The Use of Lower or Higher Than Recommended Doses of Folic Acid Supplements during Pregnancy Is Associated with Child Attentional Dysfunction at 4–5 Years of Age in the INMA Project
Abstract
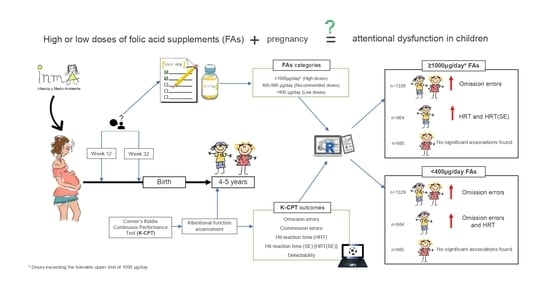
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Dietary Folate and FAs Intake during Pregnancy
2.3. Children’s Attentional Function Assessment
2.4. Other Variables
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Georgieff, M.K.; Brunette, K.E.; Tran, P.V. Early Life Nutrition and Neural Plasticity. Dev. Psychopathol. 2015, 27, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Fernstrom, J.D. Can Nutrient Supplements Modify Brain Function? Am. J. Clin. Nutr. 2000, 71, 1669S–1673S. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, E.S.; Conus, N.; Kaput, J. B Vitamin Polymorphisms and Behavior: Evidence of Associations with Neurodevelopment, Depression, Schizophrenia, Bipolar Disorder and Cognitive Decline. Neurosci. Biobehav. Rev. 2014, 47, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F.; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development-Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef] [Green Version]
- Lassi, Z.S.; Salam, R.A.; Haider, B.A.; Bhutta, Z.A. Folic Acid Supplementation during Pregnancy for Maternal Health and Pregnancy Outcomes. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Desai, A.; Sequeira, J.M.; Quadros, E.V. The Metabolic Basis for Developmental Disorders Due to Defective Folate Transport. Biochimie 2016, 126, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Kuizon, S.; Brown, W.T.; Junaid, M.A. High Gestational Folic Acid Supplementation Alters Expression of Imprinted and Candidate Autism Susceptibility Genes in a Sex-Specific Manner in Mouse Offspring. J. Mol. Neurosci. 2016, 58, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Prevention of Neural Tube Defects: Results of the Medical Research Council Vitamin Study. Available online: https://pubmed.ncbi.nlm.nih.gov/1677062/ (accessed on 14 December 2020).
- Institute of Medicine (US). Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 1998; ISBN 978-0-309-06411-8. [Google Scholar]
- Navarrete-Muñoz, E.M.; Giménez Monzó, D.; García de La Hera, M.; Climent, M.D.; Rebagliato, M.; Murcia, M.; Iñiguez, C.; Ballester, F.; Ramón, R.; Vioque, J. Folic acid intake from diet and supplements in a population of pregnant women in Valencia, Spain. Med. Clin. (Barc.) 2010, 135, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, A.; McNulty, H.; Irwin, R.E.; Walsh, C.P.; Pentieva, K. Maternal Folate Nutrition and Offspring Health: Evidence and Current Controversies. Proc. Nutr. Soc. 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.M.; Fealy, S.M.; Bisquera, A.; Smith, R.; Collins, C.E.; Evans, T.-J.; Hure, A.J. Effects of Nutritional Interventions during Pregnancy on Infant and Child Cognitive Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1265. [Google Scholar] [CrossRef] [Green Version]
