Vitamin D and Hospital Admission in Older Adults: A Prospective Association
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vitamin D Measurements
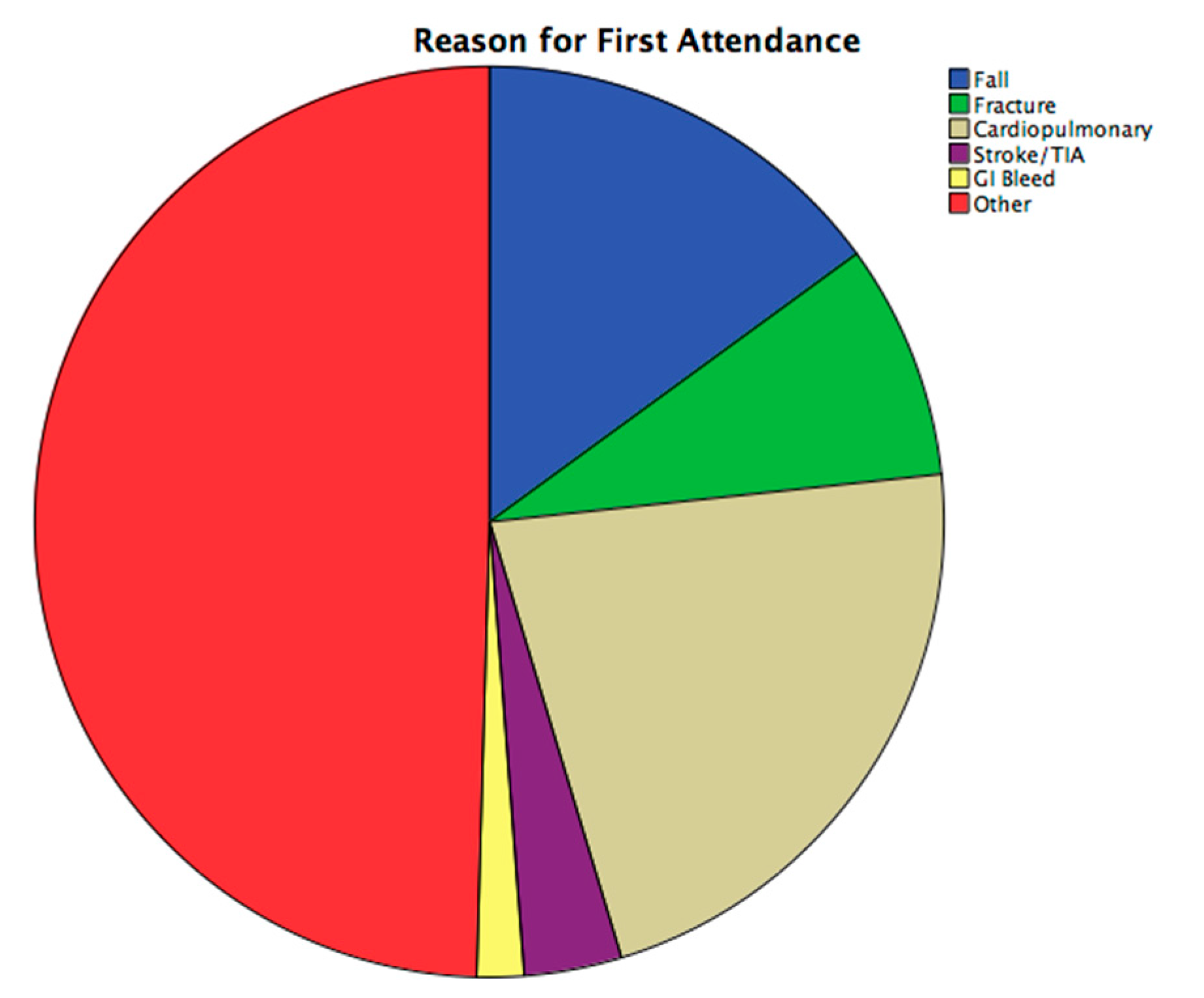
2.2. ED Attendance
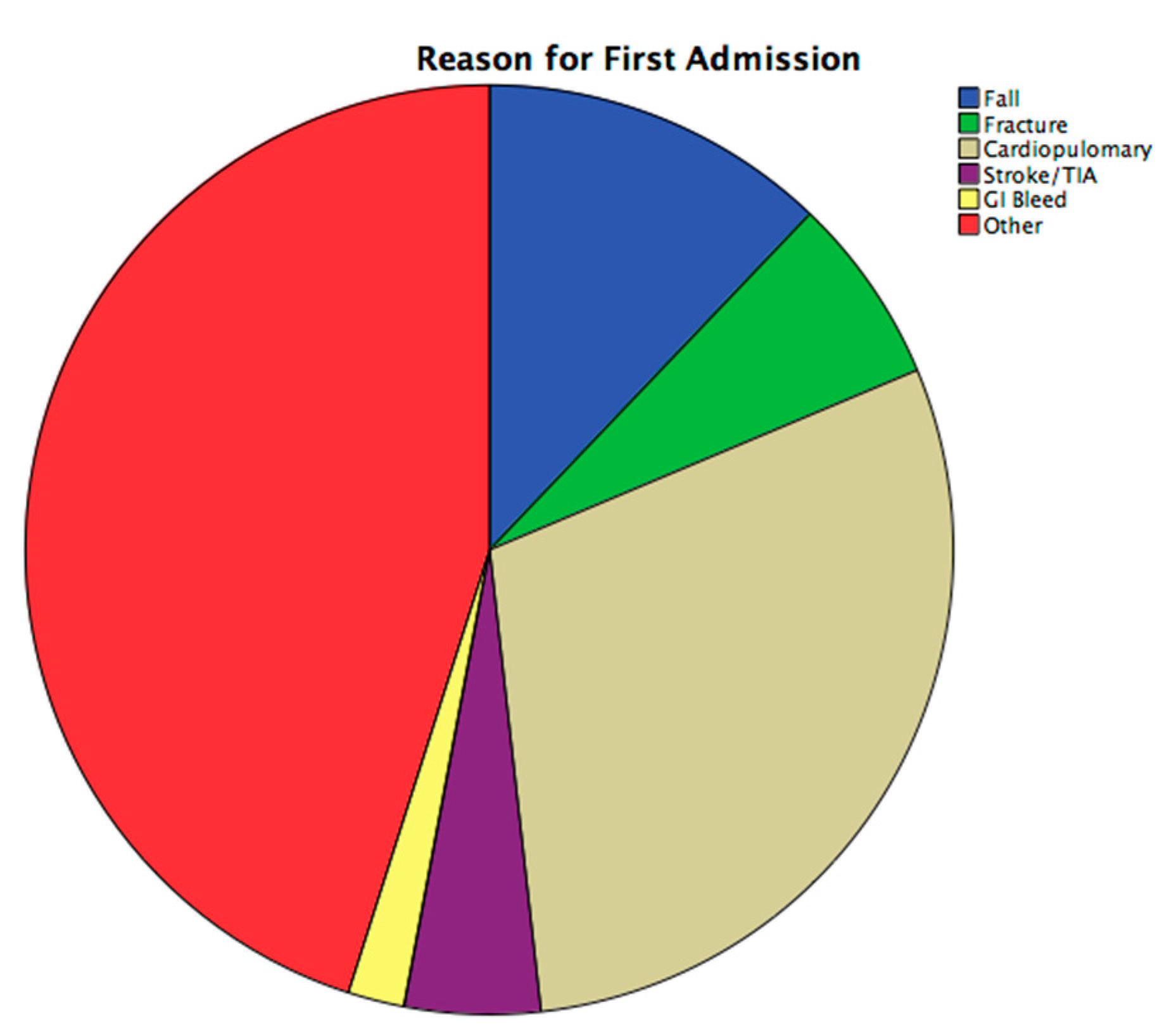
2.3. Hospital Admissions
2.4. Statistical Analysis
2.5. Covariates
3. Results
3.1. Baseline Characteristics
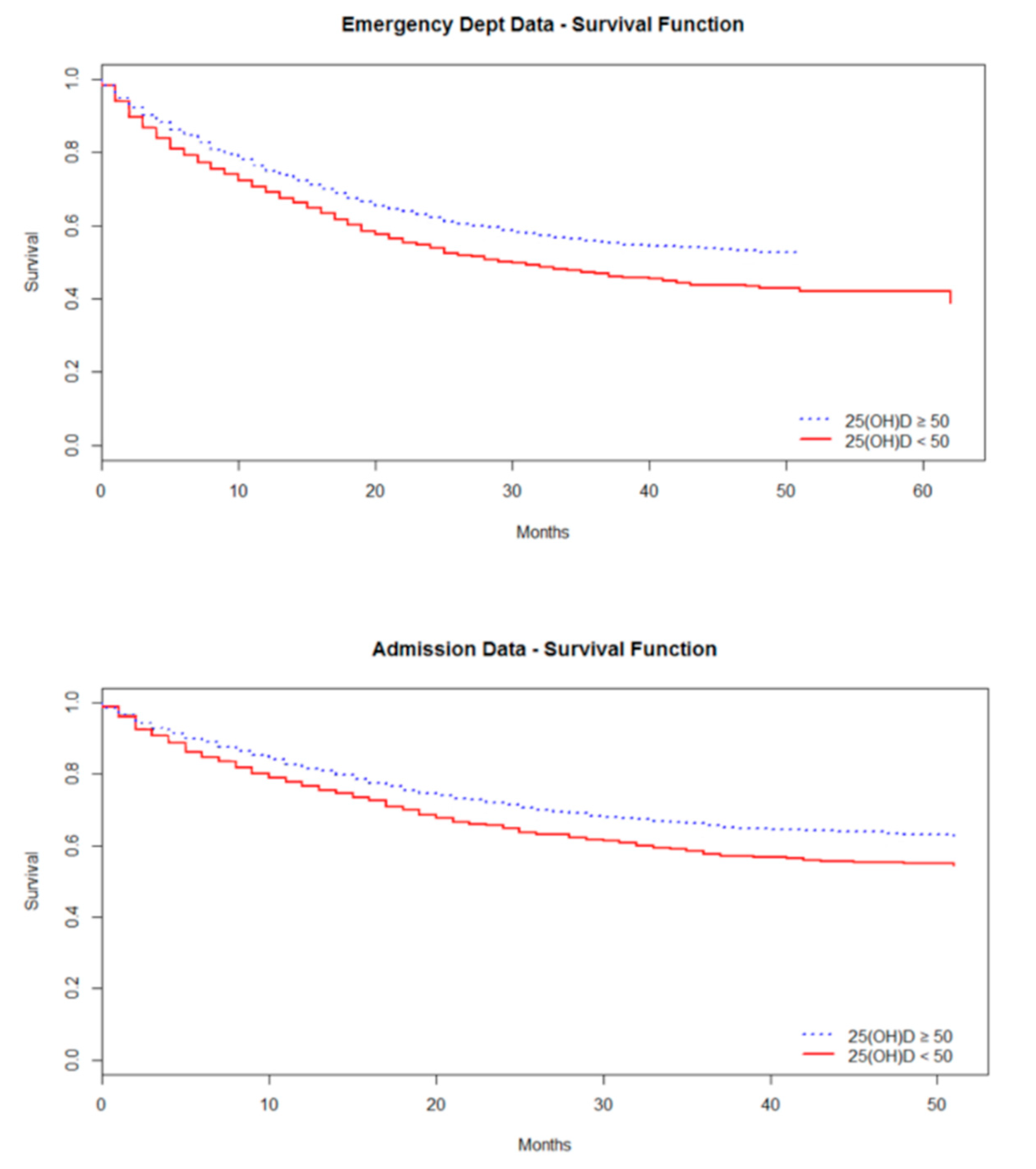
3.2. Survival Analysis: ED Attendance
3.3. Survival Analysis: Hospital Admission
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Willett, W.C.; Staehelin, H.B.; Bazemore, M.G.; Zee, R.Y.; Wong, J.B. Effect of Vitamin D on falls: A meta-analysis. JAMA 2004, 291, 1999–2006. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Willett, W.C.; Wong, J.B.; Giovannucci, E.; Dietrich, T.; Dawson-Hughes, B. Fracture prevention with vitamin D supplementation: A meta-analysis of randomized controlled trials. JAMA 2005, 293, 2257–2264. [Google Scholar] [CrossRef]
- Ginde, A.A.; Camargo, C.A., Jr.; Shapiro, N.I. Vitamin D insufficiency and sepsis severity in emergency department patients with suspected infection. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2011, 18, 551–554. [Google Scholar] [CrossRef]
- Jovanovich, A.J.; Ginde, A.A.; Holmen, J.; Jablonski, K.; Allyn, R.L.; Kendrick, J.; Chonchol, M. Vitamin D level and risk of community-acquired pneumonia and sepsis. Nutrients 2014, 6, 2196–2205. [Google Scholar] [CrossRef] [Green Version]
- Leow, L.; Simpson, T.; Cursons, R.; Karalus, N.; Hancox, R.J. Vitamin D, innate immunity and outcomes in community acquired pneumonia. Respirology 2011, 16, 611–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pletz, M.W.; Terkamp, C.; Schumacher, U.; Rohde, G.; Schutte, H.; Welte, T.; Bals, R. Vitamin D deficiency in community-acquired pneumonia: Low levels of 1,25(OH)2 D are associated with disease severity. Respir. Res. 2014, 15, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhesi, J.K.; Jackson, S.H.; Bearne, L.M.; Moniz, C.; Hurley, M.V.; Swift, C.G.; Allain, T.J. