Effect of Lactobacillus rhamnosus Probiotic in Early Pregnancy on Plasma Conjugated Bile Acids in a Randomised Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Intervention
2.4. Data Collection
2.5. Definitions
2.6. Statistical Analysis
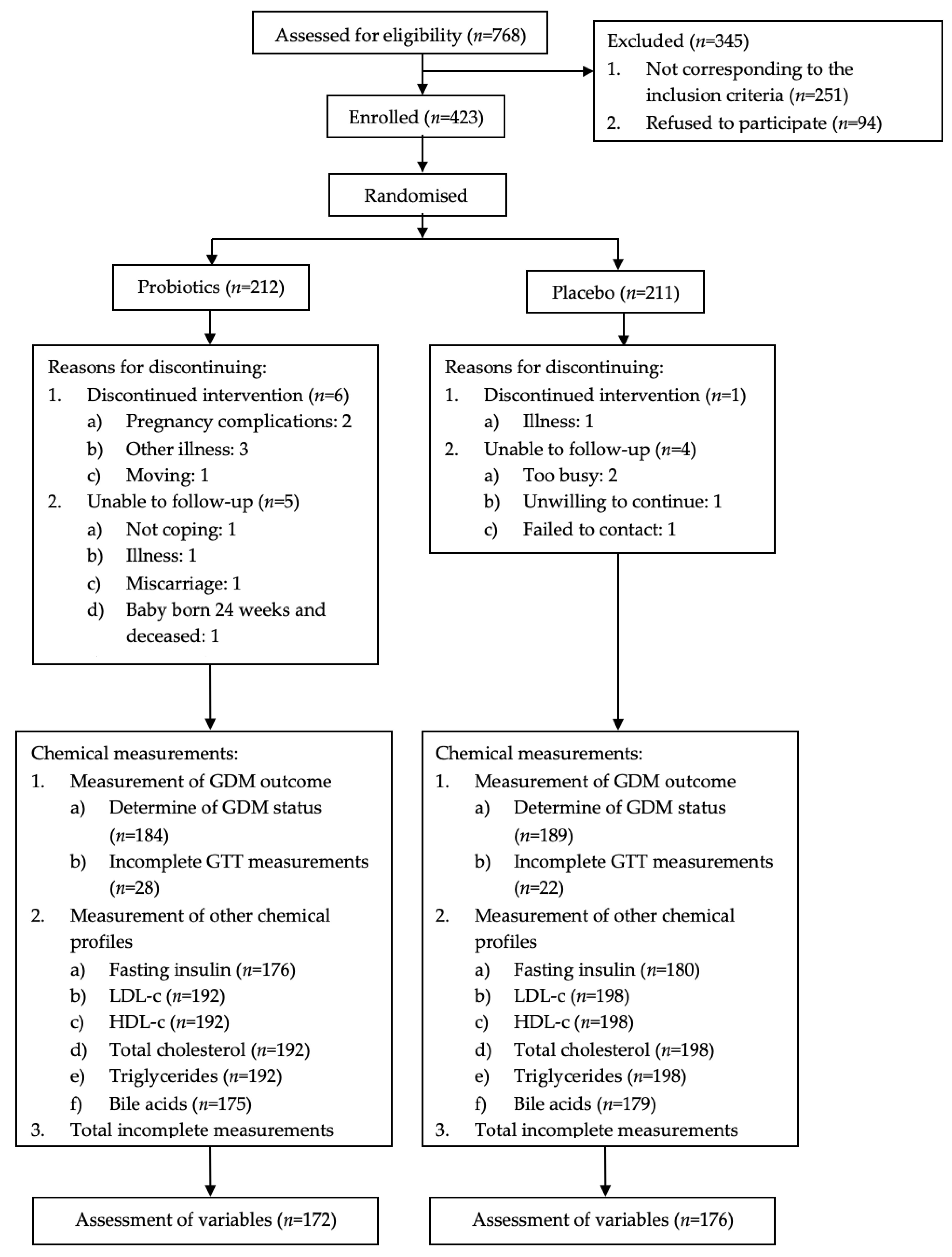
3. Results
3.1. Characteristics of the Study Population
3.2. Effect of L. rhamnosus HN001 Supplementation on GDM, Glucose, HOMA-IR, and Lipid Profiles
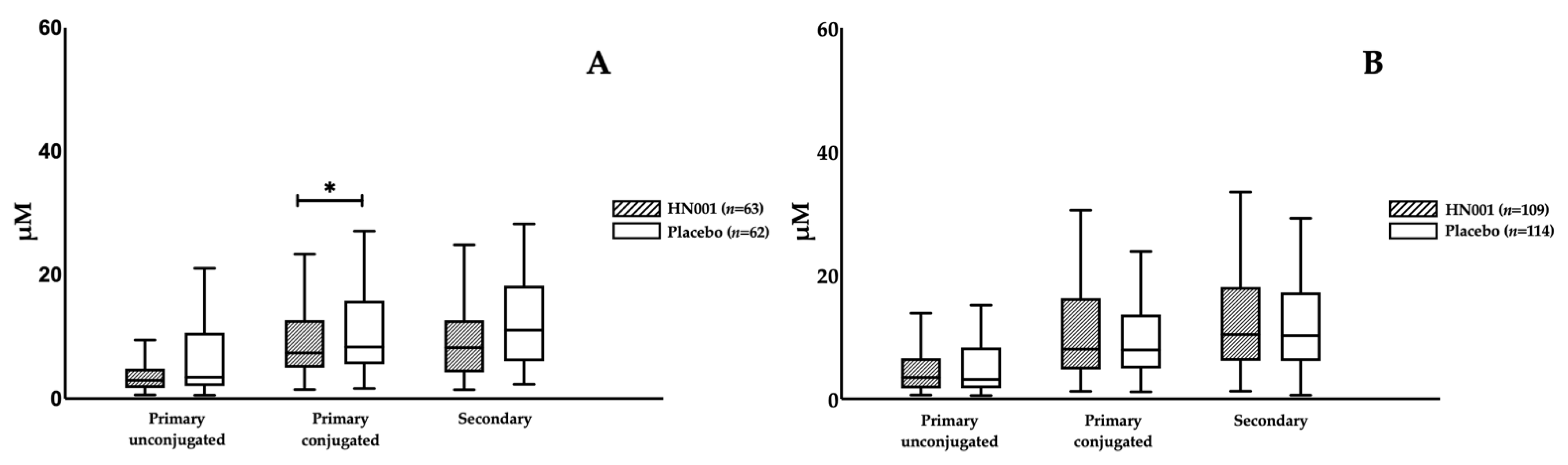
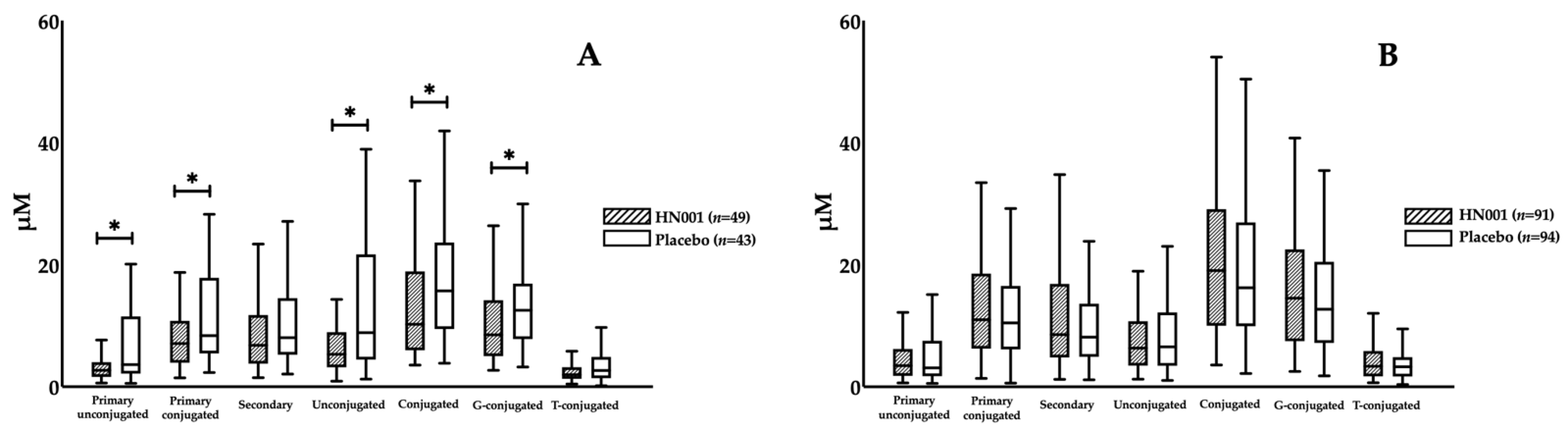
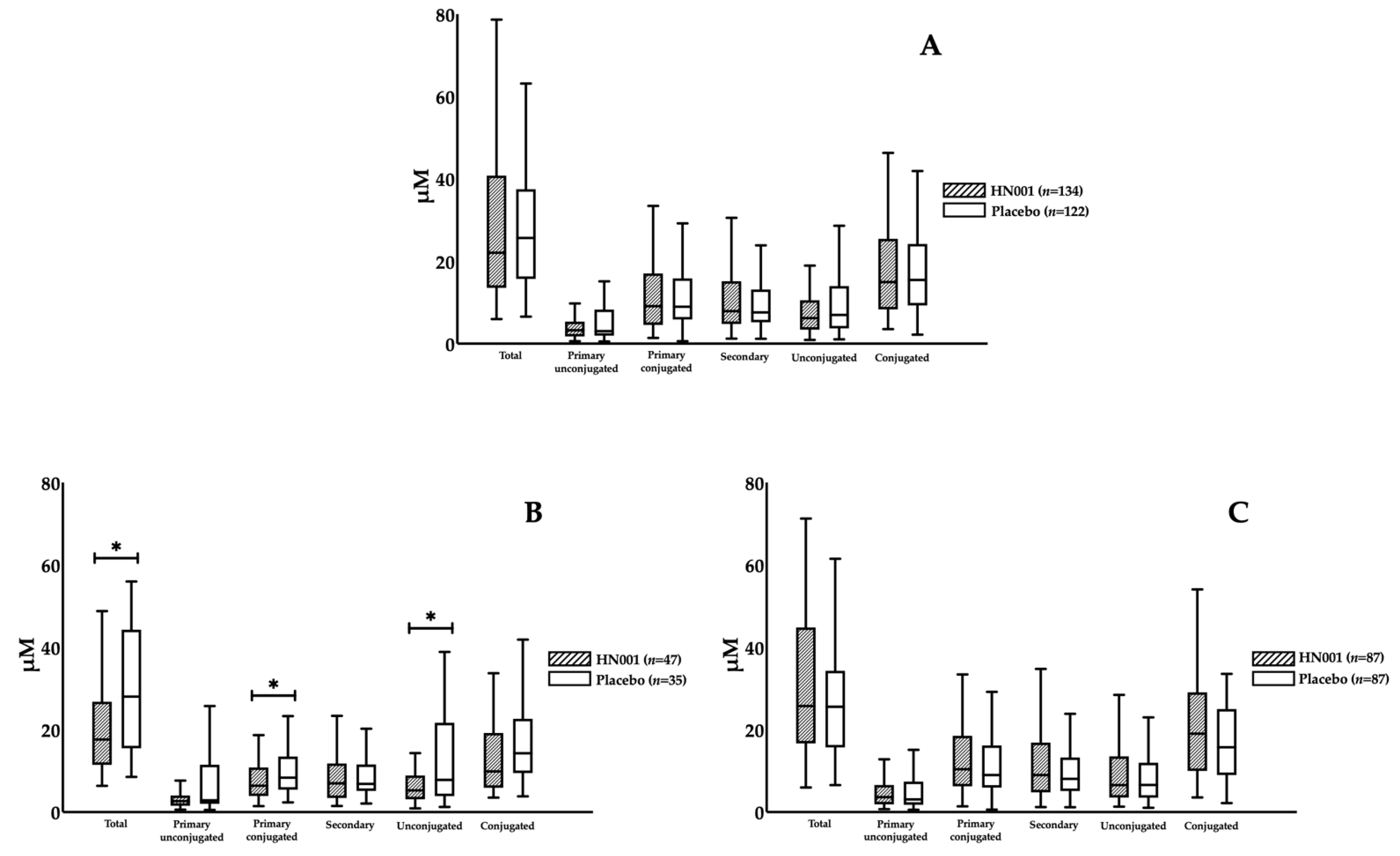
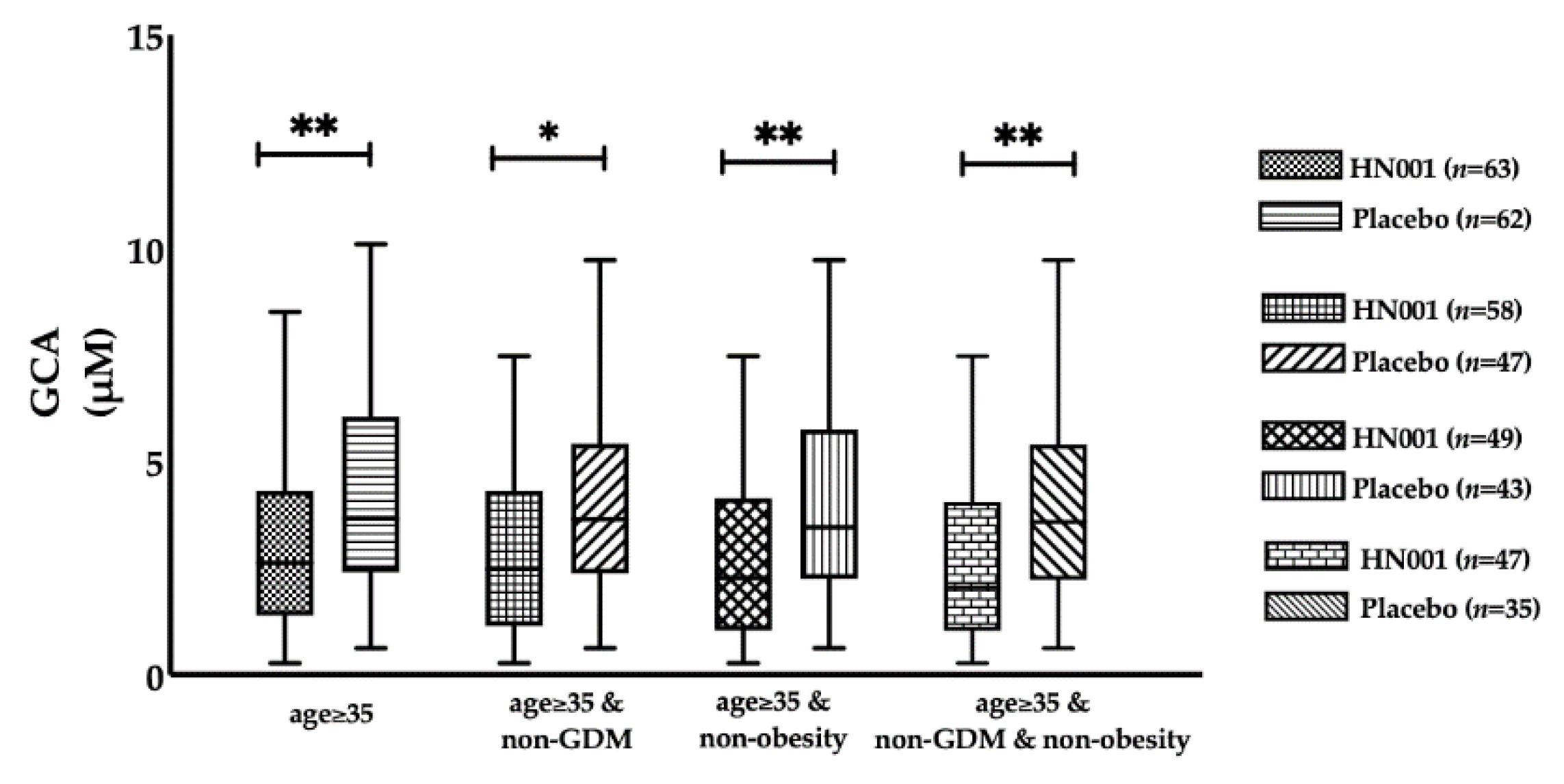
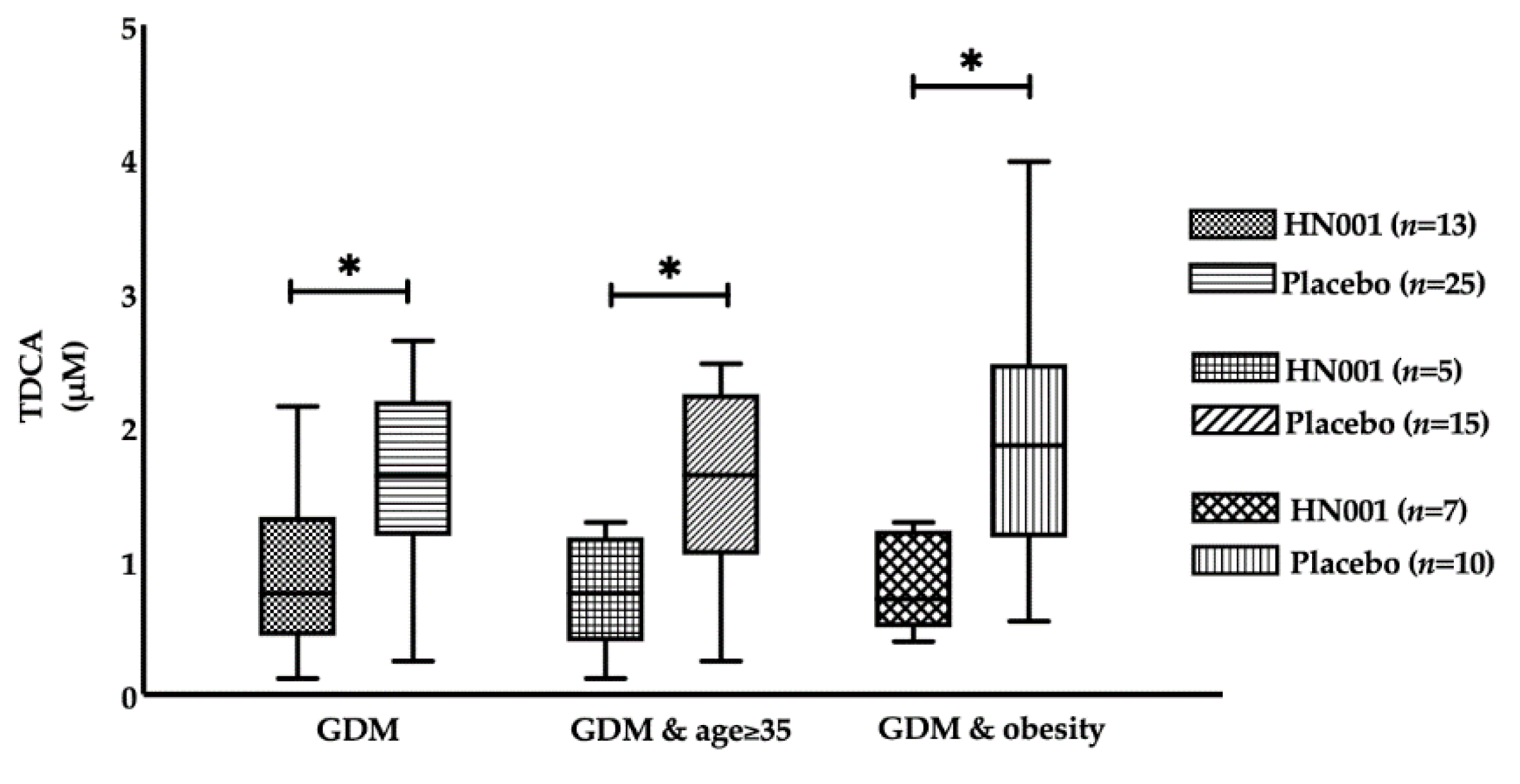
3.3. Effect of L. rhamnosus HN001 Supplementation on BAs
3.4. Correlations between Metabolic Profiles and Bile Acids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kajaa, R.; Rönnemaa, P. Gestational Diabetes: Pathogenesis and Consequences to Mother and Offspring. Rev. Diabet. Stud. 2008, 5, 194–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitanchez, D.; Yzydorczyk, C.; Simeoni, U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J. Diabetes 2015, 6, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Hotel, A.C.P.; Cordoba, A. