Nutritional Intervention Contributes to the Improvement of Symptoms Related to Quality of Life in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
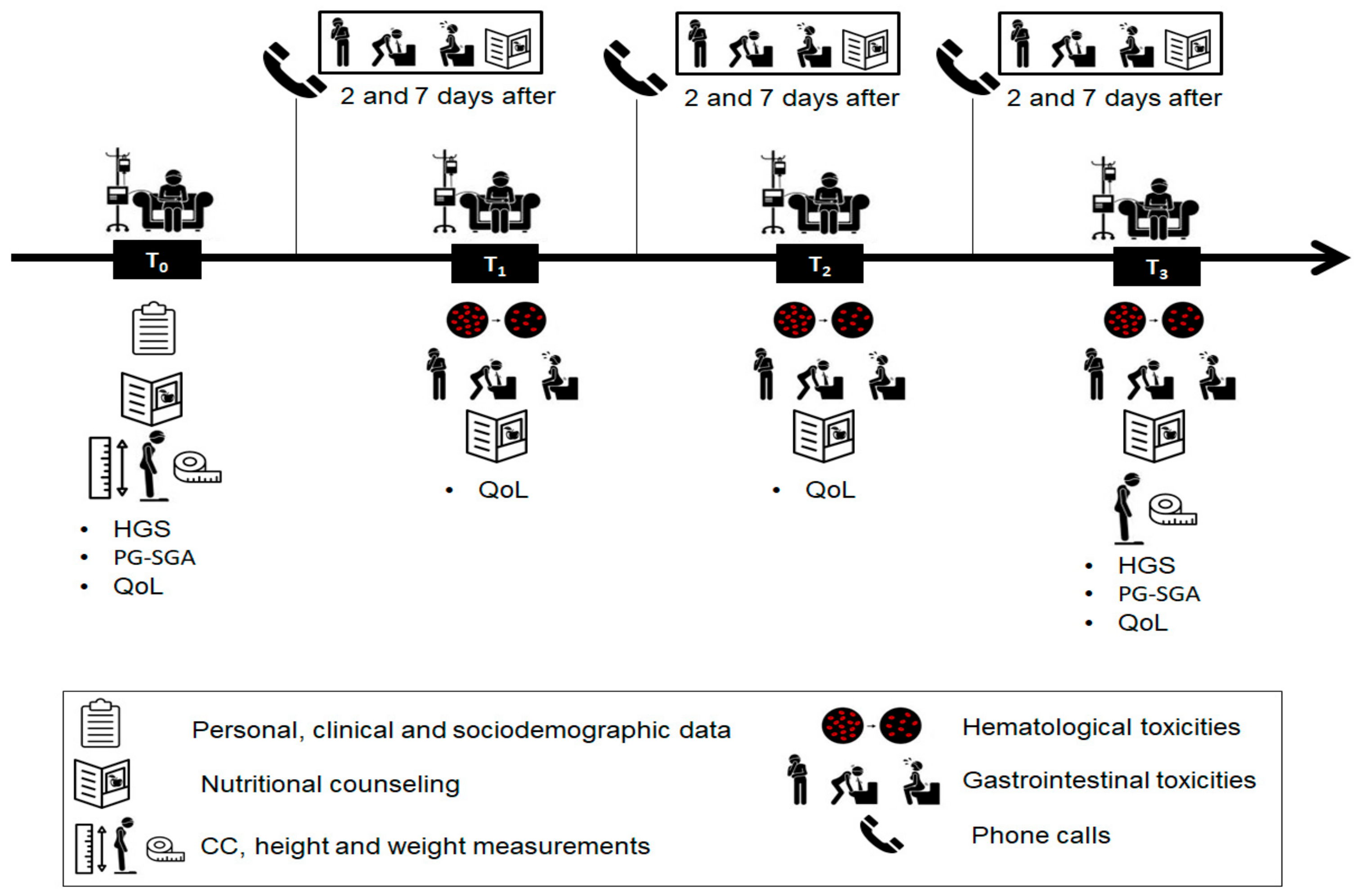
2.1. Study Design and Population
2.2. Data Collection
2.3. Nutritional Intervention
2.4. Nutritional Assessment
2.5. Quality of Life Assessment
2.6. Toxicity Assessment
2.7. Statistical Analysis
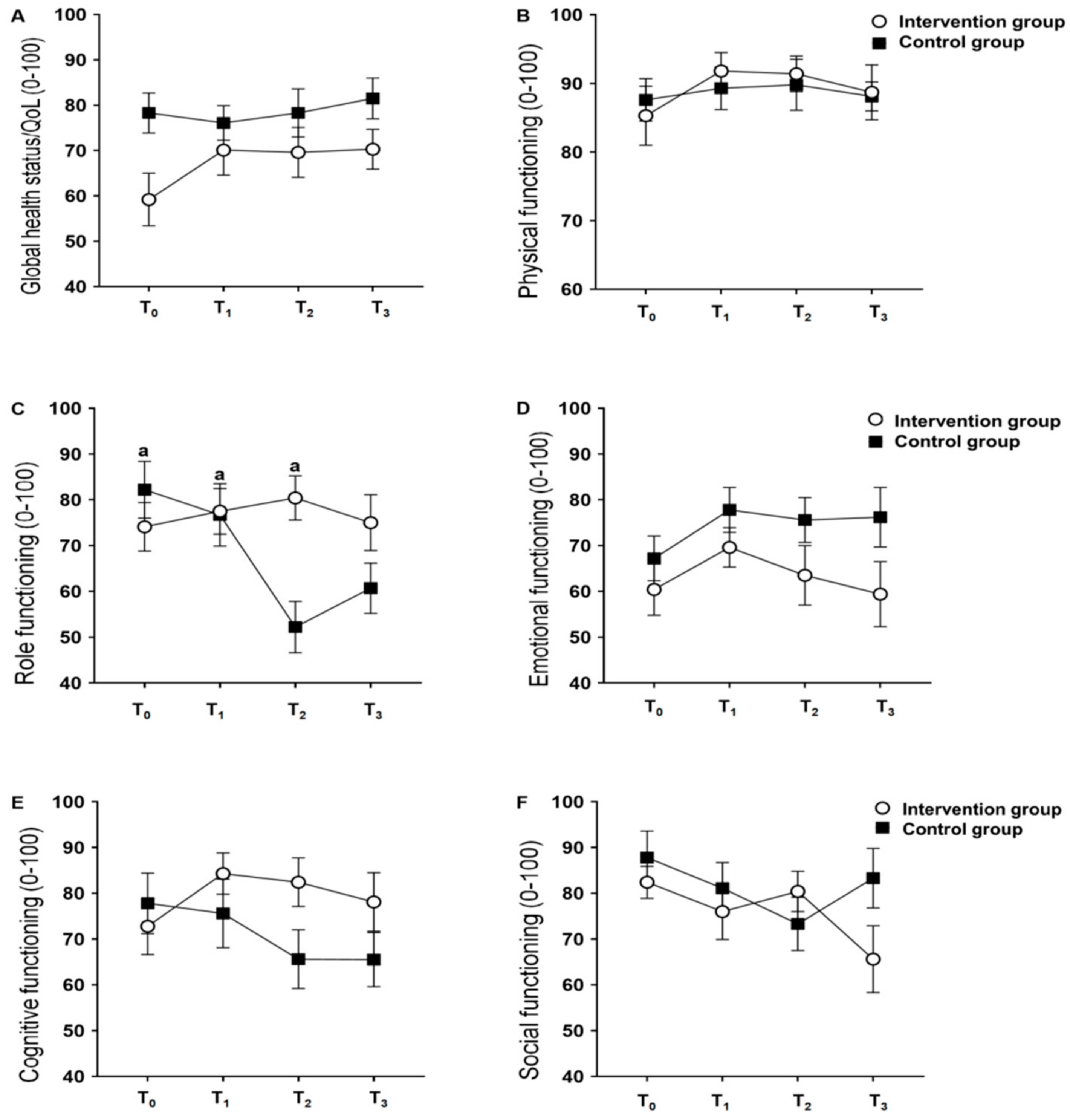
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, M.; Ciszewski, T.; Łopacka-Szatan, K.; Miotla, P.; Starosławska, E. Breast cancer risk factors. Menopausal Rev. 2015, 3, 196–202. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Da Rocha, I.M.G.; Marcadenti, A.; De Medeiros, G.O.C.; Bezerra, R.A.; Rego, J.F.D.M.; Gonzalez, M.C.; Fayh, A.P.T. Is cachexia associated with chemotherapy toxicities in gastrointestinal cancer patients? A prospective study. J. Cachex- Sarcopenia Muscle 2019, 10, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Van Soom, T.; El Bakkali, S.; Gebruers, N.; Verbelen, H.; Tjalma, W.; Van Breda, E. The effects of chemotherapy on energy metabolic aspects in cancer patients: A systematic review. Clin. Nutr. 2020, 39, 1863–1877. [Google Scholar] [CrossRef]
- Boltong, A.; Aranda, S.; Keast, R.; Wynne, R.; Francis, P.A.; Chirgwin, J.; Gough, K. A Prospective Cohort Study of the Effects of Adjuvant Breast Cancer Chemotherapy on Taste Function, Food Liking, Appetite and Associated Nutritional Outcomes. PLoS ONE 2014, 9, e103512. [Google Scholar] [CrossRef]
- De Vries, Y.C.; van den Berg, M.M.G.A.; De Vries, J.H.M.; Boesveldt, S.; De Kruif, J.T.C.M.; Buist, N.; Haringhuizen, A.; Los, M.; Sommeijer, D.W.; Timmer-Bonte, J.H.N.; et al. Differences in dietary intake during chemotherapy in breast cancer patients compared to women without cancer. Support. Care Cancer 2017, 25, 2581–2591. [Google Scholar] [CrossRef] [PubMed]
- Klepin, H.D.; Pitcher, B.N.; Ballman, K.V.; Kornblith, A.B.; Hurria, A.; Winer, E.P.; Hudis, C.; Cohen, H.J.; Muss, H.B.; Kimmick, G.G. Comorbidity, Chemotherapy Toxicity, and Outcomes Among Older Women Receiving Adjuvant Chemotherapy for Breast Cancer on a Clinical Trial: CALGB 49907 and CALGB 361004 (Alliance). J. Oncol. Pract. 2014, 10, e285–e292. [Google Scholar] [CrossRef]
- Brasil Ministério da Saúde. Diretrizes Diagnósticas e Terapêuticas Do Carcinoma de Mama; Secretaria de Atenção à Saúde. Secretaria de Ciência, Tecnologia e Insumos Estratégicos: Rio de Janeiro, Brazil, 2018.