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing 2004, 33, 589–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghafouri, H.B.; Zare, M.; Bazrafshan, A.; Modirian, E.; Mousavi, A.; Abazarian, N. The association between serum 25-hydroxyvitamin D level and recurrent falls in the elderly population: A cohort study. Electron. Physician 2016, 8, 2707–2712. [Google Scholar] [CrossRef] [Green Version]
- Scragg, R.; Waayer, D.; Stewart, A.W.; Lawes, C.M.; Toop, L.; Murphy, J.; Khaw, K.T.; Camargo, C.A., Jr. The Vitamin D Assessment (ViDA) Study: Design of a randomized controlled trial of vitamin D supplementation for the prevention of cardiovascular disease, acute respiratory infection, falls and non-vertebral fractures. J. Steroid Biochem. Mol. Biol. 2015. [Google Scholar] [CrossRef]
- Snijder, M.B.; Van Schoor, N.M.; Pluijm, S.M.; Van Dam, R.M.; Visser, M.; Lips, P. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J. Clin. Endocrinol. Metab. 2006, 91, 2980–2985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunadian, V.; Ford, G.A.; Bawamia, B.; Qiu, W.; Manson, J.E. Vitamin D deficiency and coronary artery disease: A review of the evidence. Am. Heart J. 2014, 167, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Cumhur Cure, M.; Cure, E.; Yuce, S.; Yazici, T.; Karakoyun, I.; Efe, H. Mean platelet volume and vitamin D level. Ann. Lab. Med. 2014, 34, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; O’Keefe, J.H.; Bell, D.; Hensrud, D.D.; Holick, M.F. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J. Am. Coll. Cardiol. 2008, 52, 1949–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feart, C.; Helmer, C.; Merle, B.; Herrmann, F.R.; Annweiler, C.; Dartigues, J.F.; Delcourt, C.; Samieri, C. Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer’s disease in older adults. Alzheimer Dement. J. Alzheimer Assoc. 2017. [Google Scholar] [CrossRef] [PubMed]
