39–44. [Google Scholar]
- Zheng, Y.; Danilenko, D.M.; Valdez, P.; Kasman, I.; Eastham-Anderson, J.; Wu, J.; Ouyang, W. Interleukin-22, a TH 17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007, 445, 648–651. [Google Scholar]
- Yano, S.; Umeda, D.; Yamashita, T.; Ninomiya, Y.; Sumida, M.; Fujimura, Y.; Yamada, K.; Tachibana, H. Dietary flavones suppresses IgE and Th2 cytokines in OVA-immunized BALB/c mice. Eur. J. Nutr. 2007, 46, 257–263. [Google Scholar]
- Wang, J.; Pae, M.; Meydani, S.N.; Wu, D. Green tea epigallocatechin-3-gallate modulates differentiation of naïve CD4+ T cells into specific lineage effector cells. J. Mol. Med. 2013, 91, 485–495. [Google Scholar]
- Xuzhu, G.; Komai-Koma, M.; Leung, B.P.; Howe, H.S.; McSharry, C.; McInnes, I.B.; Xu, D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann. Rheum. Dis. 2012, 71, 129–135. [Google Scholar]
- Ahmad, S.F.; Zoheir, K.M.A.; Abdel-Hamied, H.E.; Ashour, A.E.; Bakheet, S.A.; Attia, S.M.; Abd-Allah, A.R.A. Grape seed proanthocyanidin extract has potent anti-arthritic effects on collagen-induced arthritis by modifying the T cell balance. Int. Immunopharmacol. 2013, 17, 79–87. [Google Scholar]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264. 7 macrophages via the suppression of MAPKs activation. J. Funct. Foods 2018, 43, 62–69. [Google Scholar]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W.; Yang, M.; Hou, C. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264. 7 macrophages via the suppression of TLR4/NF-κB pathway activation. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef]
- Nallathambi, R.; Poulev, A.; Zuk, J.B.; Raskin, I. Proanthocyanidin-Rich Grape Seed Extract Reduces Inflammation and Oxidative Stress and Restores Tight Junction Barrier Function in Caco-2 Colon Cells. Nutrients 2020, 12, 1623. [Google Scholar]
- Wang, Y.; Wang, Y.; Shen, W.; Wang, Y.; Cao, Y.; Nuerbulati, N.; Chen, W.; Lu, G.; Xiao, W.; Qi, R. Grape Seed Polyphenols Ameliorated Dextran Sulfate Sodium-Induced Colitis via Suppression of Inflammation and Apoptosis. Pharmacology 2020, 105, 9–18. [Google Scholar]
- Li, Y.; Rahman, S.U.; Huang, Y.; Zhang, Y.; Ming, P.; Zhu, L.; Chu, X.; Li, J.; Feng, S.; Wang, X. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. J. Nutr. Biochem. 2020, 78, 108324. [Google Scholar]
- Abdollahi, E.; Momtazi, A.A.; Johnston, T.P.; Sahebkar, A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: A nature-made jack-of-all-trades? J. Cell. Physiol. 2018, 233, 830–848. [Google Scholar]
- Zheng, M.; Zhang, Q.; Joe, Y.; Lee, B.H.; Kwon, K.B.; Ryter, S.W.; Chung, H.T. Curcumin induces apoptotic cell death of activated human CD4+ T cells via increasing endoplasmic reticulum stress and mitochondrial dysfunction. Int. Immunopharmacol. 2013, 15, 517–523. [Google Scholar]
- Busch, F.; Mobasheri, A.; Shayan, P.; Lueders, C.; Stahlmann, R.; Shakibaei, M. Resveratrol modulates interleukin-1β-induced phosphatidylinositol 3-kinase and nuclear factor κB signaling pathways in human tenocytes. J. Biol. Chem. 2012, 287, 38050–38063. [Google Scholar]
- Venkatachalam, K.; Mummidi, S.; Cortez, D.M.; Prabhu, S.D.; Valente, A.J.; Chandrasekar, B. Resveratrol inhibits high glucose-induced PI3K/Akt/ERK-dependent interleukin-17 expression in primary mouse cardiac fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2078–H2087. [Google Scholar]
- Wu, D.; Wang, J.; Pae, M.; Meydani, S.N. Green tea EGCG, T cells, and T cell-mediated autoimmune diseases. Mol. Asp. Med. 2012, 33, 107–118. [Google Scholar]