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention 2001, 5, 1–10. [Google Scholar]
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F.; Arab, A. Effects of a multispecies probiotic mixture on glycemic control and inflammatory status in women with gestational diabetes: A randomized controlled clinical trial. J. Nutr. Metab. 2016, 2016, 5190846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamali, M.; Dadkhah, F.; Sadrkhanlou, M.; Jamilian, M.; Ahmadi, S.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. 2016, 42, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Wickens, K.L.; Barthow, C.A.; Murphy, R.; Abels, P.R.; Maude, R.M.; Stone, P.R.; Mitchell, E.A.; Stanley, T.V.; Purdie, G.L.; Kang, J.M.; et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: A randomised controlled trial. Br. J. Nutr. 2017, 117, 804–813. [Google Scholar] [CrossRef] [Green Version]
- Molinaro, A.; Wahlström, A.; Marschall, H.-U. Role of bile acids in metabolic control. Trends Endocrinol. Metab. 2018, 29, 31–41. [Google Scholar] [CrossRef]
- McIlvride, S.; Dixon, P.H.; Williamson, C. Bile acids and gestation. Mol. Asp. Med. 2017, 56, 90–100. [Google Scholar] [CrossRef]
- Majewska, A.; Godek, B.; Bomba-Opon, D.; Wielgos, M. Association between intrahepatic cholestasis in pregnancy and gestational diabetes mellitus. A retrospective analysis. Ginekol. Polska 2019, 90, 458–463. [Google Scholar] [CrossRef]
- Thakare, R.; Alamoudi, J.A.; Gautam, N.; Rodrigues, A.D.; Alnouti, Y. Species differences in bile acids I. Plasma and urine bile acid composition. J. Appl. Toxicol. 2018, 38, 1323–1335. [Google Scholar] [CrossRef]
- Kong, M.; Lu, Z.; Zhong, C.; Gao, Q.; Zhou, X.; Chen, R.; Xiong, G.; Hao, L.; Yang, X.; Yang, N. A higher level of total bile acid in early mid-pregnancy is associated with an increased risk of gestational diabetes mellitus: A prospective cohort study in Wuhan, China. J. Endocrinol. Investig. 