- Veena, S.R.; Gale, C.R.; Krishnaveni, G.V.; Kehoe, S.H.; Srinivasan, K.; Fall, C.H. Association between Maternal Nutritional Status in Pregnancy and Offspring Cognitive Function during Childhood and Adolescence; a Systematic Review. BMC Pregnancy Childbirth 2016, 16, 220. [Google Scholar] [CrossRef] [Green Version]
- Navarrete-Muñoz, E.M.; Valera-Gran, D.; García de la Hera, M.; Gimenez-Monzo, D.; Morales, E.; Julvez, J.; Riaño, I.; Tardón, A.; Ibarluzea, J.; Santa-Marina, L.; et al. Use of High Doses of Folic Acid Supplements in Pregnant Women in Spain: An INMA Cohort Study. BMJ Open 2015, 5, e009202. [Google Scholar] [CrossRef] [PubMed]
- Valera-Gran, D.; García de la Hera, M.; Navarrete-Muñoz, E.M.; Fernandez-Somoano, A.; Tardón, A.; Julvez, J.; Forns, J.; Lertxundi, N.; Ibarluzea, J.M.; Murcia, M.; et al. Folic Acid Supplements during Pregnancy and Child Psychomotor Development after the First Year of Life. JAMA Pediatr. 2014, 168, e142611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valera-Gran, D.; Navarrete-Muñoz, E.M.; Garcia de la Hera, M.; Fernández-Somoano, A.; Tardón, A.; Ibarluzea, J.; Balluerka, N.; Murcia, M.; González-Safont, L.; Romaguera, D.; et al. Effect of Maternal High Dosages of Folic Acid Supplements on Neurocognitive Development in Children at 4-5 y of Age: The Prospective Birth Cohort Infancia y Medio Ambiente (INMA) Study. Am. J. Clin. Nutr. 2017, 106, 878–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttenlocher, P.R.; Dabholkar, A.S. Regional Differences in Synaptogenesis in Human Cerebral Cortex. J. Comp. Neurol. 1997, 387, 167–178. [Google Scholar] [CrossRef]
- Tsujimoto, S. The Prefrontal Cortex: Functional Neural Development during Early Childhood. Neuroscientist 2008, 14, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Jan, Y.-N.; Jan, L.Y. Branching out: Mechanisms of Dendritic Arborization. Nat. Rev. Neurosci. 2010, 11, 316–328. [Google Scholar] [CrossRef]
- Harlan De Crescenzo, A.; Panoutsopoulos, A.A.; Tat, L.; Schaaf, Z.; Racherla, S.; Henderson, L.; Leung, K.-Y.; Greene, N.D.E.; Green, R.; Zarbalis, K.S. Deficient or Excess Folic Acid Supply During Pregnancy Alter Cortical Neurodevelopment in Mouse Offspring. Cereb. Cortex 2021, 31, 635–649. [Google Scholar] [CrossRef]
- Razza, R.A.; Martin, A.; Brooks-Gunn, J. Associations among Family Environment, Sustained Attention, and School Readiness for Low-Income Children. Dev. Psychol. 2010, 46, 1528–1542. [Google Scholar] [CrossRef] [Green Version]
- White, R.F.; Campbell, R.; Echeverria, D.; Knox, S.S.; Janulewicz, P. Assessment of Neuropsychological Trajectories in Longitudinal Population-Based Studies of Children. J. Epidemiol. Community Health 2009, 63, i15–i26. [Google Scholar] [CrossRef] [Green Version]