- Licher, S.; De Bruijn, R.; Wolters, F.J.; Zillikens, M.C.; Ikram, M.A.; Ikram, M.K. Vitamin D and the Risk of Dementia: The Rotterdam Study. J. Alzheimer Dis. JAD 2017, 60, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Olsson, E.; Byberg, L.; Karlstrom, B.; Cederholm, T.; Melhus, H.; Sjogren, P.; Kilander, L. Vitamin D is not associated with incident dementia or cognitive impairment: An 18-y follow-up study in community-living old men. Am. J. Clin. Nutr. 2017, 105, 936–943. [Google Scholar] [CrossRef]
- Alley, D.E.; Crimmins, E.M.; Karlamangla, A.; Hu, P.; Seeman, T.E. Inflammation and rate of cognitive change in high-functioning older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Brewer, L.D.; Thibault, V.; Chen, K.C.; Langub, M.C.; Landfield, P.W.; Porter, N.M. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J. Neurosci. 2001, 21, 98–108. [Google Scholar] [CrossRef]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Hayes, C.E.; Nashold, F.E.; Spach, K.M.; Pedersen, L.B. The immunological functions of the vitamin D endocrine system. Cell Mol. Biol. 2003, 49, 277–300. [Google Scholar]
- Korsching, S.; Auburger, G.; Heumann, R.; Scott, J.; Thoenen, H. Levels of nerve growth factor and its mRNA in the central nervous system of the rat correlate with cholinergic innervation. EMBO J. 1985, 4, 1389–1393. [Google Scholar] [CrossRef]
- McCarroll, K.; Beirne, A.; Casey, M.; McNulty, H.; Ward, M.; Hoey, L.; Molloy, A.; Laird, E.; Healy, M.; Strain, J.J.; et al. Determinants of 25-hydroxyvitamin D in older Irish adults. Age Ageing 2015, 44, 847–853. [Google Scholar] [CrossRef] [Green Version]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar] [PubMed]
- R Devlopment Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Del Valle, H.B.; Yaktine, A.L.; Taylor, C.L.; Ross, A.C. Dietary Reference Intakes for Calcium and Vitamin D; Institute of Medicine The National Academic Press: Washington, DC, USA, 2011; p. 1132. [Google Scholar]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl-Riise, H.; Spielau, U.; Ranhoff, A.H.; Gudbrandsen, O.A.; Dierkes, J. Vitamin D supplementation and its influence on muscle strength and mobility in community-dwelling older persons: A systematic review and meta-analysis. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2017, 30, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Huang, P.; Liu, P.; Hao, Q.; Chen, S.; Dong, B.; Wang, J. Association of vitamin D deficiency and frailty: A systematic review and meta-analysis. Maturitas 2016, 94, 70–76. [Google Scholar] [CrossRef]
