Macronutrient Sensing in the Oral Cavity and Gastrointestinal Tract: Alimentary Tastes
Abstract
:1. Introduction
2. Taste
2.1. Basic Taste
2.2. Measuring Taste
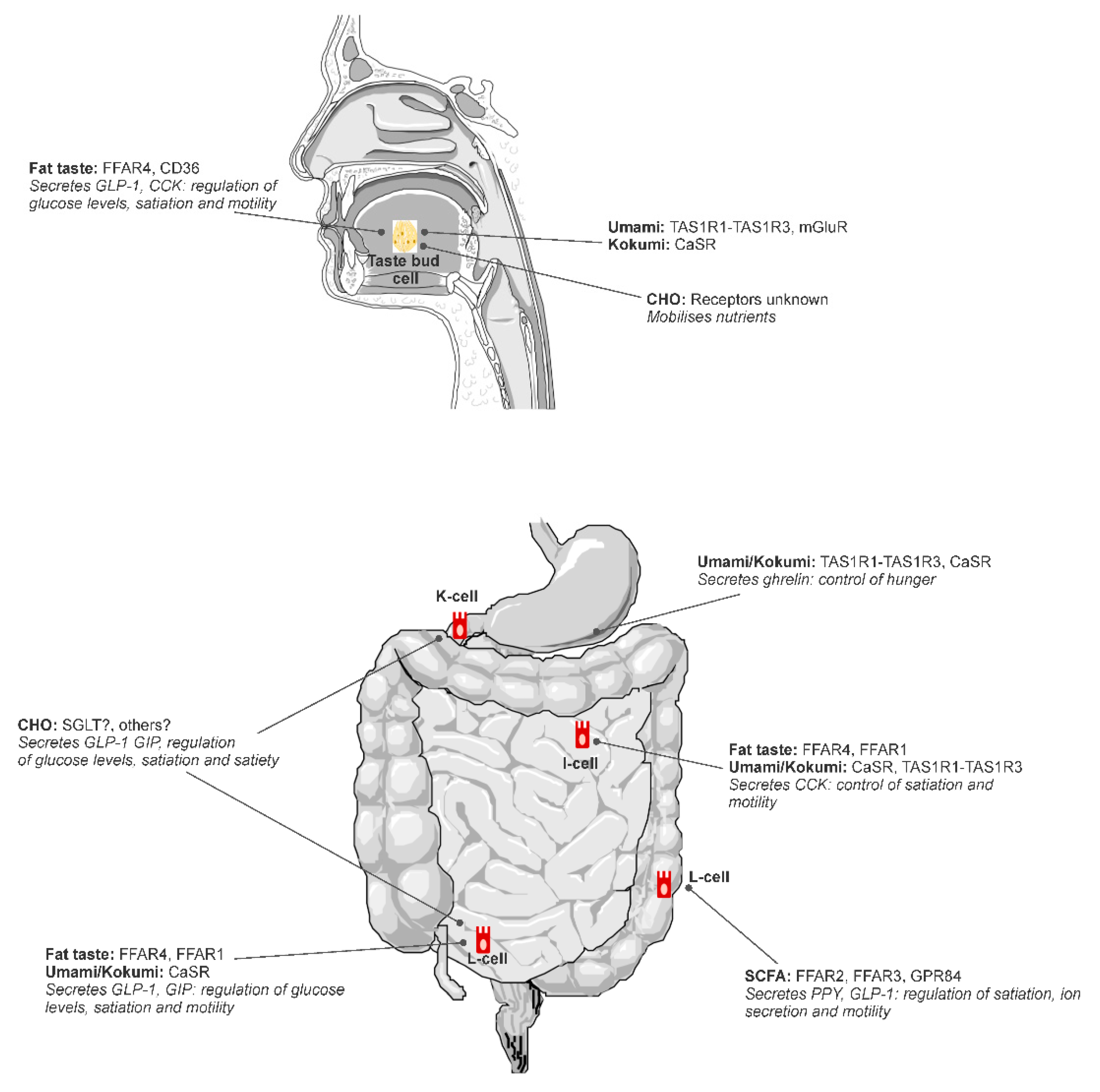
3. Beyond Basic Tastes: Alimentary Tastes
4. Macronutrient Fat: Fat Taste and Mouthfeel
4.1. Triacylglycerol
4.2. Fatty Acid Sensing
4.3. Individual Differences in Fatty Acid Sensing and Implications
4.4. Fatty Acid Sensing, Satiety, and Diet
5. Macronutrient Protein: Umami and Kokumi Tastes
5.1. Sensing Glutamate and γ-Glutamyl Peptides Throughout the Alimentary Canal
5.2. Behavioral and Health Outcomes of Umami/Kokumi Stimuli
6. Macronutrient Carbohydrate: Sweet and Carbohydrate Taste
6.1. Carbohydrate Taste Psychophysics
6.2. Behavioral and Health Outcomes of Carbohydrate Taste
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beauchamp, G.K. Basic Taste: A Perceptual Concept. J. Agric. Food Chem. 2019, 67, 13860–13869. [Google Scholar] [CrossRef]
- Hartley, I.E.; Liem, D.G.; Keast, R. Umami as an ‘Alimentary’ Taste. A New Perspective on Taste Classification. Nutrients 2019, 11, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhari, N.; Roper, S.D. The cell biology of taste. J. Cell Biol. 2010, 190, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrashekar, J.; Hoon, M.A.; Ryba, N.J.P.; Zuker, C.S. The receptors and cells for mammalian taste. Nature 2006, 444, 288. [Google Scholar] [CrossRef]
- Breslin, P.A.S. An Evolutionary Perspective on Food Review and Human Taste. Curr. Biol. 2013, 23, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keast, R.S.J.; Roper, J. A Complex Relationship among Chemical Concentration, Detection Threshold, and Suprathreshold Intensity of Bitter Compounds. Chem. Senses 2007, 32, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Keast, R. Effects of sugar and fat consumption on sweet and fat taste. Curr. Opin. Behav. Sci. 2016, 9, 55–60. [Google Scholar] [CrossRef]
- Hayes, J.E.; Keast, R.S.J. Two decades of supertasting: Where do we stand? Physiol. Behav. 2011, 104, 1072–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, J.; Bolhuis, D.P.; Cicerale, S.; Hayes, J.E.; Keast, R. The Relationships Between Common Measurements of Taste Function. Chemosens. Percept. 2015, 8, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, G.; Chandrashekar, J.; Hoon, M.A.; Feng, L.; Zhao, G.; Ryba, N.J.P.; Zuker, C.S. An amino-acid taste receptor. Nature 2002, 416, 199. [Google Scholar] [CrossRef]
- Liu, D.; Archer, N.; Duesing, K.; Hannan, G.; Keast, R. Mechanism of fat taste perception: Association with diet and obesity. Prog. Lipid Res. 2016, 63, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Ohsu, T.; Amino, Y.; Nagasaki, H.; Yamanaka, T.; Takeshita, S.; Hatanaka, T.; Maruyama, Y.; Miyamura, N.; Eto, Y. Involvement of the Calcium-sensing Receptor in Human Taste Perception. J. Biol. Chem. 2010, 285, 1016–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- San Gabriel, A.; Uneyama, H. Amino acid sensing in the gastrointestinal tract. Amino Acids 2013, 45, 451–461. [Google Scholar] [CrossRef]
- Daly, K.; Al-Rammahi, M.; Moran, A.; Marcello, M.; Ninomiya, Y.; Shirazi-Beechey, S.P. Sensing of amino acids by the gut-expressed taste receptor T1R1-T1R3 stimulates CCK secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G271–G282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wauson, E.M.; Lorente-Rodríguez, A.; Cobb, M.H. Minireview: Nutrient Sensing by G Protein-Coupled Receptors. Mol. Endocrinol. 2013, 27, 1188–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norton, M.; Murphy, K.G. Targeting gastrointestinal nutrient sensing mechanisms to treat obesity. Curr. Opin. Pharmacol. 2017, 37, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Uneyama, H.; Niijima, A.; San Gabriel, A.; Torii, K. Luminal amino acid sensing in the rat gastric mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G1163–G1170. [Google Scholar] [CrossRef]