- Prado, E.L.; Dewey, K.G. Nutrition and Brain Development in Early Life. Nutr. Rev. 2014, 72, 267–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosales, F.J.; Reznick, J.S.; Zeisel, S.H. Understanding the Role of Nutrition in the Brain and Behavioral Development of Toddlers and Preschool Children: Identifying and Addressing Methodological Barriers. Nutr. Neurosci. 2009, 12, 190–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryan, J.; Osendarp, S.; Hughes, D.; Calvaresi, E.; Baghurst, K.; Van Klinken, J.-W. Nutrients for Cognitive Development in School-Aged Children. Nutr. Rev. 2004, 62, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Rueda, M.; Pozuelos, J.; Cómbita, L. Cognitive Neuroscience of Attention From Brain Mechanisms to Individual Differences in Efficiency. AIMS Neurosci. 2015, 2, 183–202. [Google Scholar] [CrossRef]
- Naninck, E.F.G.; Stijger, P.C.; Brouwer-Brolsma, E.M. The Importance of Maternal Folate Status for Brain Development and Function of Offspring. Adv. Nutr. 2019, 10, 502–519. [Google Scholar] [CrossRef]
- Guxens, M.; Ballester, F.; Espada, M.; Fernández, M.F.; Grimalt, J.O.; Ibarluzea, J.; Olea, N.; Rebagliato, M.; Tardón, A.; Torrent, M.; et al. Cohort Profile: The INMA–INfancia y Medio Ambiente (Environment and Childhood) Project. Int. J. Epidemiol. 2012, 41, 930–940. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Palacios, S.; Navarrete-Muñoz, E.-M.; García-de-la-Hera, M.; Torres-Collado, L.; Santa-Marina, L.; Amiano, P.; Lopez-Espinosa, M.-J.; Tardon, A.; Riano-Galan, I.; Vrijheid, M.; et al. Sugar-Containing Beverages Consumption and Obesity in Children Aged 4–5 Years in Spain: The INMA Study. Nutrients 2019, 11, 1772. [Google Scholar] [CrossRef] [Green Version]
- Vioque, J.; Navarrete-Muñoz, E.-M.; Gimenez-Monzó, D.; García-de-la-Hera, M.; Granado, F.; Young, I.S.; Ramón, R.; Ballester, F.; Murcia, M.; Rebagliato, M.; et al. Reproducibility and Validity of a Food Frequency Questionnaire among Pregnant Women in a Mediterranean Area. Nutr. J. 2013, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Agriculture (USDA). Agriculture Research Service 2010 USDA National Nutrient Database for Standard Reference; Release 23; Nutrient Data Laboratory: Washington, DC, USA, 2010.
- Palma, I.; Farran, A.; Cantós, D. Tablas de Composición de Alimentos Por Medidas Caseras de Consumo Habitual En España; Centre d’Ensenyament Superior de Nutrició i Dietètica (CESNID); Primera.; Mc Graw-Hill Interamericana: Madrid, Spain, 2008. [Google Scholar]
- Conners, C.K. Conner’s Kiddie Continuous Performance Test (K-CPT V.5); Multi Health Syst: New York, NY, USA, 2006. [Google Scholar]
- Conners, C.K.; Staff, M. Conner’s Kiddie Continuous Performance Test (K-CPT): In Computer Program for Windows Technical Guide and Software Manual; Multi Health Syst: Toronto, ON, Canada, 2001. [Google Scholar]
- Conners, C.K. Conners’ Continuous Performance Test II: In Computer Program for Windows Technical Guide and Software Manual; MHS Staff, Ed.; North Tonawanda: New York, NY, USA, 2000. [Google Scholar]