- Bak, M.-J.; Truong, V.L.; Kang, H.-S.; Jun, M.; Jeong, W.-S. Anti-inflammatory effect of procyanidins from wild grape (Vitis amurensis) seeds in LPS-induced RAW 264.7 cells. Oxidative Med. Cell. Longev. 2013, 2013, 409321. [Google Scholar]
- Chu, H.; Tang, Q.; Huang, H.; Hao, W.; Wei, X. Grape-seed proanthocyanidins inhibit the lipopolysaccharide-induced inflammatory mediator expression in RAW264. 7 macrophages by suppressing MAPK and NF-κb signal pathways. Environ. Toxicol. Pharmacol. 2016, 41, 159–166. [Google Scholar]
- Armutcu, F.; Akyol, S.; Ustunsoy, S.; Turan, F.F. Therapeutic potential of caffeic acid phenethyl ester and its anti-inflammatory and immunomodulatory effects. Exp. Ther. Med. 2015, 9, 1582–1588. [Google Scholar]
- Juman, S.; Yasui, N.; Ikeda, K.; Ueda, A.; Sakanaka, M.; Negishi, H.; Miki, T. Caffeic acid phenethyl ester suppresses the production of pro-inflammatory cytokines in hypertrophic adipocytes through lipopolysaccharide-stimulated macrophages. Biol. Pharm. Bull. 2012, 35, 1941–1946. [Google Scholar]
- Zhang, M.; Zhou, J.; Wang, L.; Li, B.; Guo, J.; Guan, X.; Han, Q.; Zhang, H. Caffeic acid reduces cutaneous tumor necrosis factor alpha (TNF-α), IL-6 and IL-1β levels and ameliorates skin edema in acute and chronic model of cutaneous inflammation in mice. Biol. Pharm. Bull. 2014, 37, 347–354. [Google Scholar]
- Shimizu, M. Multifunctions of dietary polyphenols in the regulation of intestinal inflammation. J. Food Drug Anal. 2017, 25, 93–99. [Google Scholar]
- Singh, D.; Srivastava, S.; Pradhan, M.; Kanwar, J.R.; Singh, M.R. Inflammatory bowel disease: Pathogenesis, causative factors, issues, drug treatment strategies, and delivery approaches. Crit. Rev. Ther. Drug Carr. Syst. 2015, 32, 181–214. [Google Scholar]
- Center for Disease Control and Prevention. Inflammatory Bowel Disease. 2012. Available online: https://www.cdc.gov/ibd/data-statistics.htm (accessed on 10 December 2020).
- Fukata, M.; Arditi, M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013, 6, 451–463. [Google Scholar]
- Huang, S.; Zhao, L.; Kim, K.; Lee, D.S.; Hwang, D.H. Inhibition of Nod2 signaling and target gene expression by curcumin. Mol. Pharmacol. 2008, 74, 274–281. [Google Scholar]
- Shibata, T.; Nakashima, F.; Honda, K.; Lu, Y.-J.; Kondo, T.; Ushida, Y.; Aizawa, K.; Suganuma, H.; Oe, S.; Tanaka, H. Toll-like receptors as a target of food-derived anti-inflammatory compounds. J. Biol. Chem. 2014, 289, 32757–32772. [Google Scholar]
- Youn, H.S.; Lee, J.Y.; Fitzgerald, K.A.; Young, H.A.; Akira, S.; Hwang, D.H. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: Molecular targets are TBK1 and RIP1 in TRIF complex. J. Immunol. 2005, 175, 3339–3346. [Google Scholar]
- Nunes, S.; Danesi, F.; Del Rio, D.; Silva, P. Resveratrol and inflammatory bowel disease: The evidence so far. Nutr. Res. Rev. 2018, 31, 85–97. [Google Scholar]
- Kostyuk, V.A.; Potapovich, A.I.; Suhan, T.O.; de Luca, C.; Korkina, L.G. Antioxidant and signal modulation properties of plant polyphenols in controlling vascular inflammation. Eur. J. Pharmacol. 2011, 658, 248–256. [Google Scholar]
- Mackenzie, G.G.; Delfino, J.M.; Keen, C.L.; Fraga, C.G.; Oteiza, P.I. Dimeric procyanidins are inhibitors of NF-κB–DNA binding. Biochem. Pharmacol. 2009, 78, 1252–1262. [Google Scholar]
- Wang, H.-K.; Yeh, C.-H.; Iwamoto, T.; Satsu, H.; Shimizu, M.; Totsuka, M. Dietary flavonoid naringenin induces regulatory T cells via an aryl hydrocarbon receptor mediated pathway. J. Agric. Food Chem. 2012, 60, 2171–2178. [Google Scholar]