- Depoortere, I. Taste receptors of the gut: Emerging roles in health and disease. Gut 2014, 63, 179–190. [Google Scholar] [CrossRef]
- Hooper, L.; Abdelhamid, A.; Bunn, D.; Brown, T.; Summerbell, C.D.; Skeaff, C.M. Effects of total fat intake on body weight. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [Green Version]
- Blundell, J.E.; Macdiarmid, J.I. Fat as a risk factor for overconsumption: Satiation, satiety, and patterns of eating. J. Am. Diet. Assoc. 1997, 97, S63–S69. [Google Scholar] [CrossRef]
- Cotton, J.; Burley, V.; Blundell, J. Fat and satiety: Effect of fat in combination with either protein or carbohydrate. Int. J. Obes. 1993, 17, 63. [Google Scholar]
- Bolhuis, D.P.; Costanzo, A.; Newman, L.P.; Keast, R.S.J. Salt Promotes Passive Overconsumption of Dietary Fat in Humans. J. Nutr. 2015, 146, 838–845. [Google Scholar] [CrossRef] [Green Version]
- Heinze, J.M.; Preissl, H.; Fritsche, A.; Frank, S. Controversies in fat perception. Physiol. Behav. 2015, 152, 479–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Running, C.A.; Craig, B.A.; Mattes, R.D. Oleogustus: The Unique Taste of Fat. Chem. Senses 2015, 40, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Keast, R.; Costanzo, A. Is fat the sixth taste primary? Evidence and implications. Flavour 2015, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Che Man, Y.B.; Haryati, T.; Ghazali, H.M.; Asbi, B.A. Composition and thermal profile of crude palm oil and its products. J. Am. Oil Chem. Soc. 1999, 76, 237–242. [Google Scholar] [CrossRef]
- Koriyama, T.; Wongso, S.; Watanabe, K.; Abe, H. Fatty Acid Compositions of Oil Species Affect the 5 Basic Taste Perceptions. J. Food Sci. 2002, 67, 868–873. [Google Scholar] [CrossRef]
- Mukherjee, M. Human digestive and metabolic lipases—A brief review. J. Mol. Catal. B Enzym. 2003, 22, 369–376. [Google Scholar] [CrossRef]
- Drewnowski, A. Why do we like fat? J. Am. Diet. Assoc. 1997, 97, S58–S62. [Google Scholar] [CrossRef]
- Verhagen, J.V.; Kadohisa, M.; Rolls, E.T. Primate insular/opercular taste cortex: Neuronal representations of the viscosity, fat texture, grittiness, temperature, and taste of foods. J. Neurophysiol. 2004, 92, 1685–1699. [Google Scholar] [CrossRef]
- Marciani, L.; Gowland, P.A.; Spiller, R.C.; Manoj, P.; Moore, R.J.; Young, P.; Fillery-Travis, A.J. Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, C. Why liquid energy results in overconsumption. Proc. Nutr. Soc. 2011, 70, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Bolhuis, D.P.; Forde, C.G.; Cheng, Y.; Xu, H.; Martin, N.; de Graaf, C. Slow Food: Sustained Impact of Harder Foods on the Reduction in Energy Intake over the Course of the Day. PLoS ONE 2014, 9, e93370. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, D.; Degen, L.; Drewe, J.; Meuli, J.; Duebendorfen, R.; Ruckstuhl, N.; D’Amato, M.; Rovati, L.; Beglinger, C. The role of long chain fatty acids in regulating food intake and cholecystokinin release in humans. Gut 2000, 46, 688–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stillman, M.A.; Maibach, H.I.; Shallita, A.R. Relative irritancy of free fatty acids of different chain length. Contact Dermat. 1975, 1, 65–69. [Google Scholar] [CrossRef]
- Colombo, M.; Trevisi, P.; Gandolfi, G.; Bosi, P. Assessment of the presence of chemosensing receptors based on bitter and fat taste in the gastrointestinal tract of young pig1. J. Anim. Sci. 2012, 90, 128–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galindo, M.M.; Voigt, N.; Stein, J.; van Lengerich, J.; Raguse, J.-D.; Hofmann, T.; Meyerhof, W.; Behrens, M. G Protein-Coupled Receptors in Human Fat Taste Perception. Chem. Senses 2012, 37, 123–139. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Yano, T.; Adachi, T.; Koshimizu, T.A.; Hirasawa, A.; Tsujimoto, G. Cloning and characterization of the rat free fatty acid receptor GPR120: In vivo effect of the natural ligand on GLP-1 secretion and proliferation of pancreatic β cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 377, 515–522. [Google Scholar] [CrossRef]