- Conners, C.K.; Epstein, J.N.; Angold, A.; Klaric, J. Continuous performance test performance in a normative epidemiological sample. J. Abnorm. Child Psychol. 2003, 31, 555–562. [Google Scholar] [CrossRef]
- Yu, C.; Yao, W. Robust Linear Regression: A Review and Comparison. Commun. Stat. Simul. Comput. 2017, 46, 6261–6282. [Google Scholar] [CrossRef]
- Nikoloulopoulos, A.; Karlis, D. On Modeling Count Data: A Comparison of Some Well-Known Discrete Distributions. J. Stat. Comput. Simul. 2008, 78, 437–457. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottlieb, D.J.; Vezina, R.M.; Chase, C.; Lesko, S.M.; Heeren, T.C.; Weese-Mayer, D.E.; Auerbach, S.H.; Corwin, M.J. Symptoms of Sleep-Disordered Breathing in 5-Year-Old Children Are Associated with Sleepiness and Problem Behaviors. Pediatrics 2003, 112, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Liang, C.-M.; Huang, K.; Xiang, H.-Y.; Qi, J.; Feng, L.-L.; Lai, Y.-P.; Shao, S.-S.; Wu, X.-Y.; Tao, F.-B. Prenatal Serum Thallium Exposure and 36-Month-Old Children’s Attention-Deficit/Hyperactivity Disorder Symptoms: Ma’anshan Birth Cohort Study. Chemosphere 2020, 244, 125499. [Google Scholar] [CrossRef]
- Ma, S.-S.; Zhu, D.-M.; Yin, W.-J.; Hao, J.-H.; Huang, K.; Tao, F.-B.; Tao, R.-X.; Zhu, P. The Role of Neonatal Vitamin D in the Association of Prenatal Depression with Toddlers ADHD Symptoms: A Birth Cohort Study. J. Affect. Disord. 2020, 281, 390–396. [Google Scholar] [CrossRef]
- Asmussen, J.; Skovgaard, A.M.; Bilenberg, N. Trajectories of Dysregulation in Preschool Age. Eur. Child Adolesc. Psychiatry 2021. [Google Scholar] [CrossRef]
- Egeland, J.; Kovalik-Gran, I. Validity of the Factor Structure of Conners’ CPT. J. Atten. Disord. 2010, 13, 347–357. [Google Scholar] [CrossRef]
- Julvez, J.; Fortuny, J.; Mendez, M.; Torrent, M.; Ribas-Fitó, N.; Sunyer, J. Maternal Use of Folic Acid Supplements during Pregnancy and Four-Year-Old Neurodevelopment in a Population-Based Birth Cohort. Paediatr. Perinat. Epidemiol. 2009, 23, 199–206. [Google Scholar] [CrossRef]
- del Río Garcia, C.; Torres-Sánchez, L.; Chen, J.; Schnaas, L.; Hernández, C.; Osorio, E.; Portillo, M.G.; López-Carrillo, L. Maternal MTHFR 677C>T Genotype and Dietary Intake of Folate and Vitamin B(12): Their Impact on Child Neurodevelopment. Nutr. Neurosci. 2009, 12, 13–20. [Google Scholar] [CrossRef]
- Gao, Y.; Sheng, C.; Xie, R.-H.; Sun, W.; Asztalos, E.; Moddemann, D.; Zwaigenbaum, L.; Walker, M.; Wen, S.W. New Perspective on Impact of Folic Acid Supplementation during Pregnancy on Neurodevelopment/Autism in the Offspring Children A Systematic Review. PLoS ONE 2016, 11, e0165626. [Google Scholar] [CrossRef] [Green Version]