2020, 43, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, B.; Zhang, X.; Cui, Y.; Deng, L.; Shi, Z.; Shao, Y.; Ding, M. Association between serum bile acid profiles and gestational diabetes mellitus: A targeted metabolomics study. Clin. Chim. Acta 2016, 459, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Haeusler, R.A.; Astiarraga, B.; Camastra, S.; Accili, D.; Ferrannini, E. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes 2013, 62, 4184–4191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Huo, X.; Cao, Y.-F.; Li, S.-N.; Du, Z.; Shao, P.; Leng, J.; Zhang, C.; Sun, X.-Y.; Ma, R.C. Bile acid metabolites in early pregnancy and risk of gestational diabetes in Chinese women: A nested case-control study. EBioMedicine 2018, 35, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobini, R.; Tremaroli, V.; Ståhlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Bertéus Forslund, H.; Perkins, R.; Bäckhed, F. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2017, 19, 579–589. [Google Scholar] [CrossRef]
- Barthow, C.; Wickens, K.; Stanley, T.; Mitchell, E.A.; Maude, R.; Abels, P.; Purdie, G.; Murphy, R.; Stone, P.; Kang, J.; et al. The Probiotics in Pregnancy Study (PiP Study): Rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy Childbirth 2016, 16, 133. [Google Scholar] [CrossRef] [Green Version]
- Tagliacozzi, D.; Mozzi, A.F.; Casetta, B.; Bertucci, P.; Bernardini, S.; Di Ilio, C.; Urbani, A.; Federici, G. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: A simple and rapid one-step method. Clin. Chem. Lab. Med. 2003, 41, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Nemati, R.; Lu, J.; Dokpuang, D.; Booth, M.; Plank, L.D.; Murphy, R. Increased Bile Acids and FGF19 After Sleeve Gastrectomy and Roux-en-Y Gastric Bypass Correlate with Improvement in Type 2 Diabetes in a Randomized Trial. Obes. Surg. 2018, 28, 2672–2686. [Google Scholar] [CrossRef]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Health. Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: A Clinical Practice Guideline; Ministry of Health: Wellington, New Zealand, 2014.