- Dano, D.; Hénon, C.; Sarr, O.; Ka, K.; Ba, M.; Badiane, A.; Thiam, I.; Diene, P.; Diop, M.; Dem, A.; et al. Quality of Life During Chemotherapy for Breast Cancer in a West African Population in Dakar, Senegal: A Prospective Study. J. Glob. Oncol. 2019, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A.; Lio, R.M.S.; Quattrocchi, A.; Degrassi, F.; Catalano, F.; Basile, G.; Agodi, A. The Effects of Diet and Dietary Interventions on the Quality of Life among Breast Cancer Survivors: A Cross-Sectional Analysis and a Systematic Review of Experimental Studies. Cancers 2020, 12, 322. [Google Scholar] [CrossRef]
- Mohammadi, S.; Sulaiman, S.; Koon, P.B.; Amani, R.; Hosseini, S.M. Association of Nutritional Status with Quality of Life in Breast Cancer Survivors. Asian Pac. J. Cancer Prev. 2013, 14, 7749–7755. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Al-Wassia, R.; Alkhayyat, S.S.; Baig, M.; Al-Saati, B.A. Assessment of quality of life (QoL) in breast cancer patients by using EORTC QLQ-C30 and BR-23 questionnaires: A tertiary care center survey in the western region of Saudi Arabia. PLoS ONE 2019, 14, e0219093. [Google Scholar] [CrossRef]
- Al Zahrani, A.M.; Alalawi, Y.; Yagoub, U.; Saud, N.; Siddig, K. Quality of life of women with breast cancer undergoing treatment and follow-up at King Salman Armed Forces Hospital in Tabuk, Saudi Arabia. Breast Cancer Targets Ther. 2019, 11, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Villar, R.R.; Fernández, S.P.; Garea, C.C.; Pillado, M.T.S.; Barreiro, V.B.; Martín, C.G. Quality of life and anxiety in women with breast cancer before and after treatment. Rev. Latino-Am. Enferm. 2017, 25, e2958. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Haghighat, S.; Lahiji, M.R.; Razmpoosh, E.; Chamari, M.; Abdollahi, R.; Asgari, M.; Zarrati, M. Randomized Study of the Effect of Dietary Counseling During Adjuvant Chemotherapy on Chemotherapy Induced Nausea and Vomiting, and Quality of Life in Patients with Breast Cancer Randomized Study of the Effect of Dietary Counseling During Adjuvant Chemoth. Nutr. Cancer 2018, 71, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, R.; Najafi, S.; Razmpoosh, E.; Shoormasti, R.S.; Haghighat, S.; Lahiji, M.R.; Chamari, M.; Asgari, M.; Cheshmazar, E.; Zarrati, M. The Effect of Dietary Intervention Along with Nutritional Education on Reducing the Gastrointestinal Side Effects Caused by Chemotherapy Among Women with Breast Cancer. Nutr. Cancer 2019, 71, 922–930. [Google Scholar] [CrossRef]
- Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA). Consenso Nacional de Nutrição Oncológica, 2nd ed.; INCA: Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Arends, J.J.; Baracos, V.V.; Bertz, H.H.; Bozzetti, F.; Calder, P.P.; Deutz, N.; Erickson, N.N.; Laviano, A.A.; Lisanti, M.M.; Lobo, D.N.D.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Borges, L.R.; Silveira, D.H.; Assunção, M.C.F.; Orolandi, S.P. Validation of a Portuguese version of patient-generated subjective global assessment. Rev. Bras. Nutr. Clin. 2010, 25, 102–108. [Google Scholar]
- WHO. Physical Status: The Use and Interpretation of Anthropometry; Report of a WHO Expert Committee; Technical Report Series No. 854; World Health Organization: Ganeva, Switzerland, 1995; Volume 854, pp. 1–452. [Google Scholar]
- Barbosa-Silva, T.G.; Bielemann, R.M.; Gonzalez, M.C.; Menezes, A.M.B. Prevalence of sarcopenia among community-dwelling elderly of a medium-sized South American city: Results of theCOMO VAI? study. J. Cachex- Sarcopenia Muscle 2015, 7, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Sprangers, M.A.G.; Groenvold, M.; Arraras, J.I.; Franklin, J.; Velde, A.T.; Muller, M.; Franzini, L.; Williams, A.; De Haes, H.C.; Hopwood, P.; et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. J. Clin. Oncol. 1996, 14, 2756–2768. [Google Scholar] [CrossRef] [PubMed]
- Fayers, P.; Aaronson, N.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. EORTC QLQ-C30 Scoring Manual, 3rd ed.; EORTC Quality of Life Group: Brussels, Belgium, 2001. [Google Scholar]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE); Version 5.0; National Institutes of Health; National Cancer Institute: Bethesda, MD, USA, 2017.
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Leinert, E.; Singer, S.; Janni, W.; Harbeck, N.; Weissenbacher, T.; Rack, B.; Augustin, D.; Wischnik, A.; Kiechle, M.; Ettl, J.; et al. The Impact of Age on Quality of Life in Breast Cancer Patients Receiving Adjuvant Chemotherapy: A Comparative Analysis from the Prospective Multicenter Randomized ADEBAR trial. Clin. Breast Cancer 2017, 17, 100–106. [Google Scholar] [CrossRef]
- Tiezzi, M.F.B.D.M.; De Andrade, J.M.; Romão, A.P.M.S.; Tiezzi, D.G.; Lerri, M.R.; Carrara, H.A.H.; Lara, L.A.S. Quality of Life in Women With Breast Cancer Treated With or Without Chemotherapy. Cancer Nurs. 2017, 40, 108–116. [Google Scholar] [CrossRef]