- Barua, S.; Kuizon, S.; Chadman, K.K.; Flory, M.J.; Brown, W.T.; Junaid, M.A. Single-Base Resolution of Mouse Offspring Brain Methylome Reveals Epigenome Modifications Caused by Gestational Folic Acid. Epigenetics Chromatin 2014, 7, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barua, S.; Chadman, K.K.; Kuizon, S.; Buenaventura, D.; Stapley, N.W.; Ruocco, F.; Begum, U.; Guariglia, S.R.; Brown, W.T.; Junaid, M.A. Increasing Maternal or Post-Weaning Folic Acid Alters Gene Expression and Moderately Changes Behavior in the Offspring. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J.; Rosenberg, I.H. Excessive Folic Acid Intake and Relation to Adverse Health Outcome. Biochimie 2016, 126, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Lertxundi, A.; Andiarena, A.; Martínez, M.D.; Ayerdi, M.; Murcia, M.; Estarlich, M.; Guxens, M.; Sunyer, J.; Julvez, J.; Ibarluzea, J. Prenatal Exposure to PM2.5 and NO2 and Sex-Dependent Infant Cognitive and Motor Development. Environ. Res. 2019, 174, 114–121. [Google Scholar] [CrossRef]
- Barnard, H.; Rao, R.; Xu, Y.; Froehlich, T.; Epstein, J.; Lanphear, B.P.; Yolton, K. Association of the Conners’ Kiddie Continuous Performance Test (K-CPT) Performance and Parent-Report Measures of Behavior and Executive Functioning. J. Atten. Disord. 2018, 22, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Taki, Y.; Sato, K.; Hashizume, H.; Sassa, Y.; Takeuchi, H.; Thyreau, B.; He, Y.; Evans, A.C.; Li, X.; et al. Topological Organization of Functional Brain Networks in Healthy Children: Differences in Relation to Age, Sex, and Intelligence. PLoS ONE 2013, 8, e55347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheelock, M.D.; Hect, J.L.; Hernandez-Andrade, E.; Hassan, S.S.; Romero, R.; Eggebrecht, A.T.; Thomason, M.E. Sex Differences in Functional Connectivity during Fetal Brain Development. Dev. Cognit. Neurosci. 2019, 36, 100632. [Google Scholar] [CrossRef]
- Rubia, K.; Hyde, Z.; Halari, R.; Giampietro, V.; Smith, A. Effects of Age and Sex on Developmental Neural Networks of Visual-Spatial Attention Allocation. Neuroimage 2010, 51, 817–827. [Google Scholar] [CrossRef]




| All Cohorts (n = 1329) | Valencia (n = 518) | Sabadell (n = 301) | Asturias (n = 246) | Gipuzkoa (n = 264) | p1 | |
|---|---|---|---|---|---|---|
| Mother’s age, year | 31 (28–34) | 30 (28–33) | 31 (28–34) | 31 (29–35) | 31 (29–33) | <0.001 |
| Educational level | ||||||
| Primary or less | 285 (21.4) | 144 (27.8) | 73 (24.3) | 40 (16.3) | 28 (10.6) | <0.001 |
| Secondary | 561 (42.2) | 226 (43.6) | 121 (40.2) | 111 (45.1) | 103 (39.0) | |
| University | 483 (36.3) | 148 (28.6) | 107 (35.5) | 95 (38.6) | 133 (50.4) | |
| Social class | ||||||
| I + II (high) | 312 (23.5) | 99 (19.1) | 70 (23.3) | 60 (24.4) | 83 (31.4) | <0.001 |
| III | 368 (27.7) | 138 (26.6) | 100 (33.2) | 56 (22.8) | 74 (28.0) | |
| IV + V (low) | 649 (48.8) | 281 (54.2) | 131 (43.5) | 130 (52.8) | 107 (40.5) | |
| Parity ≥ 1 | 564 (42.4) | 234 (45.2) | 129 (42.9) | 92 (37.4) | 109 (41.3) | 0.230 |
| Overall tobacco exposition during pregnancy, yes | 810 (61.0) | 370 (71.4) | 179 (59.5) | 108 (43.9) | 153 (58.0) | <0.001 |