- Liu, D.; Costanzo, A.; Evans, M.D.M.; Archer, N.S.; Nowson, C.; Duesing, K.; Keast, R. Expression of the candidate fat taste receptors in human fungiform papillae and the association with fat taste function. Br. J. Nutr. 2018, 120, 64–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cvijanovic, N.; Feinle-Bisset, C.; Young, R.L.; Little, T.J. Oral and intestinal sweet and fat tasting: Impact of receptor polymorphisms and dietary modulation for metabolic disease. Nutr. Rev. 2015, 73, 318–334. [Google Scholar] [CrossRef]
- Steinert, R.E.; Beglinger, C. Nutrient sensing in the gut: Interactions between chemosensory cells, visceral afferents and the secretion of satiation peptides. Physiol. Behav. 2011, 105, 62–70. [Google Scholar] [CrossRef]
- Abdoul-Azize, S.; Selvakumar, S.; Sadou, H.; Besnard, P.; Khan, N.A. Ca2+ signaling in taste bud cells and spontaneous preference for fat: Unresolved roles of CD36 and GPR120. Biochimie 2014, 96, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Cartoni, C.; Yasumatsu, K.; Ohkuri, T.; Shigemura, N.; Yoshida, R.; Godinot, N.; Le Coutre, J.; Ninomiya, Y.; Damak, S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 2010, 30, 8376–8382. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.; Passilly-Degrace, P.; Chevrot, M.; Ancel, D.; Sparks, S.M.; Drucker, D.J.; Besnard, P. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: Putative involvement of GPR120 and impact on taste sensitivity. J. Lipid Res. 2012, 53, 2256–2265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90–94. [Google Scholar] [CrossRef]
- Ichimura, A.; Hirasawa, A.; Poulain-Godefroy, O.; Bonnefond, A.; Hara, T.; Yengo, L.; Kimura, I.; Leloire, A.; Liu, N.; Iida, K.; et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 2012, 483, 350–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Verme, J.; Gaetani, S.; Fu, J.; Oveisi, F.; Burton, K.; Piomelli, D. Regulation of food intake by oleoylethanolamide. Cell. Mol. Life Sci. 2005, 62, 708–716. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, G.J.; Fu, J.; Astarita, G.; Li, X.; Gaetani, S.; Campolongo, P.; Cuomo, V.; Piomelli, D. The Lipid Messenger OEA Links Dietary Fat Intake to Satiety. Cell Metab. 2008, 8, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundaresan, S.; Shahid, R.; Riehl, T.E.; Chandra, R.; Nassir, F.; Stenson, W.F.; Liddle, R.A.; Abumrad, N.A. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin. FASEB J. 2013, 27, 1191–1202. [Google Scholar] [CrossRef] [Green Version]
- Edfalk, S.; Steneberg, P.; Edlund, H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 2008, 57, 2280–2287. [Google Scholar] [CrossRef] [Green Version]
- Liou, A.P.; Lu, X.; Sei, Y.; Zhao, X.; Pechhold, S.; Carrero, R.J.; Raybould, H.E.; Wank, S. The G-protein-coupled receptor GPR40 directly mediates Long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology 2011, 140, 903–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kles, K.A.; Chang, E.B. Short-chain fatty acids impact on intestinal adaptation, inflammation, carcinoma, and failure. Gastroenterology 2006, 130, S100–S105. [Google Scholar] [CrossRef]
- Asano, M.; Hong, G.; Matsuyama, Y.; Wang, W.; Izumi, S.; Izumi, M.; Toda, T.; Kudo, T.-a. Association of Oral Fat Sensitivity with Body Mass Index, Taste Preference, and Eating Habits in Healthy Japanese Young Adults. Tohoku J. Exp. Med. 2016, 238, 93–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costanzo, A.; Orellana, L.; Nowson, C.; Duesing, K.; Keast, R. Fat Taste Sensitivity Is Associated with Short-Term and Habitual Fat Intake. Nutrients 2017, 9, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Ruiz, N.R.; López-Díaz, J.A.; Wall-Medrano, A.; Jiménez-Castro, J.A.; Angulo, O. Oral fat perception is related with body mass index, preference and consumption of high-fat foods. Physiol. Behav. 2014, 129, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Running, C.A.; Mattes, R.D.; Tucker, R.M. Fat taste in humans: Sources of within- and between-subject variability. Prog. Lipid Res. 2013, 52, 438–445. [Google Scholar] [CrossRef]