- Josefowicz, S.Z.; Lu, L.-F.; Rudensky, A.Y. Regulatory T cells: Mechanisms of differentiation and function. Annu. Rev. Immunol. 2012, 30, 531–564. [Google Scholar]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar]
- Magrone, T.; Jirillo, E. The interplay between the gut immune system and microbiota in health and disease: Nutraceutical intervention for restoring intestinal homeostasis. Curr. Pharm. Des. 2013, 19, 1329–1342. [Google Scholar]
- Rahman, S.U.; Li, Y.; Huang, Y.; Zhu, L.; Feng, S.; Wu, J.; Wang, X. Treatment of inflammatory bowel disease via green tea polyphenols: Possible application and protective approaches. Inflammopharmacology 2018, 26, 319–330. [Google Scholar]
- Barbalho, S.M.; Bosso, H.; Salzedas-Pescinini, L.M.; de Alvares Goulart, R. Green tea: A possibility in the therapeutic approach of inflammatory bowel diseases?: Green tea and inflammatory bowel diseases. Complementary Ther. Med. 2019, 43, 148–153. [Google Scholar]
- Hollebeeck, S.; Larondelle, Y.; Schneider, Y.-J.; During, A. The use of pomegranate (Punica granatum l.) phenolic compounds as potential natural prevention against IBDs. Inflamm. Bowel Dis. Adv. Pathog. Manag. Intech-Publ. Belg. 2012, 275–300. [Google Scholar] [CrossRef]
- Kim, H.; Venancio, V.P.; Fang, C.; Dupont, A.W.; Talcott, S.T.; Mertens-Talcott, S.U. Mango (Mangifera indica L.) polyphenols reduce IL-8, GRO, and GM-SCF plasma levels and increase Lactobacillus species in a pilot study in patients with inflammatory bowel disease. Nutr. Res. 2020, 75, 85–94. [Google Scholar]
- Scarano, A.; Butelli, E.; De Santis, S.; Cavalcanti, E.; Hill, L.; De Angelis, M.; Giovinazzo, G.; Chieppa, M.; Martin, C.; Santino, A. Combined dietary anthocyanins, flavonols, and stilbenoids alleviate inflammatory bowel disease symptoms in mice. Front. Nutr. 2018, 4, 75. [Google Scholar]
- Devereux, G. The increase in the prevalence of asthma and allergy: Food for thought. Nat. Rev. Immunol. 2006, 6, 869–874. [Google Scholar]
- Tai, A.; Volkmer, R.; Burton, A. Prevalence of asthma symptoms and atopic disorders in preschool children and the trend over a decade. J. Asthma 2009, 46, 343–346. [Google Scholar]
- Singh, A.; Holvoet, S.; Mercenier, A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clin. Exp. Allergy 2011, 41, 1346–1359. [Google Scholar]
- Gong, J.; Chen, S.-S. Polyphenolic antioxidants inhibit peptide presentation by antigen-presenting cells. Int. Immunopharmacol. 2003, 3, 1841–1852. [Google Scholar]
- Kim, J.-Y.; Kina, T.; Iwanaga, Y.; Noguchi, H.; Matsumura, K.; Hyon, S.-H. Tea polyphenol inhibits allostimulation in mixed lymphocyte culture. Cell Transplant. 2007, 16, 75–83. [Google Scholar]
- Iwamura, C.; Shinoda, K.; Yoshimura, M.; Watanabe, Y.; Obata, A.; Nakayama, T. Naringenin chalcone suppresses allergic asthma by inhibiting the type-2 function of CD4 T cells. Allergol. Int. 2010, 59, 67–73. [Google Scholar]
- Zuercher, A.W.; Holvoet, S.; Weiss, M.; Mercenier, A. Polyphenol-enriched apple extract attenuates food allergy in mice. Clin. Exp. Allergy 2010, 40, 942–950. [Google Scholar]
- Aires, V.; Adote, S.; Hirchami, A.; Moutairou, K.; Boustani, E.-S.E.; Khan, N.A. Modulation of intracellular calcium concentrations and T cell activation by prickly pear polyphenols. Mol. Cell. Biochem. 2004, 260, 103–110. [Google Scholar]
- Schoene, N.W.; Kelly, M.A.; Polansky, M.M.; Anderson, R.A. A polyphenol mixture from cinnamon targets p38 MAP kinase-regulated signaling pathways to produce G2/M arrest. J. Nutr. Biochem. 2009, 20, 614–620. [Google Scholar]