- Avenell, A.; Mak, J.C.; O’Connell, D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst. Rev. 2014, 4, CD000227. [Google Scholar] [CrossRef] [PubMed]
- Boonen, S.; Lips, P.; Bouillon, R.; Bischoff-Ferrari, H.A.; Vanderschueren, D.; Haentjens, P. Need for additional calcium to reduce the risk of hip fracture with vitamin d supplementation: Evidence from a comparative metaanalysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2007, 92, 1415–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amrein, K.; Litonjua, A.A.; Moromizato, T.; Quraishi, S.A.; Gibbons, F.K.; Pieber, T.R.; Camargo, C.A., Jr.; Giovannucci, E.; Christopher, K.B. Increases in pre-hospitalization serum 25(OH)D concentrations are associated with improved 30-day mortality after hospital admission: A cohort study. Clin. Nutr. 2016, 35, 514–521. [Google Scholar] [CrossRef]
- Beauchet, O.; Launay, C.; De Decker, L.; Fantino, B.; Kabeshova, A.; Annweiler, C. Who is at risk of long hospital stay among patients admitted to geriatric acute care unit? Results from a prospective cohort study. J. Nutr. Health Aging 2013, 17, 695–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graedel, L.; Merker, M.; Felder, S.; Kutz, A.; Haubitz, S.; Faessler, L.; Kaeslin, M.; Huber, A.; Mueller, B.; Schuetz, P. Vitamin D Deficiency Strongly Predicts Adverse Medical Outcome Across Different Medical Inpatient Populations: Results from a Prospective Study. Medicine 2016, 95, e3533. [Google Scholar] [CrossRef]
- Visser, M.; Deeg, D.J.; Puts, M.T.; Seidell, J.C.; Lips, P. Low serum concentrations of 25-hydroxyvitamin D in older persons and the risk of nursing home admission. Am. J. Clin. Nutr. 2006, 84, 616–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinovschi, A.; Masoero, M.; Bellocchia, M.; Ciuffreda, A.; Solidoro, P.; Mattei, A.; Mercante, L.; Heffler, E.; Rolla, G.; Bucca, C. Severe vitamin D deficiency is associated with frequent exacerbations and hospitalization in COPD patients. Respir. Res. 2014, 15, 131. [Google Scholar] [CrossRef] [Green Version]