- Tucker, R.M.; Nuessle, T.M.; Garneau, N.L.; Smutzer, G.; Mattes, R.D. No Difference in Perceived Intensity of Linoleic Acid in the Oral Cavity between Obese and Nonobese Individuals. Chem. Senses 2015, 40, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.P.; Keast, R.S.J. The Test-Retest Reliability of Fatty Acid Taste Thresholds. Chemosens. Percept. 2013, 6, 70–77. [Google Scholar] [CrossRef]
- Newman, L.P.; Bolhuis, D.P.; Torres, S.J.; Keast, R.S. Dietary fat restriction increases fat taste sensitivity in people with obesity. Obesity 2016, 24, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.E.; Keast, R.S. Recent fat intake modulates fat taste sensitivity in lean and overweight subjects. Int. J. Obes. 2012, 36, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, A.; Nowson, C.; Orellana, L.; Bolhuis, D.; Duesing, K.; Keast, R. Effect of dietary fat intake and genetics on fat taste sensitivity: A co-twin randomized controlled trial. Am. J. Clin. Nutr. 2018, 107, 683–694. [Google Scholar] [CrossRef]
- Costanzo, A.; Liu, D.; Nowson, C.; Duesing, K.; Archer, N.; Bowe, S.; Keast, R. A low-fat diet up-regulates expression of fatty acid taste receptor gene FFAR4 in fungiform papillae in humans: A co-twin randomised controlled trial. Br. J. Nutr. 2019, 122, 1212–1220. [Google Scholar] [CrossRef]
- Costanzo, A.; Russell, C.G.; Lewin, S.; Keast, R. A Fatty Acid Mouth Rinse Decreases Self-Reported Hunger and Increases Self-Reported Fullness in Healthy Australian Adults: A Randomized Cross-Over Trial. Nutrients 2020, 12, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French, S.J.; Conlon, C.A.; Mutuma, S.T.; Arnold, M.; Read, N.W.; Meijer, G.; Francis, J. The effects of intestinal infusion of long-chain fatty acids on food intake in humans. Gastroenterology 2000, 119, 943–948. [Google Scholar] [CrossRef]
- Mattes, R.D. Fat taste and lipid metabolism in humans. Physiol. Behav. 2005, 86, 691–697. [Google Scholar] [CrossRef]
- Brennan, I.M.; Luscombe-Marsh, N.D.; Seimon, R.V.; Otto, B.; Horowitz, M.; Wishart, J.M.; Feinle-Bisset, C. Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide YY, and ghrelin, and energy intake in lean and obese men. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Love-Gregory, L.; Klein, S.; Abumrad, N.A. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J. Lipid Res. 2012, 53, 561–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, J.E.; Seimon, R.V.; Otto, B.; Keast, R.S.; Clifton, P.M.; Feinle-Bisset, C. Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. Am. J. Clin. Nutr. 2011, 93, 703–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keast, R.S.; Azzopardi, K.M.; Newman, L.P.; Haryono, R.Y. Impaired oral fatty acid chemoreception is associated with acute excess energy consumption. Appetite 2014, 80, 1–6. [Google Scholar] [CrossRef]
- Collier, G.; O’Dea, K. The effect of coingestion of fat on the glucose, insulin, and gastric inhibitory polypeptide responses to carbohydrate and protein. Am. J. Clin. Nutr. 1983, 37, 941–944. [Google Scholar] [CrossRef]