- Lindsay, K.L.; Walsh, C.A.; Brennan, L.; McAuliffe, F.M. Probiotics in pregnancy and maternal outcomes: A systematic review. J. Matern. Fetal Neonatal Med. 2013, 26, 772–778. [Google Scholar] [CrossRef]
- Dolatkhah, N.; Hajifaraji, M.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Mehrabi, Y.; Abbasi, M.M. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J. Health Popul. Nutr. 2015, 33, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Rad, M.N.; Foroushani, A.R.; Khorammian, H.; Esmaillzadeh, A. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: A randomized controlled trial. Eur. J. Clin. Nutr. 2013, 67, 71. [Google Scholar] [CrossRef]
- Laitinen, K.; Poussa, T.; Isolauri, E. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: A randomised controlled trial. Br. J. Nutr. 2008, 101, 1679–1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsay, K.L.; Brennan, L.; Kennelly, M.A.; Maguire, O.C.; Smith, T.; Curran, S.; Coffey, M.; Foley, M.E.; Hatunic, M.; Shanahan, F. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: A randomized controlled trial. Am. J. Obstet. Gynecol. 2015, 212, e491–e496. [Google Scholar]
- Hoppu, U.; Isolauri, E.; Koskinen, P.; Laitinen, K. Maternal dietary counseling reduces total and LDL cholesterol postpartum. Nutrition 2014, 30, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Feng, Q.; Zheng, S.; Xiao, X. The effects of probiotics supplementation on metabolic health in pregnant women: An evidence based meta-analysis. PLoS ONE 2018, 13, e0197771. [Google Scholar] [CrossRef] [PubMed]
- Rad, A.H.; Mehrabany, E.V.; Alipoor, B.; Mehrabany, L.V.; Javadi, M. Do probiotics act more efficiently in foods than in supplements? Nutrition 2012, 28, 733. [Google Scholar]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao J-z Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 1–12. [Google Scholar]
- Gao, X.; Zhang, M.; Xue, J.; Huang, J.; Zhuang, R.; Zhou, X.; Zhang, H.; Fu, Q.; Hao, Y. Body mass index differences in the gut microbiota are gender specific. Front. Microbiol. 2018, 9, 1250. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, Q.; Huang, W.; Yan, Q.; Chen, Y.; Zhang, L.; Tian, Z.; Liu, T.; Yuan, X.; Liu, C. Gestational Diabetes Mellitus Is Associated with Reduced Dynamics of Gut Microbiota during the First Half of Pregnancy. Msystems 2020, 5, e00109-20. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, Z.; Deng, T.K.; Luo, H.; Tang, X.; Liao, Y.; Wang, D.; Zhou, J.; Yang, P.; Su, Y. Probiotics supplementation during human pregnancy affects the gut microbiome and immune status. Front. Cell. Infect. Microbiol. 2019, 9, 254. [Google Scholar] [CrossRef] [Green Version]
- Maghsoodi, N.; Shaw, N.; Cross, G.F.; Alaghband-Zadeh, J.; Wierzbicki, A.S.; Pinkney, J.; Millward, A.; Vincent, R.P. Bile acid metabolism is altered in those with insulin resistance after gestational diabetes mellitus. Clin. Biochem. 2019, 64, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wewalka, M.; Patti, M.-E.; Barbato, C.; Houten, S.M.; Goldfine, A.B. Fasting serum taurine-conjugated bile acids are elevated in type 2 diabetes and do not change with intensification of insulin. J. Clin. Endocrinol. Metab. 2014, 99, 1442–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culpepper, T.; Rowe, C.; Rusch, C.; Burns, A.; Federico, A.; Girard, S.-A.; Tompkins, T.; Nieves, C.; Dennis-Wall, J.; Christman, M. Three probiotic strains exert different effects on plasma bile acid profiles in healthy obese adults: Randomised, double-blind placebo-controlled crossover study. Benef. Microbes 2019, 10, 497–509. [Google Scholar] [CrossRef] [PubMed]