- Kawai, K.; Tsuno, N.H.; Kitayama, J.; Sunami, E.; Takahashi, K.; Nagawa, H. Catechin inhibits adhesion and migration of peripheral blood B cells by blocking CD11b. Immunopharmacol. Immunotoxicol. 2011, 33, 391–397. [Google Scholar]
- Yano, S.; Umeda, D.; Maeda, N.; Fujimura, Y.; Yamada, K.; Tachibana, H. Dietary apigenin suppresses IgE and inflammatory cytokines production in C57BL/6N mice. J. Agric. Food Chem. 2006, 54, 5203–5207. [Google Scholar]
- Chung, S.-Y.; Champagne, E.T. Reducing the allergenic capacity of peanut extracts and liquid peanut butter by phenolic compounds. Food Chem. 2009, 115, 1345–1349. [Google Scholar]
- Wang, C.-C.; Lin, Y.-R.; Liao, M.-H.; Jan, T.-R. Oral supplementation with areca-derived polyphenols attenuates food allergic responses in ovalbumin-sensitized mice. Bmc Complementary Altern. Med. 2013, 13, 154. [Google Scholar]
- Bansode, R.R.; Randolph, P.D.; Plundrich, N.J.; Lila, M.A.; Williams, L.L. Peanut protein-polyphenol aggregate complexation suppresses allergic sensitization to peanut by reducing peanut-specific IgE in C3H/HeJ mice. Food Chem. 2019, 299, 125025. [Google Scholar]
- Peérot, M.; Lupi, R.; Guyot, S.; Delayre-Orthez, C.; Gadonna-Widehem, P.; Theébaudin, J.-Y.; Bodinier, M.; Larreé, C. Polyphenol interactions mitigate the immunogenicity and allergenicity of gliadins. J. Agric. Food Chem. 2017, 65, 6442–6451. [Google Scholar]
- Anderson, K.C.; Teuber, S.S. Ellagic acid and polyphenolics present in walnut kernels inhibit in vitro human peripheral blood mononuclear cell proliferation and alter cytokine production. Ann. N. Y. Acad. Sci. 2010, 1190, 86–96. [Google Scholar]
- Labuckas, D.O.; Maestri, D.M.; Perello, M.; Martínez, M.L.; Lamarque, A.L. Phenolics from walnut (Juglans regia L.) kernels: Antioxidant activity and interactions with proteins. Food Chem. 2008, 107, 607–612. [Google Scholar]
- Graff, J.C.; Jutila, M.A. Differential regulation of CD11b on γδ T cells and monocytes in response to unripe apple polyphenols. J. Leukoc. Biol. 2007, 82, 603–607. [Google Scholar]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar]
- Tominaga, T.; Kawaguchi, K.; Kanesaka, M.; Kawauchi, H.; Jirillo, E.; Kumazawa, Y. Suppression of type-I allergic responses by oral administration of grape marc fermented with Lactobacillus plantarum. Immunopharmacol. Immunotoxicol. 2010, 32, 593–599. [Google Scholar]
- Katsarou, A.; Davoy, E.; Xenos, K.; Armenaka, M.; Theoharides, T.C. Effect of an antioxidant (quercetin) on sodium-lauryl-sulfate-induced skin irritation. Contact Dermat. 2000, 42, 85–89. [Google Scholar]
- Neukam, K.; Stahl, W.; Tronnier, H.; Sies, H.; Heinrich, U. Consumption of flavanol-rich cocoa acutely increases microcirculation in human skin. Eur. J. Nutr. 2007, 46, 53–56. [Google Scholar]
- Mehrbani, M.; Choopani, R.; Fekri, A.; Mehrabani, M.; Mosaddegh, M.; Mehrabani, M. The efficacy of whey associated with dodder seed extract on moderate-to-severe atopic dermatitis in adults: A randomized, double-blind, placebo-controlled clinical trial. J. Ethnopharmacol. 2015, 172, 325–332. [Google Scholar]
- Kojima, T.; Akiyama, H.; Sasai, M.; Taniuchi, S.; Goda, Y.; Toyoda, M.; Kobayashi, Y. Anti-allergic effect of apple polyphenol on patients with atopic dermatitis: A pilot study. Allergol. Int. 2000, 49, 69–73. [Google Scholar]