- Gibbons, C.; Caudwell, P.; Finlayson, G.; Webb, D.-L.; Hellström, P.M.; Näslund, E.; Blundell, J.E. Comparison of Postprandial Profiles of Ghrelin, Active GLP-1, and Total PYY to Meals Varying in Fat and Carbohydrate and Their Association With Hunger and the Phases of Satiety. J. Clin. Endocrinol. Metab. 2013, 98, E847–E855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helou, N.; Obeid, O.; Azar, S.T.; Hwalla, N. Variation of Postprandial PYY3–36Response following Ingestion of Differing Macronutrient Meals in Obese Females. Ann. Nutr. Metab. 2008, 52, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Tannous dit El Khoury, D.; Obeid, O.; Azar, S.T.; Hwalla, N. Variations in Postprandial Ghrelin Status following Ingestion of High-Carbohydrate, High-Fat, and High-Protein Meals in Males. Ann. Nutr. Metab. 2006, 50, 260–269. [Google Scholar] [CrossRef]
- Van der Klaauw, A.A.; Keogh, J.M.; Henning, E.; Trowse, V.M.; Dhillo, W.S.; Ghatei, M.A.; Farooqi, I.S. High protein intake stimulates postprandial GLP1 and PYY release. Obesity 2013, 21, 1602–1607. [Google Scholar] [CrossRef]
- Robertson, M.D.; Jackson, K.G.; Williams, C.M.; Fielding, B.A.; Frayn, K.N. Prolonged effects of modified sham feeding on energy substrate mobilization. Am. J. Clin. Nutr. 2001, 73, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Wisén, O.; Björvell, H.; Cantor, P.; Johansson, C.; Theodorsson, E. Plasma concentrations of regulatory peptides in obseity following modified sham feeding (MSF) and a liquid test meal. Regul. Pept. 1992, 39, 43–54. [Google Scholar] [CrossRef]
- Heinze, J.M.; Costanzo, A.; Baselier, I.; Fritsche, A.; Frank-Podlech, S.; Keast, R. Detection thresholds for four different fatty stimuli are associated with increased dietary intake of processed high-caloric food. Appetite 2018, 123, 7–13. [Google Scholar] [CrossRef]
- Kindleysides, S.; Beck, K.; Walsh, D.; Henderson, L.; Jayasinghe, S.; Golding, M.; Breier, B. Fat Sensation: Fatty Acid Taste and Olfaction Sensitivity and the Link with Disinhibited Eating Behaviour. Nutrients 2017, 9, 879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, J.E.; Feinle-Bisset, C.; Golding, M.; Delahunty, C.; Clifton, P.M.; Keast, R.S. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br. J. Nutr. 2010, 104, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.E.; Newman, L.P.; Keast, R.S. Oral sensitivity to oleic acid is associated with fat intake and body mass index. Clin. Nutr. 2011, 30, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Little, T. Oral and gastrointestinal sensing of dietary fat and appetite regulation in humans: Modification by diet and obesity. Front. Neurosci. 2010, 1, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feltrin, K.L.; Little, T.J.; Meyer, J.H.; Horowitz, M.; Smout, A.J.P.M.; Wishart, J.; Pilichiewicz, A.N.; Rades, T.; Chapman, I.M.; Feinle-Bisset, C. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, 524–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feltrin, K.L.; Little, T.J.; Meyer, J.H.; Horowitz, M.; Rades, T.; Wishart, J.; Feinle-Bisset, C. Comparative effects of intraduodenal infusions of lauric and oleic acids on antropyloroduodenal motility, plasma cholecystokinin and peptide YY, appetite, and energy intake in healthy men. Am. J. Clin. Nutr. 2008, 87, 1181–1187. [Google Scholar] [CrossRef] [Green Version]
- Tucker, R.M.; Edlinger, C.; Craig, B.A.; Mattes, R.D. Associations Between BMI and Fat Taste Sensitivity in Humans. Chem. Senses 2014, 39, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Tucker, R.M.; Kaiser, K.A.; Parman, M.A.; George, B.J.; Allison, D.B.; Mattes, R.D. Comparisons of Fatty Acid Taste Detection Thresholds in People Who Are Lean vs. Overweight or Obese: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169583. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, L.R.; Beety, J.M.; Morgan, L.M.; Wright, J.W.; Howland, R.; Marks, V. Attenuated GLP-1 secretion in obesity: Cause or consequence? Gut 1996, 38, 916–919. [Google Scholar] [CrossRef] [Green Version]
- French, S.J.; Murray, B.; Rumsey, R.D.E.; Sepple, C.P.; Read, N.W. Preliminary studies on the gastrointestinal responses to fatty meals in obese people. Int. J. Obes. 1993, 17, 295–300. [Google Scholar]