- Lambert, L.K.; Balneaves, L.G.; Howard, A.F.; Gotay, C.C. Patient-reported factors associated with adherence to adjuvant endocrine therapy after breast cancer: An integrative review. Breast Cancer Res. Treat. 2018, 167, 615–633. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Feng, Z.; Xu, Y.; Zeng, G. Longitudinal Trends in Anxiety, Depression, and Quality of Life During Different Intermittent Periods of Adjuvant Breast Cancer Chemotherapy. Cancer Nurs. 2018, 41, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.E.; Hiller, L.; Dorling, L.; Vallier, A.-L.; Dunn, J.A.; Bowden, S.J.; Ingle, S.; Jones, L.; Hardy, R.; Twelves, C.; et al. A nested cohort study of 6,248 early breast cancer patients treated in neoadjuvant and adjuvant chemotherapy trials investigating the prognostic value of chemotherapy-related toxicities. BMC Med. 2015, 13, 306. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, B.; Badalamenti, G.; Armento, G.; Silletta, M.; Ceruso, M.S.; Catania, G.; Napolitano, A.; Maltese, G.; Valeri, S.; Incorvaia, L.; et al. Body Mass Index as a Risk Factor for Toxicities in Patients with Advanced Soft-Tissue Sarcoma Treated with Trabectedin. Oncology 2018, 95, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca, F.; Onesti, C.E.; Roberto, M.; Di Girolamo, M.; Botticelli, A.; Begini, P.; Strigari, L.; Marchetti, P.; Muscaritoli, M. Lean body mass wasting and toxicity in early breast cancer patients receiving anthracyclines. Oncotarget 2018, 9, 25714–25722. [Google Scholar] [CrossRef]
- Friese, C.R.; Bsn, J.M.H.; Janz, N.K.; Jagsi, R.; Morrow, M.; Li, Y.; Hamilton, A.S.; Ward, K.C.; Kurian, A.W.; Katz, S.J.; et al. Treatment-associated toxicities reported by patients with early-stage invasive breast cancer. Cancer 2017, 123, 1925–1934. [Google Scholar] [CrossRef]
- Neil-Sztramko, S.E.; Kirkham, A.A.; Hung, S.H.; Niksirat, N.; Nishikawa, K.; Campbell, K.L. Aerobic capacity and upper limb strength are reduced in women diagnosed with breast cancer: A systematic review. J. Physiother. 2014, 60, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.; Ordan, M.; Barbe, C.; Mazza, C.; Perrier, M.; Botsen, D.; Brasseur, M.; Portefaix, C.; Renard, Y.; Tallière, B.; et al. Correlation between muscle mass and handgrip strength in digestive cancer patients undergoing chemotherapy. Cancer Med. 2019, 8, 3677–3684. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Medysky, M.E.; Savin, M. Patient-reported and objectively measured physical function in older breast cancer survivors and cancer-free controls. J. Geriatr. Oncol. 