- Singh, A.; Demont, A.; Actis-Goretta, L.; Holvoet, S.; Lévêques, A.; Lepage, M.; Nutten, S.; Mercenier, A. Identification of epicatechin as one of the key bioactive constituents of polyphenol-enriched extracts that demonstrate an anti-allergic effect in a murine model of food allergy. Br. J. Nutr. 2014, 112, 358–368. [Google Scholar]
- Abril-Gil, M.; Massot-Cladera, M.; Pérez-Cano, F.J.; Castellote, C.; Franch, À.; Castell, M. A diet enriched with cocoa prevents IgE synthesis in a rat allergy model. Pharmacol. Res. 2012, 65, 603–608. [Google Scholar]
- Enomoto, T.; Nagasako-Akazome, Y.; Kanda, T.; Ikeda, M.; Dake, Y. Clinical effects of apple polyphenols on persistent allergic rhinitis: A randomized double-blind placebo-controlled parallel arm study. J. Investig. Allergol. Clin. Immunol. 2006, 16, 283. [Google Scholar]
- Belcaro, G.; Luzzi, R.; Cesinaro, P.D.R.; Cesarone, M.R.; Dugall, M.; Feragalli, B.; Errichi, B.M.; Ippolito, E.; Grossi, M.G.; Hosoi, M. Pycnogenol® improvements in asthma management. Panminerva Med. 2011, 53, 57–64. [Google Scholar]
- Kishi, K.; Saito, M.; Saito, T.; Kumemura, M.; Okamatsu, H.; Okita, M.; Takazawa, K. Clinical efficacy of apple polyphenol for treating cedar pollinosis. Biosci. Biotechnol. Biochem. 2005, 69, 829–832. [Google Scholar]
- Spergel, J.M. Epidemiology of atopic dermatitis and atopic march in children. Immunol. Allergy Clin. 2010, 30, 269–280. [Google Scholar]
- Katiyar, S.K.; Elmets, C.A.; Agarwal, R.; Mukhtar, H. Protection against ultraviolet-B radiation-induced local and systemic suppression of contact hypersensitivity and edema responses in C3H/HeN mice by green tea polyphenols. Photochem. Photobiol. 1995, 62, 855–861. [Google Scholar]
- Ichikawa, D.; Matsui, A.; Imai, M.; Sonoda, Y.; Kasahara, T. Effect of various catechins on the IL-12p40 production by murine peritoneal macrophages and a macrophage cell line, J774. 1. Biol. Pharm. Bull. 2004, 27, 1353–1358. [Google Scholar]
- Meydani, M. Potential health benefits of avenanthramides of oats. Nutr. Rev. 2009, 67, 731–735. [Google Scholar]
- Guo, W.; Wise, M.L.; Collins, F.W.; Meydani, M. Avenanthramides, polyphenols from oats, inhibit IL-1β-induced NF-κB activation in endothelial cells. Free Radic. Biol. Med. 2008, 44, 415–429. [Google Scholar]
- Papaliodis, D.; Boucher, W.; Kempuraj, D.; Theoharides, T.C. The flavonoid luteolin inhibits niacin-induced flush. Br. J. Pharmacol. 2008, 153, 1382–1387. [Google Scholar]
- Djukanovic, R.; Roche, W.; Wilson, J.; Beasley, C.; Twentyman, O.; Howarth, P.; Holgate, S. Mucosal Inflammation In Asthma1. 2. Am. Rev. Respir. Dis. 1990, 142, 434–457. [Google Scholar]
- Bisset, L.R.; Schmid-Grendelmeier, P. Chemokines and their receptors in the pathogenesis of allergic asthma: Progress and perspective. Curr. Opin. Pulm. Med. 2005, 11, 35–42. [Google Scholar]
- Lee, M.; Kim, S.; Kwon, O.-K.; Oh, S.-R.; Lee, H.-K.; Ahn, K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int. Immunopharmacol. 2009, 9, 418–424. [Google Scholar]
- Park, H.-j.; Lee, C.-M.; Jung, I.D.; Lee, J.S.; Jeong, Y.-i.; Chang, J.H.; Chun, S.-H.; Kim, M.-J.; Choi, I.-W.; Ahn, S.-C. Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int. Immunopharmacol. 2009, 9, 261–267. [Google Scholar]
- Lim, J.-Y.; Lee, J.-H.; Lee, B.-R.; Kim, M.; Lee, Y.-M.; Kim, D.-K.; Choi, J.K. Extract of Boehmeria nivea Suppresses Mast Cell-Mediated Allergic Inflammation by Inhibiting Mitogen-Activated Protein Kinase and Nuclear Factor-κB. Molecules 2020, 25, 4178. [Google Scholar]