- van Dongen, M.V.; van den Berg, M.C.; Vink, N.; Kok, F.J.; de Graaf, C. Taste–nutrient relationships in commonly consumed foods. Br. J. Nutr. 2012, 108, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, G.K.; Pearson, P. Human development and umami taste. Physiol. Behav. 1991, 49, 1009–1012. [Google Scholar] [CrossRef]
- Naim, M.; Ohara, I.; Kare, M.R.; Levinson, M. Interaction of MSG taste with nutrition: Perspectives in consummatory behavior and digestion. Physiol. Behav. 1991, 49, 1019–1024. [Google Scholar] [CrossRef]
- Yamaguchi, S. The Synergistic Taste Effect of Monosodium Glutamate and Disodium 5′-Inosinate. J. Food Sci. 1967, 32, 473–478. [Google Scholar] [CrossRef]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.Q.; Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Erlenbach, I.; Ryba, N.J.P.; Zuker, C.S. The receptors for mammalian sweet and umami taste. Cell 2003, 115, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Bai, W.; Zeng, X.; Cui, C. Gamma glutamyl peptides: The food source, enzymatic synthesis, kokumi-active and the potential functional properties A review. Trends Food Sci. Technol. 2019, 91, 339–346. [Google Scholar] [CrossRef]
- Rhyu, M.R.; Song, A.Y.; Kim, E.Y.; Son, H.J.; Kim, Y.; Mummalaneni, S.; Qian, J.; Grider, J.R.; Lyall, V. Kokumi taste active peptides modulate salt and umami taste. Nutrients 2020, 12, 1198. [Google Scholar] [CrossRef]
- Maruyama, Y.; Yasuda, R.; Kuroda, M.; Eto, Y. Kokumi substances, enhancers of basic tastes, induce responses in calcium-sensing receptor expressing taste cells. PLoS ONE 2012, 7, e34489. [Google Scholar] [CrossRef] [Green Version]
- Amino, Y.; Nakazawa, M.; Kaneko, M.; Miyaki, T.; Miyamura, N.; Maruyama, Y.; Eto, Y. Structure-CaSR-Activity Relation of Kokumi γ-Glutamyl Peptides. Chem. Pharm. Bull. 2016, 64, 1181–1189. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.H.; Fabek, H.; Akilen, R.; Chatterjee, D.; Kubant, R. Acute effects of monosodium glutamate addition to whey protein on appetite, food intake, blood glucose, insulin and gut hormones in healthy young men. Appetite 2018, 120, 92–99. [Google Scholar] [CrossRef]
- Tey, S.L.; Salleh, N.; Henry, C.J.; Forde, C.G. Effects of Consuming Preloads with Different Energy Density and Taste Quality on Energy Intake and Postprandial Blood Glucose. Nutrients 2018, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Hosaka, H.; Kusano, M.; Zai, H.; Kawada, A.; Kuribayashi, S.; Shimoyama, Y.; Nagoshi, A.; Maeda, M.; Kawamura, O.; Mori, M. Monosodium glutamate stimulates secretion of glucagon-like peptide-1 and reduces postprandial glucose after a lipid-containing meal. Aliment. Pharmacol. Ther. 2012, 36, 895–903. [Google Scholar] [CrossRef]
- Vancleef, L.; Van Den Broeck, T.; Thijs, T.; Steensels, S.; Briand, L.; Tack, J.; Depoortere, I. Chemosensory signalling pathways involved in sensing of amino acids by the ghrelin cell. Sci. Rep. 2015, 5, 15725. [Google Scholar] [CrossRef] [Green Version]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and After RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Bai, W.; Zeng, X.; Cui, C. γ-[Glu](n=1,2)-Phe/-Met/-Val stimulates gastrointestinal hormone (CCK and GLP-1) secretion by activating the calcium-sensing receptor. Food Funct. 2019, 10, 4071–4080. [Google Scholar] [CrossRef] [PubMed]
- Masic, U.; Yeomans, M.R. Umami flavor enhances appetite but also increases satiety. Am. J. Clin. Nutr. 2014, 100, 532–538. [Google Scholar] [CrossRef]
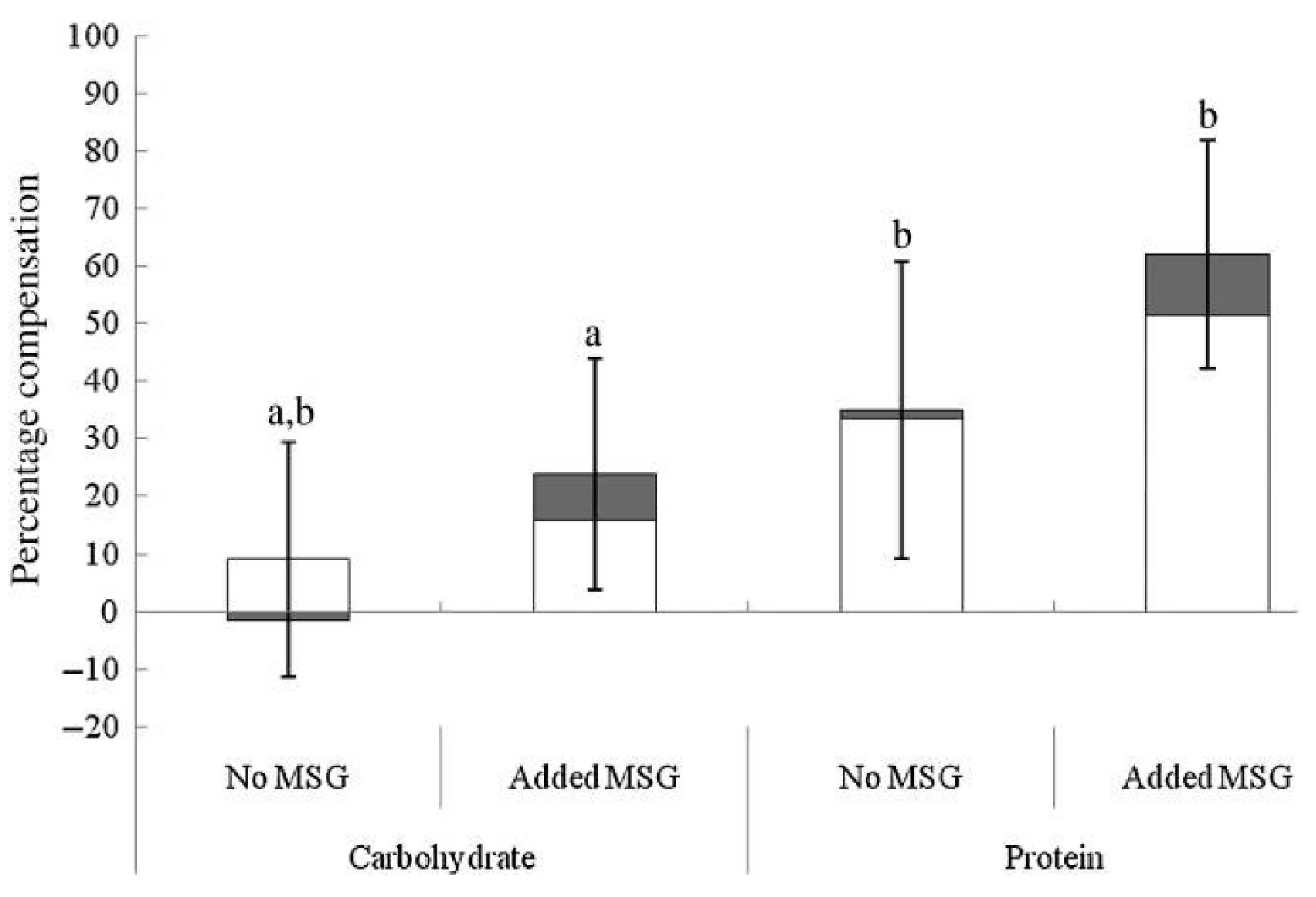
- Masic, U.; Yeomans, M.R. Monosodium glutamate delivered in a protein-rich soup improves subsequent energy compensation. J. Nutr. Sci. 2014, 3, e15. [Google Scholar] [CrossRef] [Green Version]
- Carter, B.E.; Monsivais, P.; Perrigue, M.M.; Drewnowski, A. Supplementing chicken broth with monosodium glutamate reduces hunger and desire to snack but does not affect energy intake in women. Br. J. Nutr. 2011, 106, 1441–1448. [Google Scholar] [CrossRef] [Green Version]