- Aregbesola, A.; Voutilainen, S.; Nurmi, T.; Virtanen, J.K.; Ronkainen, K.; Tuomainen, T.P. Serum 25-hydroxyvitamin D3 and the risk of pneumonia in an ageing general population. J. Epidemiol. Community Health 2013, 67, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Spiro, A.; Buttriss, J.L. Vitamin D: An overview of vitamin D status and intake in Europe. Nutr. Bull. 2014, 39, 322–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laird, E.; O’Halloran, A.M.; Carey, D.; Healy, M.; O’Connor, D.; Moore, P.; Shannon, T.; Molloy, A.M.; Kenny, R.A. The Prevalence of Vitamin D Deficiency and the Determinants of 25(OH)D Concentration in Older Irish Adults: Data From The Irish Longitudinal Study on Ageing (TILDA). J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 519–525. [Google Scholar] [CrossRef]
- Laird, E.; McNulty, H.; Ward, M.; Hoey, L.; McSorley, E.; Wallace, J.M.; Carson, E.; Molloy, A.M.; Healy, M.; Casey, M.C.; et al. Vitamin D deficiency is associated with inflammation in older Irish adults. J. Clin. Endocrinol. Metab. 2014, 99, 1807–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laird, E.; Rhodes, J.; Kenny, R.A. Vitamin D and Inflammation: Potential Implications for Severity of Covid-19. Ir. Med. J. 2020, 113, 81. [Google Scholar] [PubMed]
- McCartney, D.M.; O’Shea, P.M.; Faul, J.L.; Healy, M.J.; Byrne, G.; Griffin, T.P.; Walsh, J.B.; Byrne, D.G.; Kenny, R.A. Vitamin D and SARS-CoV-2 infection-evolution of evidence supporting clinical practice and policy development: A position statement from the Covit-D Consortium. Ir. J. Med. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristic | ED Attenders (n = 1557) | ED Non-Attenders (n = 1536) | p-Value | Admitted (n = 1269) | Not Admitted (n = 1824) | p-Value |
|---|---|---|---|---|---|---|
| Cognitive Cohort (%) | 72.7 | 37.0 | <0.001 *,b | 76.6 | 39.9 | <0.001 *,b |