- Lin, C.-H.; Shen, M.-L.; Zhou, N.; Lee, C.-C.; Kao, S.-T.; Wu, D.C. Protective Effects of the Polyphenol Sesamin on Allergen-Induced TH 2 Responses and Airway Inflammation in Mice. PLoS ONE 2014, 9, e96091. [Google Scholar]
- Khan, H.; Sureda, A.; Belwal, T.; Çetinkaya, S.; Süntar, İ.; Tejada, S.; Devkota, H.P.; Ullah, H.; Aschner, M. Polyphenols in the treatment of autoimmune diseases. Autoimmun. Rev. 2019, 18, 647–657. [Google Scholar]
- Sudres, M.; Verdier, J.; Truffault, F.; Le Panse, R.; Berrih-Aknin, S. Pathophysiological mechanisms of autoimmunity. Ann. N. Y. Acad. Sci. 2018, 1413, 59–68. [Google Scholar]
- Thomas, N.J.; Jones, S.E.; Weedon, M.N.; Shields, B.M.; Oram, R.A.; Hattersley, A.T. Frequency and phenotype of type 1 diabetes in the first six decades of life: A cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018, 6, 122–129. [Google Scholar]
- Apaya, M.K.; Kuo, T.-F.; Yang, M.-T.; Yang, G.; Hsiao, C.-L.; Chang, S.-B.; Lin, Y.; Yang, W.-C. Phytochemicals as modulators of β-cells and immunity for the therapy of type 1 diabetes: Recent discoveries in pharmacological mechanisms and clinical potential. Pharmacol. Res. 2020, 156, 104754. [Google Scholar]
- Stojanović, I.; Šavikin, K.; Đedović, N.; Živković, J.; Saksida, T.; Momčilović, M.; Koprivica, I.; Vujičić, M.; Stanisavljević, S.; Miljković, Đ. Pomegranate peel extract ameliorates autoimmunity in animal models of multiple sclerosis and type 1 diabetes. J. Funct. Foods 2017, 35, 522–530. [Google Scholar]
- Ravikumar, N.; Kavitha, C.N. Immunomodulatory effect of Quercetin on dysregulated Th1/Th2 cytokine balance in mice with both type 1 diabetes and allergic asthma. J. Appl. Pharm. Sci. 2020, 10, 80–87. [Google Scholar]
- Jeong, G.-S.; Lee, D.-S.; Song, M.-Y.; Park, B.-H.; Kang, D.-G.; Lee, H.-S.; Kwon, K.-B.; Kim, Y.-C. Butein from Rhus verniciflua protects pancreatic β cells against cytokine-induced toxicity mediated by inhibition of nitric oxide formation. Biol. Pharm. Bull. 2011, 34, 97–102. [Google Scholar]
- Bae, U.-J.; Jang, H.-Y.; Lim, J.M.; Hua, L.; Ryu, J.-H.; Park, B.-H. Polyphenols isolated from Broussonetia kazinoki prevent cytokine-induced β-cell damage and the development of type 1 diabetes. Exp. Mol. Med. 2015, 47, e160. [Google Scholar]
- Glossop, J.R.; Dawes, P.T.; Mattey, D.L. Association between cigarette smoking and release of tumour necrosis factor α and its soluble receptors by peripheral blood mononuclear cells in patients with rheumatoid arthritis. Rheumatology 2006, 45, 1223–1229. [Google Scholar]
- Mateen, S.; Moin, S.; Zafar, A.; Khan, A.Q. Redox signaling in rheumatoid arthritis and the preventive role of polyphenols. Clin. Chim. Acta 2016, 463, 4–10. [Google Scholar]
- Kloesch, B.; Becker, T.; Dietersdorfer, E.; Kiener, H.; Steiner, G. Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like synoviocytes. Int. Immunopharmacol. 2013, 15, 400–405. [Google Scholar]
- Mateen, S.; Moin, S.; Khan, A.Q.; Zafar, A.; Fatima, N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS ONE 2016, 11, e0152925. [Google Scholar]
- Yang, G.; Chang, C.-C.; Yang, Y.; Yuan, L.; Xu, L.; Ho, C.-T.; Li, S. Resveratrol alleviates rheumatoid arthritis via reducing ROS and inflammation, inhibiting MAPK signaling pathways, and suppressing angiogenesis. J. Agric. Food Chem. 2018, 66, 12953–12960. [Google Scholar]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Elhakeim, E.H. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin. Rheumatol. 2018, 37, 2035–2042. [Google Scholar]