- Noel, C.A.; Finlayson, G.; Dando, R. Prolonged Exposure to Monosodium Glutamate in Healthy Young Adults Decreases Perceived Umami Taste and Diminishes Appetite for Savory Foods. J. Nutr. 2018, 148, 980–988. [Google Scholar] [CrossRef] [Green Version]
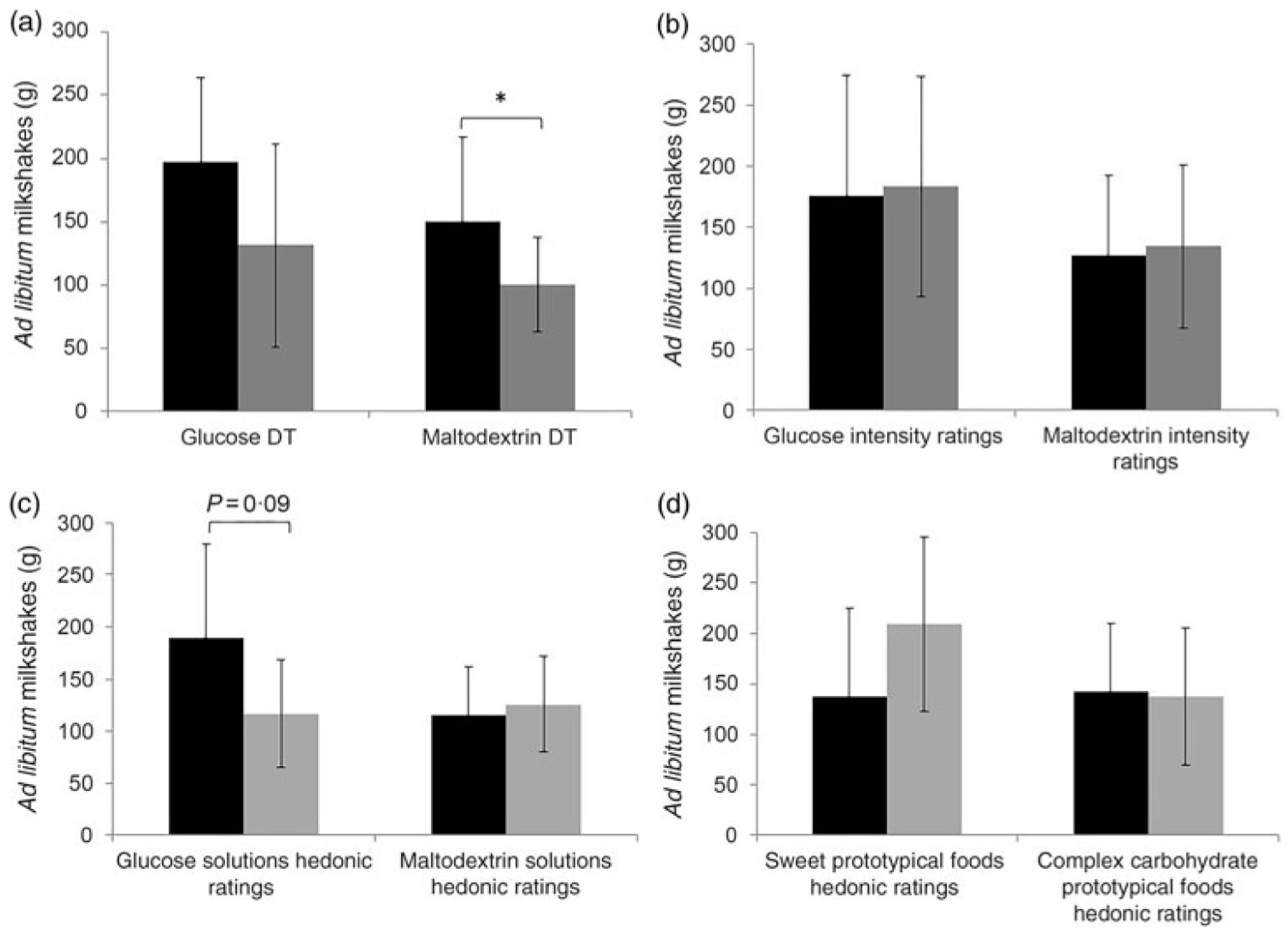
- Low, J.Y.Q.; Lacy, K.E.; McBride, R.L.; Keast, R.S.J. Associations between sweet taste function, oral complex carbohydrate sensitivity, liking and consumption of ad libitum sweet and non-sweet carbohydrate milkshakes among female adults. Br. J. Nutr. 2019, 122, 829–840. [Google Scholar] [CrossRef]
- Low, J.Y.; Lacy, K.E.; McBride, R.L.; Keast, R.S. Carbohydrate Taste Sensitivity Is Associated with Starch Intake and Waist Circumference in Adults. J. Nutr. 2017, 147, 2235–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurihara, K. Umami the Fifth Basic Taste: History of Studies on Receptor Mechanisms and Role as a Food Flavor. BioMed Res. Int. 2015, 2015, 189402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masic, U.; Yeomans, M.R. Does acute or habitual protein deprivation influence liking for monosodium glutamate? Physiol. Behav. 2017, 171, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Griffioen-Roose, S.; Mars, M.; Siebelink, E.; Finlayson, G.; Tomé, D.; de Graaf, C. Protein status elicits compensatory changes in food intake and food preferences. Am. J. Clin. Nutr. 2012, 95, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overberg, J.; Hummel, T.; Krude, H.; Wiegand, S. Differences in taste sensitivity between obese and non-obese children and adolescents. Arch. Dis. Child. 2012, 97, 1048. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Finkbeiner, S.; Beauchamp, G.K.; Mennella, J.A. Obese Women Have Lower Monosodium Glutamate Taste Sensitivity and Prefer Higher Concentrations Than Do Normal-weight Women. Obesity 2010, 18, 959–965. [Google Scholar] [CrossRef] [Green Version]
- Van Langeveld, A.W.B.; Teo, P.S.; de Vries, J.H.M.; Feskens, E.J.M.; de Graaf, C.; Mars, M. Dietary taste patterns by sex and weight status in the Netherlands. Br. J. Nutr. 2018, 119, 1195–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luscombe-Marsh, N.D.; Smeets, A.J.P.G.; Westerterp-Plantenga, M.S. Taste sensitivity for monosodium glutamate and an increased liking of dietary protein. Br. J. Nutr. 2008, 99, 904–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyaki, T.; Imada, T.; Shuzhen Hao, S.; Kimura, E. Monosodium l-glutamate in soup reduces subsequent energy intake from high-fat savoury food in overweight and obese women. Br. J. Nutr. 2016, 115, 176–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imoscopi, A.; Inelmen, E.M.; Sergi, G.; Miotto, F.; Manzato, E. Taste loss in the elderly: Epidemiology, causes and consequences. Aging Clin. Exp. Res. 2012, 24, 570–579. [Google Scholar] [CrossRef]
- Sasano, T.; Satoh-Kuriwada, S.; Shoji, N.; Iikubo, M.; Kawai, M.; Uneyama, H.; Sakamoto, M. Important Role of Umami Taste Sensitivity in Oral and Overall Health. Curr. Pharm. Des. 2014, 20, 2750–2754. [Google Scholar] [CrossRef]
- Sasano, T.; Satoh-Kuriwada, S.; Shoji, N. The important role of umami taste in oral and overall health. Flavour 2015, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Schiffman, S.S.; Miletic, I.D. Effect of taste and smell on secretion rate of salivary IgA in elderly and young persons. J. Nutr. Health Aging 1999, 3, 158–164. [Google Scholar] [PubMed]
- Mathey, M.-F.A.M.; Siebelink, E.; de Graaf, C.; Van Staveren, W.A. Flavor Enhancement of Food Improves Dietary Intake and Nutritional Status of Elderly Nursing Home Residents. J. Gerontol. Ser. A 2001, 56, M200–M205. [Google Scholar] [CrossRef]
- Valenzuela, C.; Aguilera, J.M. Effects of maltodextrin on hygroscopicity and crispness of apple leathers. J. Food Eng. 2015, 144, 1–9. [Google Scholar] [CrossRef]