| Bone Cohort (%) | 27.3 | 63.0 | <0.001 *,b | 23.4 | 60.1 | <0.001 *,b |
| Age (years), Mean (SD) | 79.2 (±7.8) | 73.7 (±8.2) | <0.001 *,a | 80.0 (±7.6) | 74.1 (±8.3) | <0.001 *,a |
| Sex, female (%) | 70.0 | 79.9 | <0.001 *,b | 68.5 | 79.8 | <0.001 *,b |
| Education (years), Mean (SD) | 10.0 (±2.5) | 12.0 (±3.5) | <0.001 *,c | 10.0 (±2.5) | 11.0 (±3.4) | <0.001 *,c |
| Living Alone (%) | 43.7 | 36.1 | <0.001 *,b | 45.0 | 36.4 | <0.001 *,b |
| Current Smoker (%) | 13.0 | 12.6 | 0.747 b | 12.9 | 12.8 | 0.912 b |
| Previous Smoker (%) | 43.9 | 37.7 | <0.001 *,b | 44.5 | 38.2 | <0.001 *,b |
| Current Alcohol (%) | 52.7 | 61.4 | <0.001 *,b | 51.0 | 61.2 | <0.001 *,b |
| Previous Alcohol (%) | 22.9 | 14.6 | <0.001 *,b | 24.2 | 15.0 | <0.001 *,b |
| 25(OH)D (nmol/L), Mean (SD) | 59.1 (±33.3) | 70.6 (±31.9) | <0.001 *,c | 58.4 (±33.6) | 69.3 (±32.0) | <0.001 *,c |
| 25(OH)D (nmol/L), Median (Range) | 55.0 (2.2–185.0) | 71.9 (3.7–288.0) | <0.001 *,c | 53.9 (2.2–185.0) | 70.4 (3.7–288.0) | <0.001 *,c |
| Vitamin D supplementation (%) | 62.8 | 67.3 | 0.012 *,b | 62.4 | 66.9 | 0.012 *,b |
| MMSE, Median (Range) | 27.0 (6.0–30.0) | 27.0 (5.0–30.0) | 0.074 c | 27.0 (6.0–30.0) | 27.0 (5.0–30.0) | 0.033 c |
| HADS, Median (Range) | 2.0 (0–21.0) | 2.0 (0–21.0) | 0.574 c | 2.0 (0–21.0) | 2.0 (0–21.0) | 0.309 c |
| CES-D, Mean (SD) | 6.6 (±7.4) | 5.9 (±7.1) | 0.014 *,a | 6.5 (±7.3) | 6.0 (±7.2) | 0.056 a |
| TUG (seconds), Median (Range) | 16.0 (4.0–140.0) | 12.0 (3.0–68.0) | <0.001 *,c | 16.0 (4.0–140.0) | 12.0 (3.0–68.0) | <0.001 *,c |
| BMI (kg/m2), Mean (SD) | 26.9 (±5.3) | 26.6 (±5.3) | 0.109 a | 26.9 (±5.4) | 26.6 (±5.3) | 0.875 a |
| Waist–Hip Ratio, Mean (SD) | 0.9 (±0.1) | 0.9 (±0.1) | 0.022 a | 0.9 (±0.1) | 0.9 (±0.1) | 0.68 a |
| IADL, Mean (SD) | 22.3 (±4.6) | 23.1 (±4.6) | <0.001 *,a | 22.4 (±4.7) | 23.0 (±4.6) | <0.001 *,a |
| PSMS, Median (Range) | 24.0 (10.0–24.0) | 24.0 (9.0–24.0) | 0.292 c | 24.0 (10.0–24.0) | 24.0 (9.0–24.0) | 0.203 c |
| Hypertension (%) ** | 83.1 | 75.5 | <0.001 *,b | 83.2 | 76.6 | <0.001 *,b |