| Missing values | 26 (1.3) | 6 (1.2) | 6 (2.0) | 12 (4.9) | 2 (0.8) | |
| Prepregnancy mother’s BMI, kg/m2 | ||||||
| <18.5–25 | 982 (73.9) | 368 (71.0) | 232 (77.1) | 172 (69.9) | 210 (79.5) | 0.045 |
| >25–30 | 251 (18.9) | 103 (19.9) | 50 (16.6) | 58 (23.6) | 40 (15.2) | |
| >30 | 96 (7.2) | 47 (9.1) | 19 (6.3) | 16 (6.5) | 14 (5.3) | |
| Prepregnancy father’s BMI, kg/m2 | ||||||
| <18.5–25 | 569 (42.8) | 226 (43.6) | 142 (47.2) | 75 (30.5) | 126 (47.7) | <0.001 |
| >25–30 | 574 (43.2) | 233 (45.0) | 112 (37.2) | 120 (48.8) | 109 (41.3) | |
| >30 | 164 (12.5) | 59 (11.4) | 43 (14.3) | 42 (17.1) | 20 (7.6) | |
| Missing values | 22 (1.7) | 0 (0.0) | 4 (1.3) | 9 (3.7) | 9 (3.4) | |
| Child’s sex, male | 664 (50.0) | 267 (51.5) | 153 (50.8) | 121 (49.2) | 123 (46.6) | 0.600 |
| Child’s sex, female | 665 (50.0) | 251 (48.5) | 148 (49.2) | 125 (50.8) | 141 (53.4) | |
| Age at K-CPT examination, year | 4.6 (4.4–5.7) | 5.8 (5.7–5.8) | 4.5 (4.4–4.6) | 4.4 (4.3–4.5) | 4.5 (4.4–4.5) | <0.001 |
| Dietary folate, µg/day | ||||||
| First period | 294 (237–359) | 294 (234–360) | 282 (231–343) | 312 (245–370) | 307 (246–365) | 0.003 |
| Second period | 291 (235–358) | 278 (220–353) | 289 (233–348) | 300 (242–365) | 302 (259–369) | <0.001 |
| FAs µg/day | ||||||
| First period | ||||||
| <400 | 742 (55.8) | 309 (59.7) | 190 (63.1) | 112 (45.5) | 131 (49.6) | <0.001 |
| 400–999 | 199 (15.0) | 80 (15.4) | 35 (11.6) | 42 (17.1) | 42 (15.9) | |
| ≥1000 | 388 (29.2) | 129 (24.9) | 76 (25.2) | 92 (37.4) | 91 (34.5) | |
| Second period | ||||||
| <400 | 683 (51.4) | 169 (32.6) | 252 (83.7) | 66 (26.8) | 196 (74.2) | <0.001 |
| 400–999 | 405 (30.5) | 246 (47.5) | 35 (11.6) | 88 (35.8) | 36 (13.6) | |
| ≥1000 | 241 (18.1) | 103 (19.9) | 14 (4.7) | 92 (37.4) | 32 (12.1) | |
| K-CPT outcomes | ||||||
| HRT, ms | 705 (633–792) | 669 (604–735) | 718 (647–803) | 758 (678–843) | 733 (659–845) | <0.001 |
| HRT(SE), ms | 28 (20.6–37.9) | 22.9 (17.2–30.7) | 32.6 (24.7–43.6) | 31.6 (24.1–40.0) | 31.3 (22.9–41.6) | <0.001 |
| Detectability, no unit | 0.6 (0.3–0.9) | 0.5 (0.3–0.8) | 0.5 (0.3–0.8) | 0.7 (0.3–1.0) | 0.6 (0.3–0.9) | 0.011 |
| Omissions, number | 18 (9–34) | 10 (5–20.75) | 24 (13–37) | 23 (13–37.75) | 31 (16.75–50) | <0.001 |
| Commissions, number | 21 (13–29) | 22 (15–29) | 22 (15–31) | 16.5 (10–30.75) | 18 (11–28) | <0.001 |
| HRT | HRT(SE) | Detectability | Omissions | Commissions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β 1 (95% CI) | p | I2 | β 1 (95% CI) | p | I2 | β 1 (95% CI) | p | I2 | IRR 1 (95% CI) | p | I2 | IRR 1 (95% CI) | p | I2 | |
| First period | |||||||||||||||
| FAs, µg/day | |||||||||||||||
| 400–999 | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||
| <400 | 14.56 (−3.26; 32.38) | 0.109 | 0.0 | 1.01 (−0.69; 2.70) | 0.244 | 0.0 | 0.03 (−0.11; 0.16) | 0.685 | 75.5 | 1.14 (1.01; 1.29) | 0.035 | 0.0 | 0.96 (0.83; 1.12) | 0.633 | 66.1 |