2019, 10, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Lintermans, A.; Van Asten, K.; Wildiers, H.; Laenen, A.; Paridaens, R.; Weltens, C.; Verhaeghe, J.; Vanderschueren, D.; Smeets, A.; Van Limbergen, E.; et al. A prospective assessment of musculoskeletal toxicity and loss of grip strength in breast cancer patients receiving adjuvant aromatase inhibitors and tamoxifen, and relation with BMI. Breast Cancer Res. Treat. 2014, 146, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Extermann, M.; Leeuwenburgh, C.; Samiian, L.; Sehovic, M.; Xu, J.; Cubitt, C.; Jacobsen, P.B.; Pahor, M.; Grobmyer, S.R.; Manini, T.M. Impact of chemotherapy on medium-term physical function and activity of older breast cancer survivors, and associated biomarkers. J. Geriatr. Oncol. 2017, 8, 69–75. [Google Scholar] [CrossRef]
- Guo, C.-B.; Zhang, W.; Ma, D.-Q.; Zhang, K.-H.; Huang, J.-Q. Hand grip strength: An indicator of nutritional state and the mix of postoperative complications in patients with oral and maxillofacial cancers. Br. J. Oral Maxillofac. Surg. 1996, 34, 325–327. [Google Scholar] [CrossRef]
- Lakenman, P.; Ottens-Oussoren, K.; Nierop, J.W.-V.; Van Der Peet, N.; De Van Der Schueren, M.A.E. Handgrip Strength Is Associated with Treatment Modifications During Neoadjuvant Chemoradiation in Patients with Esophageal Cancer. Nutr. Clin. Pract. 2017, 32, 652–657. [Google Scholar] [CrossRef]
- Celis-Morales, C.A.; Welsh, P.; Lyall, D.M.; Steell, L.; Petermann, F.; Anderson, J.; Iliodromiti, S.; Sillars, A.; Graham, N.; Mackay, D.F.; et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: Prospective cohort study of half a million UK Biobank participants. BMJ 2018, 361, k1651. [Google Scholar] [CrossRef] [PubMed]

| Variables | Intervention Group (n = 19) | Control Group (n = 15) | p |
|---|---|---|---|
| Age (years) | 44.3 ± 9.2 | 45.5 ± 8.6 | 0.697 |
| Ethnicity, n (%) | |||
| Caucasian | 5 (62.5) | 3 (37.5) | 1.000 |
| Non-Caucasian | 14 (53.8) | 12 (46.2) | |
| Education, n (%) | |||
| Up to elementary school | 4 (50.0) | 4 (50.0) | 1.000 |
| High school/Higher | 15 (57.7) | 11 (42.3) | |
| Income | |||
| Up to 1 MW | 7 (53.8) | 6 (46.2) | 0.933 |
| >1 MW | 10 (58.8) | 7 (41.2) | |
| Not informed | 2 (50.0) | 2 (50.0) | |
| Reproductive history, n (%) | |||
| With children | 14 (48.3) | 15 (51.7) | 0.053 |
| Without children | 5 (100.0) | 0 (0.0) | |
| Breastfeeding, n (%) | |||
| Yes | 13 (52.0) | 12 (48.0) | 0.697 |
| No | 6 (66.7) | 3 (33.3) | |
| Menopause, n (%) | |||
| Yes | 5 (55.6) | 4 (44.4) | 1.000 |
| No | 14 (41.2) | 11 (44.0) | |
| Physical activity, n (%) | |||