| Vascular Disease (%) ^ | 30.4 | 26.6 | 0.019 *,b | 29.6 | 27.8 | 0.267 b |
| Diabetes (%) | 11.5 | 8.4 | 0.003 *,b | 11.4 | 9.0 | 0.026 *,b |
| Atrial Fibrillation (%) | 16.0 | 14.6 | 0.270 b | 15.7 | 15.0 | 0.602 b |
| ED Attendance | β Coefficient | HR | 95% CI | pValue |
| Model 1 | −3.570 | 0.996 | 0.995–0.998 | <0.001 * |
| Model 2 | −3.644 | 0.996 | 0.994–0.998 | <0.001 * |
| Model 3 | −3.868 | 0.996 | 0.994–0.998 | <0.001 * |
| Hospital Admission | β Coefficient | HR | 95% CI | pValue |
| Model 1 | −3.485 | 0.997 | 0.995–0.998 | <0.001 * |
| Model 2 | −3.768 | 0.996 | 0.994–0.998 | <0.001 * |
| Model 3 | −4.035 | 0.996 | 0.994–0.998 | <0.001 * |
| ED Attendance | β Coefficient | RR | pValue | |
| Model 1 | −0.2519 | 0.77 | <0.001 * | |
| Model 2 | −0.2667 | 0.77 | <0.001 * | |
| Model 3 | −0.2740 | 0.76 | <0.001 * | |
| Hospital Admission | β Coefficient | RR | pValue | |
| Model 1 | −0.2521 | 0.77 | <0.0001 * | |
| Model 2 | −0.2574 | 0.77 | 0.0004 * | |
| Model 3 | −0.2638 | 0.77 | 0.0003 * | |
| Length of Stay | β Coefficient | 95% CI | p Value |
|---|---|---|---|
| Model 1 | −0.74 | −0.27–−0.03 | 0.015 * |
| Model 2 | −0.95 | −0.33–−0.56 | 0.006 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beirne, A.; McCarroll, K.; Walsh, J.B.; Casey, M.; Laird, E.; McNulty, H.; Ward, M.; Hoey, L.; Molloy, A.M.; Healy, M.; et al. Vitamin D and Hospital Admission in Older Adults: A Prospective Association. Nutrients 2021, 13, 616. https://doi.org/10.3390/nu13020616
Beirne A, McCarroll K, Walsh JB, Casey M, Laird E, McNulty H, Ward M, Hoey L, Molloy AM, Healy M, et al. Vitamin D and Hospital Admission in Older Adults: A Prospective Association. Nutrients. 2021; 13(2):616. https://doi.org/10.3390/nu13020616
Chicago/Turabian StyleBeirne, Avril, Kevin McCarroll, James Bernard Walsh, Miriam Casey, Eamon Laird, Helene McNulty, Mary Ward, Leane Hoey, Anne M. Molloy, Martin Healy, and et al. 2021. "Vitamin D and Hospital Admission in Older Adults: A Prospective Association" Nutrients 13, no. 2: 616. https://doi.org/10.3390/nu13020616