| Subset Titles | Definitions | Number of Participants (n) | ||
|---|---|---|---|---|
| HN001 | Placebo | Total | ||
| Age ≥ 35 years | Older women | 63 | 62 | 125 |
| Age < 35 years | Young women | 109 | 114 | 223 |
| GDM | Women with GDM | 13 | 25 | 38 |
| Age ≥ 35 and GDM | Older women with GDM | 5 | 15 | 20 |
| Age ≥ 35 and non-GDM | Older women without GDM | 58 | 47 | 105 |
| Age < 35 and non-GDM | Young women without GDM | 101 | 104 | 205 |
| Age ≥ 35 and non-obese | Non-obese older women | 49 | 43 | 92 |
| Age < 35 and non-obese | Non-obese young women | 91 | 94 | 185 |
| non-GDM and non-obese | Non-obese women without GDM | 134 | 122 | 256 |
| Age ≥ 35 and non-GDM and non-obese | Non-obese older women without GDM | 47 | 35 | 82 |
| Age < 35 and non-GDM and non-obese | Non-obese young women without GDM | 87 | 87 | 174 |
| GDM and obese | Obese women with GDM | 7 | 10 | 17 |
| HN001 (n = 172) | Placebo (n = 176) | p-Value | |
|---|---|---|---|
| Baseline Characteristics | |||
| Age (years) | 33.1 ± 4.2 | 33.8 ± 4.3 | 0.144 |
| Weight (kg) | 68.4 (63.0–79.1) | 71.1 (63.3–81.9) | 0.194 |
| Waist circumference (cm) | 86.4 (79.8–93.8) | 86.8 (80.6–99.1) | 0.146 |
| BMI (kg m−2) | 25.1 (22.9–28.6) | 25.8 (23.0–30.0) | 0.209 |
| Obesity statistics | 32 (18.6%) | 39 (22.2%) | 0.412 |
| Diagnosed GDM (IADPSG) | 13 (7.6%) | 25 (14.2%) | 0.047 |
| Diagnosed GDM (NZ definition) | 3 (1.7%) | 10 (5.7%) | 0.053 |
| Fasting metabolic variables | |||
| Fasting glucose (mmol L−1) | 4.3 (4.1–4.5) | 4.4 (4.1–4.6) | 0.040 |
| 1-h glucose (mmol L−1) | 6.6 (5.6–7.7) | 6.7 (5.7–8.1) | 0.258 |
| 2-h glucose (mmol L−1) | 5.5 (4.9–6.3) | 5.5 (4.7–6.5) | 0.791 |
| Insulin (pmol L−1) | 64.37 (48.58–92.43) | 60.09 (41.93–86.00) | 0.134 |
| HOMA-IR | 1.74 (1.24–2.49) | 1.64 (1.12–2.45) | 0.363 |
| LDL-c (mmol L−1) | 3.76 (3.20–4.57) | 3.66 (3.00–4.56) | 0.287 |
| HDL-c (mmol L−1) | 1.92 (1.60–2.19) | 1.93 (1.69–2.18) | 0.450 |
| Total cholesterol (mmol L−1) | 6.26 (5.50–7.05) | 6.11 (5.33–6.98) | 0.426 |
| Triglycerides (mmol L−1) | 1.72 (1.34–2.07) | 1.61 (1.30–1.98) | 0.264 |
| Fasting bile acids | |||
| CA (μM) | 1.86 (0.77–4.23) | 1.61 (0.84–7.04) | 0.511 |
| CDCA (μM) | 1.10 (0.50–1.91) | 1.24 (0.63–1.89) | 0.413 |
| GCA (μM) | 3.41 (1.62–5.63) | 3.48 (2.24–6.06) | 0.243 |
| GCDCA (μM) | 4.24 (2.26–7.44) | 4.39 (2.70–7.89) | 0.433 |
| TCDCA (μM) | 1.29 (0.69–2.63) | 1.64 (0.76–3.10) | 0.133 |
| DCA (μM) | 1.03 (0.52–1.94) | 1.00 (0.51–1.78) | 0.981 |
| UDCA (μM) | 0.93 (0.47–1.71) | 0.82 (0.41–1.76) | 0.366 |
| GDCA (μM) | 3.07 (1.37–6.09) | 3.18 (1.74–5.77) | 0.488 |
| GUDCA (μM) | 0.36 (0.19–0.75) | 0.43 (0.22–0.76) | 0.480 |
| TDCA (μM) | 1.13 (0.50–1.95) | 1.15 (0.55–1.87) | 0.759 |
| TUDCA (μM) | 0.06 (0.02–0.12) | 0.05 (0.03–0.10) | 0.907 |
| TLCA (μM) | 0.06 (0.04–0.09) | 0.06 (0.04–0.10) | 0.459 |
| THDCA (μM) | 0.03 (0.01–0.07) | 0.03 (0.01–0.06) | 0.662 |
| non-GDM | GDM | |||||
|---|---|---|---|---|---|---|
| HN001 (n = 159) | Placebo (n = 151) | p-Value | HN001 (n = 13) | Placebo (n = 25) | p-Value | |
| Fasting metabolic variables | ||||||
| Fasting glucose (mmol L−1) | 4.2 (4.1–4.5) | 4.3 (4.1–4.5) | 0.186 | 4.9 (4.2–5.1) | 4.8 (4.5–5.3) | 0.536 |