| ≥1000 | 9.06 (−11.02; 29.14) | 0.376 | 0.0 | 1.73 (−0.13; 3.60) | 0.068 | 0.0 | −0.00 (−0.16; 0.15) | 0.986 | 79.0 | 1.16 (1.02; 1.33) | 0.027 | 0.0 | 0.99 (0.83; 1.19) | 0.941 | 72.3 |
| Second period | |||||||||||||||
| FAs, µg/day | |||||||||||||||
| 400–999 | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||
| <400 | 3.80 (−11.23; 18.83) | 0.620 | 0.0 | −1.25 (−2.70; 0.21) | 0.094 | 9.3 | 0.02 (−0.04; 0.08) | 0.455 | 0.0 | 0.91 (0.82; 1.02) | 0.097 | 0.0 | 0.97 (0.91; 1.04) | 0.426 | 0.0 |
| ≥1000 | −1.39 (−21.45; 18.67) | 0.892 | 0.0 | 0.41 (−3.25; 4.07) | 0.825 | 52.7 | −0.02 (−0.09; 0.05) | 0.568 | 0.0 | 1.02 (0.90; 1.16) | 0.773 | 6.3 | 1.03 (0.95; 1.12) | 0.442 | 0.0 |
| Entire pregnancy | |||||||||||||||
| FAs, µg/day | |||||||||||||||
| 400–999 | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||
| <400 | −9.52 (−38.06; 19.02) | 0.513 | 52.4 | −0.41 (−1.98; 1.16) | 0.607 | 22.0 | −0.03 (−0.20; 0.15) | 0.775 | 86.9 | 0.95 (0.77; 1.17) | 0.624 | 70.0 | 1.05 (0.85; 1.29) | 0.666 | 83.9 |
| ≥1000 | −10.94 (−30.28; 8.41) | 0.268 | 1.0 | −0.07 (−1.83; 1.69) | 0.934 | 41.1 | −0.01 (−0.17; 0.14) | 0.894 | 76.9 | 0.98 (0.80; 1.19) | 0.838 | 56.8 | 1.02 (0.83; 1.26) | 0.826 | 79.1 |
| HRT | HRT(SE) | Detectability | Omissions | Commissions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β 1 (95% CI) | p | I2 | β 1 (95% CI) | p | I2 | β1 (95% CI) | p | I2 | IRR 1 (95% CI) | p | I2 | IRR 1 (95% CI) | p | I2 | |
| BOYS | |||||||||||||||
| First period | |||||||||||||||
| FAs, µg/day | |||||||||||||||
| 400–999 | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||
| <400 | 34.36 (10.01; 58.71) | 0.006 | 0.0 | 2.02 (−0.56; 4.59) | 0.124 | 0.0 | 0.05 (−0.03; 0.14) | 0.202 | 16.5 | 1.22 (1.01; 1.47) | 0.036 | 8.6 | 0.92 (0.83; 1.03) | 0.153 | 0.0 |
| ≥1000 | 33.18 (6.10; 60.25) | 0.016 | 0.0 | 3.31 (0.53; 6.09) | 0.019 | 3.7 | 0.04 (−0.05; 0.13) | 0.395 | 44.8 | 1.25 (0.91; 1.72) | 0.169 | 60.9 | 0.97 (0.80; 1.18) | 0.752 | 57.4 |
| Second period | |||||||||||||||
| FAs, µg/day | |||||||||||||||
| 400–999 | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||
| <400 | −1.96 (−22.06; 18.13) | 0.848 | 0.0 | −2.15 (−4.47; 0.17) | 0.069 | 0.0 | 0.06 (−0.10; 0.22) | 0.490 | 65.3 | 0.92 (0.78; 1.09) | 0.346 | 0.0 | 1.00 (0.91; 1.09) | 0.919 | 37.1 |
| ≥1000 | −6.03 (−57.93; 45.87) | 0.820 | 54.6 | 1.01 (−1.76; 3.79) | 0.474 | 15.2 | −0.07 (−0.15; 0.02) | 0.121 | 9.2 | 0.96 (0.80; 1.16) | 0.687 | 0.0 | 1.10 (0.99; 1.23) | 0.072 | 49.4 |
| Entire pregnancy | |||||||||||||||
| FAs, µg/day | |||||||||||||||
| 400–999 | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||
| <400 | −19.19 (−56.07; 17.69) | 0.308 | 53.2 | −0.72 (−2.99; 1.56) | 0.537 | 0.0 | −0.06 (−0.20; 0.09) | 0.450 | 61.4 | 0.95 (0.70; 1.27) | 0.713 | 71.7 | 1.07 (0.92; 1.25) | 0.405 | 58.2 |
| ≥1000 | −21.48 (−47.27; 4.30) | 0.102 | 0.0 | −0.11 (−2.68; 2.46) | 0.935 | 47.9 | −0.05 (−0.19; 0.10) | 0.521 | 51.6 | 1.00 (0.73; 1.37) | 1.000 | 68.0 | 1.05 (0.84; 1.30) | 0.686 | 71.4 |