- Trumbo, P.R.; Appleton, K.M.; de Graaf, K.; Hayes, J.E.; Baer, D.J.; Beauchamp, G.K.; Dwyer, J.T.; Fernstrom, J.D.; Klurfeld, D.M.; Mattes, R.D.; et al. Perspective: Measuring Sweetness in Foods, Beverages, and Diets: Toward Understanding the Role of Sweetness in Health. Adv. Nutr. 2020. [Google Scholar] [CrossRef]
- Feigin, M.B.; Sclafani, A.; Sunday, S.R. Species differences in polysaccharide and sugar taste preferences. Neurosci. Biobehav. Rev. 1987, 11, 231–240. [Google Scholar] [CrossRef]
- Hettinger, T.P.; Frank, M.E.; Myers, W.E. Are the tastes of polycose and monosodium glutamate unique? Chem. Senses 1996, 21, 341–347. [Google Scholar] [CrossRef] [Green Version]
- De Araujo, I.E.; Lin, T.; Veldhuizen, M.G.; Small, D.M. Metabolic regulation of brain response to food cues. Curr. Biol. 2013, 23, 878–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeomans, M.R.; Leitch, M.; Gould, N.J.; Mobini, S. Differential hedonic, sensory and behavioral changes associated with flavor–nutrient and flavor–flavor learning. Physiol. Behav. 2008, 93, 798–806. [Google Scholar] [CrossRef]
- Yeomans, M.R. Flavour–nutrient learning in humans: An elusive phenomenon? Physiol. Behav. 2012, 106, 345–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sclafani, A. Starch and sugar tastes in rodents: An update. Brain Res. Bull. 1991, 27, 383–386. [Google Scholar] [CrossRef]
- Sclafani, A. The sixth taste? Appetite 2004, 43, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.; Mann, S. Carbohydrate taste preferences in rats: Glucose, sucrose, maltose, fructose and polycose compared. Physiol. Behav. 1987, 40, 563–568. [Google Scholar] [CrossRef]
- Brietzke, C.; Franco-Alvarenga, P.E.; Coelho-Junior, H.J.; Silveira, R.; Asano, R.Y.; Pires, F.O. Effects of Carbohydrate Mouth Rinse on Cycling Time Trial Performance: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.; Bridge, M.; Jones, D. Carbohydrate sensing in the human mouth: Effects on exercise performance and brain activity. J. Physiol. 2009, 587, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Lapis, T.J.; Penner, M.H.; Lim, J. Evidence that humans can taste glucose polymers. Chem. Senses 2014, 39, 737–747. [Google Scholar] [CrossRef] [Green Version]
- Lapis, T.J.; Penner, M.H.; Lim, J. Humans can taste glucose oligomers independent of the hT1R2/hT1R3 sweet taste receptor. Chem. Senses 2016, 41, 755–762. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Pullicin, A.J. Oral carbohydrate sensing: Beyond sweet taste. Physiol. Behav. 2019, 202, 14–25. [Google Scholar] [CrossRef]
- Low, J.Y.Q.; Lacy, K.E.; McBride, R.L.; Keast, R.S.J. Evidence supporting oral sensitivity to complex carbohydrates independent of sweet taste sensitivity in humans. PLoS ONE 2017, 12, e0188784. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Chambers, E.S. Oral carbohydrate sensing and exercise performance. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 447–451. [Google Scholar] [CrossRef]
- Low, J.Y.Q.; Lacy, K.E.; McBride, R.L.; Keast, R.S.J. The Associations Between Oral Complex Carbohydrate Sensitivity, BMI, Liking, and Consumption of Complex Carbohydrate Based Foods. J. Food. Sci. 2018, 83, 2227–2236. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keast, R.; Costanzo, A.; Hartley, I. Macronutrient Sensing in the Oral Cavity and Gastrointestinal Tract: Alimentary Tastes. Nutrients 2021, 13, 667. https://doi.org/10.3390/nu13020667
Keast R, Costanzo A, Hartley I. Macronutrient Sensing in the Oral Cavity and Gastrointestinal Tract: Alimentary Tastes. Nutrients. 2021; 13(2):667. https://doi.org/10.3390/nu13020667
Chicago/Turabian StyleKeast, Russell, Andrew Costanzo, and Isabella Hartley. 2021. "Macronutrient Sensing in the Oral Cavity and Gastrointestinal Tract: Alimentary Tastes" Nutrients 13, no. 2: 667. https://doi.org/10.3390/nu13020667