| Perform | 3 (60.0) | 2 (40.0) | 1.000 |
| Do not perform | 16 (55.2) | 13 (44.8) | |
| PG-SGA, n (%) | |||
| A | 17 (60.7) | 11 (39.4) | 0.370 |
| B e C | 2 (33.3) | 4 (66.7) | |
| BMI classification, n (%) | |||
| Low weight | 0 (0.0) | 1 (100.0) | 0.371 |
| Eutrophic | 6 (60.0) | 4 (40.0) | |
| Overweight | 10 (66.7) | 5 (33.3) | |
| Obesity | 3 (37.5) | 5 (62.5) | |
| Reduced HGS (<16 kgF), n (%) | |||
| Yes | 2 (40.0) | 4 (60.0) | 1.000 |
| No | 16 (55.2) | 13 (44.8) | |
| Reduced CC (<33 cm), n (%) | |||
| Yes | 2 (33.3) | 4 (66.7) | 0.336 |
| No | 9 (64.3) | 5 (35.7) | |
| Comorbidities, n (%) | |||
| With comorbidities | 3 (60.0) | 2 (40.0) | 1.000 |
| No comorbidities | 16 (55.2) | 13 (44.8) | |
| Staging (TNM) | |||
| II | 12 (54.5) | 10 (45.5) | 0.666 |
| III | 6 (54.5) | 5 (45.5) | |
| Undefined | 1 (100.0) | 0 (0.0) | |
| Molecular subtype | |||
| Luminal A | 5 (62.5) | 3 (37.5) | 0.481 |
| Luminal B | 9 (60.0) | 6 (40.0) | |
| HER2+ | 3 (75.0) | 1 (25.0) | |
| Negative triple | 1 (20.0) | 4 (80.0) | |
| Undefined | 1 (50.0) | 1 (50.0) |
| Variables | Intervention Group (n = 19) | Control Group (n = 15) | ||||
|---|---|---|---|---|---|---|
| T0 | T3 | p | T0 | T3 | p | |
| Weight (kg) | 63.8 ± 5.7 | 62.9 ± 5.7 | 0.189 | 69.4 ± 19.4 | 68.4 ± 18.2 | 0.184 |
| BMI (kg/m2) | 26.9 ± 2.5 | 25.8 ± 2.7 | 0.215 | 27.8 ± 6.1 | 27.4 ± 5.8 | 0.245 |
| CC (cm) | 34.6 ± 2.2 | 34.4 ± 2.1 | 0.319 | 36.1 ± 5.7 | 35.9 ± 5.4 | 0.89 |
| HGS (kgF) | 19.2 ± 4.6 | 17.8 ± 4.9 | 0.125 | 21.6 ± 59 | 18.8 ± 4.0 | 0.009 |
| Symptom Scales | Intervention Group (n = 19) | Control Group (n = 15) | pInteraction | ptime | pgroup | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T0 | T1 | T2 | T3 | ||||
| Fatigue | 27.0 ± 3.8 | 28.6 ± 7.0 | 27.2 ± 6.1 | 32.1 ± 7.3 | 29.3 ± 7.5 | 26.7 ± 5.5 | 26.9 ± 4.2 | 33.3 ± 5.2 | 0.948 | 0.446 | 0.962 |
| Cohen’s ds | 0.4 | 0.3 | 0.06 | 0.19 | |||||||
| Nausea/vomiting | 28.6 ± 5.5 | 44.4 ± 22.7 | 33.3 ± 0.1 | 16.7 ± 0.1 | 27.8 ± 9.1 | 16.7 ± 0.1 a | 21.4 ± 2.8 a | 23.3 ± 3.7 | <0.001 | 0.018 | 0.065 |
| Cohen’s ds | 0.11 | 1.64 | 6.37 | 2.67 | |||||||
| Pain | 38.1 ± 6.2 | 28.6 ± 8.7 | 41.7 ± 9.3 | 43.7 ± 9.3 | 38.9 ± 9.4 | 26.7 ± 7.9 | 45.8 ± 16.0 | 38.1 ± 8.0 | 0.936 | 0.653 | 0.932 |
| Cohen’s ds | 0.10 | 0.23 | 0.32 | 0.64 | |||||||
| Insomnia | 63.3 ± 7.4 | 53.3 ± 7.3 | 53.3 ± 11.9 | 52.4 ± 9.2 | 73.3 ± 11.2 | 61.9 ± 10.5 | 70.8 ± 7.1 | 52.4 ± 6.2 | 0.872 | 0.266 | 0.160 |
| Cohen’s ds | 1.08 | 0.97 | 1.74 | 0.00 | |||||||