| GIRLS | |||||||||||||||
| First period | |||||||||||||||
| FAs, µg/day | |||||||||||||||
| 400–999 | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||
| <400 | 6.20 (−20.88; 25.53) | 0.654 | 0.0 | 0.70 (−1.82; 3.22) | 0.586 | 0.0 | 0.04 (−0.17; 0.26) | 0.688 | 77.8 | 1.11 (0.94; 1.30) | 0.227 | 0.0 | 0.98 (0.75; 1.27) | 0.855 | 76.0 |
| ≥1000 | −4.77 (−35.07; 25.53) | 0.758 | 0.0 | 0.38 (−2.44; 3.20) | 0.791 | 0.0 | 0.00 (−0.15; 0.15) | 0.998 | 50.3 | 1.10 (0.92; 1.31) | 0.302 | 0.0 | 0.99 (0.87; 1.13) | 0.847 | 39.6 |
| Second period | |||||||||||||||
| FAs, µg/day | |||||||||||||||
| 400–999 | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||
| <400 | 13.04 (−9.90; 35.98) | 0.265 | 0.0 | −0.65 (−2.52; 1.21) | 0.493 | 20.8 | 0.04 (−0.04; 0.12) | 0.357 | 0.0 | 0.86 (0.74; 1.00) | 0.045 | 0.9 | 0.95 (0.86; 1.06) | 0.358 | 5.3 |
| ≥1000 | −4.36 (−34.59; 25.86) | 0.777 | 0.0 | −0.65 (−3.46; 2.17) | 0.654 | 29.3 | 0.03 (−0.07; 0.13) | 0.534 | 0.0 | 1.02 (0.86; 1.22) | 0.792 | 0.0 | 0.97 (0.86; 1.11) | 0.669 | 0.0 |
| Entire pregnancy | |||||||||||||||
| FAs, µg/day | |||||||||||||||
| 400–999 | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||
| <400 | 10.59 (−15.21; 36.40) | 0.421 | 0.1 | 0.28 (−2.14; 2.69) | 0.822 | 0.0 | 0.03 (−0.19; 0.25) | 0.774 | 80.0 | 1.00 (0.85; 1.18) | 0.976 | 0.0 | 1.03 (0.87; 1.10) | 0.837 | 79.5 |
| ≥1000 | −3.07 (−32.22; 26.08) | 0.837 | 22.4 | −0.18 (−2.83; 2.48) | 0.896 | 0.0 | 0.03 (−0.13; 0.20) | 0.710 | 52.6 | 0.97 (0.81; 1.17) | 0.772 | 0.0 | 1.00 (0.80; 1.27) | 0.937 | 53.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Compañ Gabucio, L.M.; García de la Hera, M.; Torres Collado, L.; Fernández-Somoano, A.; Tardón, A.; Guxens, M.; Vrijheid, M.; Rebagliato, M.; Murcia, M.; Ibarluzea, J.; et al. The Use of Lower or Higher Than Recommended Doses of Folic Acid Supplements during Pregnancy Is Associated with Child Attentional Dysfunction at 4–5 Years of Age in the INMA Project. Nutrients 2021, 13, 327. https://doi.org/10.3390/nu13020327
Compañ Gabucio LM, García de la Hera M, Torres Collado L, Fernández-Somoano A, Tardón A, Guxens M, Vrijheid M, Rebagliato M, Murcia M, Ibarluzea J, et al. The Use of Lower or Higher Than Recommended Doses of Folic Acid Supplements during Pregnancy Is Associated with Child Attentional Dysfunction at 4–5 Years of Age in the INMA Project. Nutrients. 2021; 13(2):327. https://doi.org/10.3390/nu13020327
Chicago/Turabian StyleCompañ Gabucio, Laura María, Manuela García de la Hera, Laura Torres Collado, Ana Fernández-Somoano, Adonina Tardón, Mònica Guxens, Martine Vrijheid, Marisa Rebagliato, Mario Murcia, Jesús Ibarluzea, and et al. 2021. "The Use of Lower or Higher Than Recommended Doses of Folic Acid Supplements during Pregnancy Is Associated with Child Attentional Dysfunction at 4–5 Years of Age in the INMA Project" Nutrients 13, no. 2: 327. https://doi.org/10.3390/nu13020327