- Morinobu, A.; Biao, W.; Tanaka, S.; Horiuchi, M.; Jun, L.; Tsuji, G.; Sakai, Y.; Kurosaka, M.; Kumagai, S. (−)-Epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2008, 58, 2012–2018. [Google Scholar]
- Park, M.-K.; Park, J.-S.; Cho, M.-L.; Oh, H.-J.; Heo, Y.-J.; Woo, Y.-J.; Heo, Y.-M.; Park, M.-J.; Park, H.-S.; Park, S.-H. Grape seed proanthocyanidin extract (GSPE) differentially regulates Foxp3+ regulatory and IL-17+ pathogenic T cell in autoimmune arthritis. Immunol. Lett. 2011, 135, 50–58. [Google Scholar]
- Gonçalves, G.A.; Soares, A.A.; Correa, R.C.G.; Barros, L.; Haminiuk, C.W.I.; Peralta, R.M.; Ferreira, I.C.F.R.; Bracht, A. Merlot grape pomace hydroalcoholic extract improves the oxidative and inflammatory states of rats with adjuvant-induced arthritis. J. Funct. Foods 2017, 33, 408–418. [Google Scholar]
- Stamer, D.K.; Nizami, S.A.; Lee, F.Y.; Soung, D.Y. Whole grape alleviates inflammatory arthritis through inhibition of tumor necrosis factor. J. Funct. Foods 2017, 35, 458–465. [Google Scholar]
- Rosillo, M.Á.; Alcaraz, M.J.; Sánchez-Hidalgo, M.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C.; Ferrándiz, M.L. Anti-inflammatory and joint protective effects of extra-virgin olive-oil polyphenol extract in experimental arthritis. J. Nutr. Biochem. 2014, 25, 1275–1281. [Google Scholar]
- Javadi, F.; Ahmadzadeh, A.; Eghtesadi, S.; Aryaeian, N.; Zabihiyeganeh, M.; Rahimi Foroushani, A.; Jazayeri, S. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: A double-blind, randomized controlled trial. J. Am. Coll. Nutr. 2017, 36, 9–15. [Google Scholar]
- Riccio, P. The molecular basis of nutritional intervention in multiple sclerosis: A narrative review. Complementary Ther. Med. 2011, 19, 228–237. [Google Scholar]
- Nair, M.P.; Mahajan, S.; Reynolds, J.L.; Aalinkeel, R.; Nair, H.; Schwartz, S.A.; Kandaswami, C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-κβ system. Clin. Vaccine Immunol. 2006, 13, 319–328. [Google Scholar]
- Verbeek, R.; Plomp, A.C.; van Tol, E.A.F.; van Noort, J.M. The flavones luteolin and apigenin inhibit in vitro antigen-specific proliferation and interferon-gamma production by murine and human autoimmune T cells. Biochem. Pharmacol. 2004, 68, 621–629. [Google Scholar]
- Sternberg, Z.; Chadha, K.; Lieberman, A.; Hojnacki, D.; Drake, A.; Zamboni, P.; Rocco, P.; Grazioli, E.; Weinstock-Guttman, B.; Munschauer, F. Quercetin and interferon-β modulate immune response (s) in peripheral blood mononuclear cells isolated from multiple sclerosis patients. J. Neuroimmunol. 2008, 205, 142–147. [Google Scholar]
- Hendriks, J.J.A.; de Vries, H.E.; van der Pol, S.M.A.; van den Berg, T.K.; van Tol, E.A.F.; Dijkstra, C.D. Flavonoids inhibit myelin phagocytosis by macrophages; a structure–activity relationship study. Biochem. Pharmacol. 2003, 65, 877–885. [Google Scholar]
- Fonseca-Kelly, Z.; Nassrallah, M.; Uribe, J.; Khan, R.S.; Dine, K.; Dutt, M.; Shindler, K.S. Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Front. Neurol. 2012, 3, 84. [Google Scholar]
- Ghaiad, H.R.; Nooh, M.M.; El-Sawalhi, M.M.; Shaheen, A.A. Resveratrol promotes remyelination in cuprizone model of multiple sclerosis: Biochemical and histological study. Mol. Neurobiol. 2017, 54, 3219–3229. [Google Scholar]
- Siahpoosh, A.; Majdinasab, N.; Derakhshannezhad, N.; Khalili, H.R.; Malayeri, A. Effect of grape seed on quality of life in multiple sclerosis patients. J. Contemp. Med Sci. 2018, 4. Available online: http://www.jocms.org/index.php/jcms/article/view/453/243 (accessed on 10 December 2020).