| Financial difficulties | 83.3 ± 5.6 | 83.3 ± 7.1 | 81.0 ± 6.5 | 77.8 ± 7.2 | 63.0 ± 9.7 | 70.4 ± 9.7 | 74.1 ± 10.2 | 59.3 ± 10.2 | 0.498 | 0.275 | 0.108 |
| Cohen’s ds | 2.64 | 2.43 | 0.83 | 2.14 | |||||||
| Appetite loss | 80.0 ± 11.9 | 33.3 ± 0.1 b | 50.0 ± 11.8 | 33.3 ± 0.1 b | 50.0 ± 10.4 | 33.3 ± 0.1 | 41.7 ± 7.2 | 38.9 ± 5.1 | 0.199 | <0.001 | 0.173 |
| Cohen’s ds | 2.66 | 0.00 | 0.83 | 1.65 | |||||||
| Dyspnea | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 16.7) | 0.0 (0.0 a 0.0) | - | - | - |
| Constipation | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 33.3) | 0.0 (0.0 a 33.3) | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 0.0) | - | - | - |
| Diarrhea | 0.0 (0.0 a 33.3) | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 0.0) | 0.0 (0.0 a 0.0) | - | - | - |
| Variables | T1 | T2 | T3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IG (n = 19) | CG (n = 15) | p | IG (n = 19) | CG (n = 15) | p | IG (n = 19) | CG (n = 15) | p | ||
| Hematological toxicities | ||||||||||
| Anemia | ||||||||||
| Yes | 5 (41.7) | 7 (58.3) | 0.218 | 8 (47.1) | 9 (52.9) | 0.464 | 11 (52.4) | 10 (47.6) | 0.907 | |
| No | 14 (63.6) | 8 (36.4) | 9 (60.0) | 6 (40.0) | 6 (54.5) | 5 (45.5) | ||||
| Leukopenia | ||||||||||
| Yes | 7 (58.3) | 5 (41.7) | 0.832 | 5 (62.5) | 3 (37.5) | 0.691 | 3 (27.3) | 8 (72.7) | 0.034 | |
| No | 12 (54.5) | 10 (45.5) | 12 (50.0) | 12 (50.0) | 14 (66.7) | 7 (33.3) | ||||
| Neutropenia | ||||||||||
| Yes | 7 (70.0) | 3 (30.0) | 0.451 | 4 (80.0) | 1 (20.0) | 0.338 | 2 (28.6) | 5 (71.4) | 0.209 | |
| No | 12 (50.0) | 12 (50.0) | 13 (48.1) | 14 (51.9) | 15 (60.0) | 10 (40.0) | ||||
| Thrombocytopenia | ||||||||||
| Yes | 0 (0.0) | 1 (100.0) | 0.441 | - | - | - | 0 (0.0) | 1 (100.0) | 0.469 | |
| No | 19 (57.6) | 14 (42.4) | 15 (46.9) | 17 (53.1) | 17 (54.8) | 14 (45.2) | ||||
| Gastrointestinal toxicities | ||||||||||
| Nausea | ||||||||||
| Yes | 19 (55.9) | 15 (44.1) | - | 16 (51.6) | 15 (48.4) | 1.000 | 16 (51.6) | 15 (48.4) | 1.000 | |
| No | - | - | 1 (100.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | ||||
| Vomiting | ||||||||||
| Yes | 10 (45.5) | 12 (54.5) | 0.097 | 3 (50.0) | 3 (50.0) | 1000 | 3 (37.5) | 5 (62.5) | 0.423 | |
| No | 9 (75.0) | 3 (25.0) | 14 (53.8) | 12 (46.2) | 14 (58.3) | 10 (41.7) | ||||
| Diarrhea | ||||||||||
| Yes | 2 (33.3) | 4 (66.7) | 0.370 | 1 (50.0) | 1 (50.0) | 1.000 | - | - | - | |
| No | 17 (60.7) | 11 (39.3) | 16 (53.3) | 14 (46.7) | 17 (53.1) | 15 (46.9) | ||||
| Anorexia | ||||||||||
| Yes | 11 (52.4) | 10 (47.6) | 0.728 | 11 (52.4) | 10 (47.6) | 0.907 | 8 (44.4) | 10 (55.6) | 0.265 | |
| No | 8 (61.5) | 5 (38.5) | 6 (54.5) | 5 (45.5) | 9 (64.3) | 5 (35.7) | ||||
| Abdominal pain | ||||||||||
| Yes | 7 (43.8) | 9 (56.3) | 0.179 | 3 (27.3) | 8 (72.2) | 0.034 | 2 (28.6) | 5 (71.4) | 0.209 | |
| No | 12 (66.7) | 6 (33.3) | 14 (66.7) | 7 (33.3) | 15 (60.0) | 10 (40.0) | ||||
| Constipation | ||||||||||
| Yes | 5 (38.5) | 8 (61.5) | 0.107 | 7 (50.0) | 7 (50.0) | 0.755 | 2 (25.0) | 6 (75.0) | 0.106 | |
| No | 14 (66.7) | 7 (33.3) | 10 (55.6) | 8 (44.4) | 15 (62.5) | 9 (37.5) | ||||
| Mucositis | ||||||||||
| Yes | 0 (0.0) | 2 (100.0) | 0.187 | 3 (42.9) | 4 (57.1) | 0.678 | 3 (42.9) | 4 (57.1) | 0.678 | |
| No | 19 (59.4) | 13 (40.6) | 14 (56.0) | 11 (44.0) | 14 (56.0) | 11 (44.0) | ||||
| Weight loss | ||||||||||
| Yes | 1 (100.0) | 0 (0.0) | 1.000 | - | - | - | 0 (0.0) | 1 (100.0) | 0.469 | |
| No | 18 (54.5) | 15 (45.5) | 17 (53.1) | 15 (46.9) | 17 (54.8) | 14 (45.2) | ||||
| Presence of dose-limiting toxicity, n (%) | ||||||||||
| Yes | 0 (0.0) | 2 (100.0) | 0.187 | 3 (100.0) | 0 (0.0) | 0.229 | 0 (0.0) | 2 (100.0) | 0.212 | |
| No | 19 (59.4) | 13 (40.6) | 14 (48.3) | 15 (51.7) | 17 (56.7) | 13 (43.3) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, A.P.S.; da Silva, L.C.; Fayh, A.P.T. Nutritional Intervention Contributes to the Improvement of Symptoms Related to Quality of Life in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy: A Randomized Clinical Trial. Nutrients 2021, 13, 589. https://doi.org/10.3390/nu13020589
de Souza APS, da Silva LC, Fayh APT. Nutritional Intervention Contributes to the Improvement of Symptoms Related to Quality of Life in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy: A Randomized Clinical Trial. Nutrients. 2021; 13(2):589. https://doi.org/10.3390/nu13020589
Chicago/Turabian Stylede Souza, Ana Priscilla Silva, Luciana Câmara da Silva, and Ana Paula Trussardi Fayh. 2021. "Nutritional Intervention Contributes to the Improvement of Symptoms Related to Quality of Life in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy: A Randomized Clinical Trial" Nutrients 13, no. 2: 589. https://doi.org/10.3390/nu13020589
APA Stylede Souza, A. P. S., da Silva, L. C., & Fayh, A. P. T. (2021). Nutritional Intervention Contributes to the Improvement of Symptoms Related to Quality of Life in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy: A Randomized Clinical Trial. Nutrients, 13(2), 589. https://doi.org/10.3390/nu13020589