| Polyphenols | Signaling Pathways | Immunomodulatory Responses |
|---|---|---|
| Curcumin [70,71] | Suppress NF-κB | ↓ Bcl-2 in PHA-activated Tcells |
| Suppress maturation of DCs | ||
| Inhibit IL-12, IL-8 | ||
| ↑ IL-4 | ||
| Resveratrol, Quercetin, Silibinin [72] | Altering PI3K/Akt | ↓IL-6 and IL-1 |
| Genistein [71,73] | Activate AMPK | ↓ IL-1β, IL-6, IL-8 |
| Inhibit ROS/Akt/NF-κB | ↓COX-2 | |
| EGCG [74] | Suppress NF-κB and MAPK | Inhibit Th1 and Th17 differentiation |
| ↓ Transcription factors (STAT1 and T-bet for Th1, and STAT3 and RORγt for Th17) | ||
| ↑ T-reg in lymphoid tissues and central nervous system | ||
| Proanthocyanidins Procyanidins [75,76] | Suppress NF-κB and MAPK | ↓TNF-α, IL-1β |
| Inhibit iNOS and COX-2 | ||
| Caffeic acid [77,78,79] | Suppress p38 MAPK, JNK1/2 and NF-κB | ↓ IL-1β, IL-6, TNF-α |
| ↓ Monocyte chemoattractant protein (MCP)-1 | ||
| Inhibit xanthine oxidase and COX |
| Dietary Polyphenols | Treatment and Duration | Results |
|---|---|---|
| Atopic Eczema or Dermatitis | ||
| Quercetin (pure isolated polyphenols) | 15 human subjects with contact dermatitis. Quercetin applied topically for five days | No change as compared to the control [119] |
| Cocoa flavanols (catechin, epicatechin, procyanidins) at a lower dose of 27 mg or a higher dose of 329 mg | Ten healthy women consumed a low and high dose. | The higher dose of cocoa drink reduced water loss and improved the blood circulation in the skin [120] |
| Water extract of whey powder dodder rich in quercetin | Randomized control trial (RCT) study recruited 52 subjects atopic dermatitis recruited for 30 days | Quercetin reduces allergy and inhibits the secretion of the mast cell. Elevate skin moisture and elasticity [121] |
| Apple condensed tannins (ACT) at a dose of 10 mg/kg | Apple polyphenols were investigated in subjects with atopic eczema for 8 weeks. | Reduced inflammation and itching in disease subjects compared with the control group. ACT has an anti-allergic effect [122] |
| Food Allergy | ||
| Polyphenol enriched extracts or purified epicatechin (1, 0.3 and 0.01%) | Female BALB/c mice treated with polyphenols for 8 days | Epicatechin exhibited a significant anti-allergic effect [123] |
| Polyphenol-enriched apple extract (>40%) | BALB/c mice treated with an apple extract for 7 weeks | Reduce allergenicity by protein–polyphenol interaction, decrease intestinal mast cell protease and pro-inflammatory genes, diminished cytokine secretion. [105] |
| Cocoa diet with 0.2% polyphenols | Rats received either a cocoa diet or a standard diet for 4 weeks | Cocoa diet decreased total serum immunoglobulin (Ig)E, Tumor necrosis factor (TNF)-α and interleukin (IL)-10 secretion. No effect on IL-4 synthesis [124] |
| Asthma and Rhinitis | ||
| Drinks containing apple polyphenols at low and high dose (50 mg and 100 mg) | 33 subjects having moderate or severe persistent allergic rhinitis treated with apple polyphenols for 4 weeks | Improve sneezing attacks nasal discharger and swelling of the nasal turbinate in the low-dose group and high dose group [125] |
| 100 mg pycnogenol mixture of water-soluble bioflavonoids | 76 patients with asthma | Decrease by 15.2% of the specific IgE, whereas IgG1 and IgG4 remained unchanged. Reduced the need for medication [126] |
| 500 mg/day Apple condensed tannins (ACT) and polyphenols | A double-blind comparative study on 36 subjects with rhinitis for 12 weeks | Significant improvement in sneezing scores and nasal discharge inhibited in perennial rhinitis due in the group taking polyphenols treatment [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. https://doi.org/10.3390/nu13030728
Shakoor H, Feehan J, Apostolopoulos V, Platat C, Al Dhaheri AS, Ali HI, Ismail LC, Bosevski M, Stojanovska L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients. 2021; 13(3):728. https://doi.org/10.3390/nu13030728
Chicago/Turabian StyleShakoor, Hira, Jack Feehan, Vasso Apostolopoulos, Carine Platat, Ayesha Salem Al Dhaheri, Habiba I. Ali, Leila Cheikh Ismail, Marijan Bosevski, and Lily Stojanovska. 2021. "Immunomodulatory Effects of Dietary Polyphenols" Nutrients 13, no. 3: 728. https://doi.org/10.3390/nu13030728
APA StyleShakoor, H., Feehan, J., Apostolopoulos, V., Platat, C., Al Dhaheri, A. S., Ali, H. I., Ismail, L. C., Bosevski, M., & Stojanovska, L. (2021). Immunomodulatory Effects of Dietary Polyphenols. Nutrients, 13(3), 728. https://doi.org/10.3390/nu13030728