| 1-h glucose (mmol L−1) | 6.5 (5.5–7.4) | 6.4 (5.6–7.5) | 0.949 | 10.5 (10.0–10.7) | 10.0 (8.9–10.5) | 0.355 |
| 2-h glucose (mmol L−1) | 5.5 (4.8–6.1) | 5.4 (4.7–6.1) | 0.699 | 7.0 (5.7–8.8) | 7.0 (6.5–8.8) | 0.961 |
| Insulin (pmol L−1) | 62.87 (47.71–84.66) | 55.86 (39.57–79.61) | 0.046 | 104.90 (90.32–183.80) | 84.62 (64.45–136.90) | 0.234 |
| HOMA-IR | 1.70 (1.18–2.29) | 1.47 (1.06–2.29) | 0.092 | 3.27 (1.92–5.27) | 2.81 (1.79–4.29) | 0.259 |
| LDL-c (mmol L−1) | 3.76 (3.25–4.57) | 3.67 (3.07–4.57) | 0.407 | 3.65 (2.94–4.56) | 3.61 (2.50–4.40) | 0.494 |
| HDL-c (mmol L−1) | 1.92 (1.6–2.20) | 1.93 (1.70–2.18) | 0.348 | 1.80 (1.55–2.06) | 1.78 (1.39–2.30) | 0.988 |
| Total cholesterol (mmol L−1) | 6.28 (5.52–7.06) | 6.12 (5.43–6.97) | 0.602 | 6.10 (5.50–6.88) | 6.03 (5.12–7.07) | 0.459 |
| Triglycerides (mmol L−1) | 1.66 (1.33–2.07) | 1.55 (1.30–1.96) | 0.157 | 1.95 (1.79–2.07) | 1.94 (1.39–2.43) | 0.896 |
| Fasting bile acids | ||||||
| CA (μM) | 1.94 (0.80–4.31) | 1.52 (0.83–7.07) | 0.756 | 1.48 (0.71–3.38) | 2.63 (1.08–4.21) | 0.361 |
| CDCA (μM) | 1.15 (0.53–1.89) | 1.25 (0.61–1.78) | 0.678 | 0.58 (0.32–2.31) | 1.11 (0.69–2.70) | 0.188 |
| GCA (μM) | 3.42 (1.62–5.73) | 3.43 (2.21–5.83) | 0.470 | 3.34 (1.76–4.23) | 3.78 (2.51–9.08) | 0.210 |
| GCDCA (μM) | 4.27 (2.27–7.62) | 4.35 (2.67–7.66) | 0.697 | 3.86 (2.24–5.80) | 4.73 (3.03–11.82) | 0.314 |
| TCDCA (μM) | 1.29 (0.69–2.67) | 1.61 (0.74–3.00) | 0.303 | 1.42 (1.04–2.21) | 2.68 (0.96–5.05) | 0.222 |
| DCA (μM) | 1.02 (0.52–2.01) | 0.98 (0.52–1.74) | 0.843 | 1.40 (0.53–1.70) | 1.20 (0.51–2.08) | 0.584 |
| UDCA (μM) | 0.93 (0.46–1.76) | 0.82 (0.42–1.66) | 0.363 | 1.09 (0.74–1.68) | 0.98 (0.35–1.96) | 1.000 |
| GDCA (μM) | 3.07 (1.37–6.33) | 3.00 (1.52–5.58) | 0.926 | 3.00 (1.36–3.70) | 4.13 (3.14–6.84) | 0.079 |
| GUDCA (μM) | 0.36 (0.19–0.74) | 0.44 (0.19–0.76) | 0.596 | 0.33 (0.31–0.83) | 0.43 (0.29–0.62) | 0.832 |
| TDCA (μM) | 1.14 (0.50–2.06) | 1.05 (0.52–1.66) | 0.623 | 0.76 (0.48–1.29) | 1.63 (1.19–2.17) | 0.018 |
| TUDCA (μM) | 0.06 (0.02–0.12) | 0.05 (0.03–0.09) | 0.918 | 0.06 (0.03–0.14) | 0.07 (0.02–0.16) | 0.879 |
| TLCA (μM) | 0.06 (0.04–0.09) | 0.06 (0.03–0.10) | 0.748 | 0.05 (0.03–0.08) | 0.07 (0.05–0.10) | 0.199 |
| THDCA (μM) | 0.03 (0.01–0.07) | 0.03 (0.02–0.06) | 0.435 | 0.02 (0.01–0.09) | 0.04 (0.01–0.07) | 0.345 |
| Fasting Glucose | 1-h Glucose | 2-h Glucose | Fasting Insulin | HOMA-IR | LDL-c | HDL-c | Total Cholesterol | Triglycerides | |
|---|---|---|---|---|---|---|---|---|---|
| Total | 0.136 * | 0.099 | −0.037 | 0.102 | 0.102 | −0.013 | 0.010 | −0.016 | 0.066 |
| Conjugated | 0.125 * | 0.079 | 0.031 | 0.113 * | 0.089 | −0.053 | −0.040 | −0.060 | 0.061 |
| Primary conjugated | 0.091 | 0.109 * | 0.005 | 0.123 * | 0.114 * | −0.096 | −0.032 | −0.103 | 0.035 |
| GCA | 0.088 | 0.085 | 0.021 | 0.147 ** | 0.132 * | −0.079 | −0.048 | −0.086 | 0.065 |
| GCDCA | 0.084 | 0.073 | −0.027 | 0.080 | 0.080 | −0.076 | −0.008 | −0.083 | 0.016 |
| TCDCA | 0.036 | 0.134 * | 0.021 | 0.113 * | 0.094 | −0.091 | −0.036 | −0.092 | 0.006 |
| Secondary conjugated | 0.085 | 0.074 | 0.053 | 0.076 | 0.074 | 0.007 | 0.059 | 0.011 | 0.062 |
| GUDCA | 0.145 ** | 0.069 | 0.041 | 0.107 * | 0.124 * | 0.019 | 0.000 | 0.035 | 0.141 ** |
| TDCA | 0.027 | 0.100 | 0.051 | 0.066 | 0.057 | −0.009 | 0.051 | −0.008 | −0.012 |
| G-conjugated | 0.103 | 0.088 | 0.014 | 0.105 * | 0.104 | −0.047 | 0.009 | −0.050 | 0.050 |
| T-conjugated | 0.030 | 0.125 * | 0.019 | 0.075 | 0.058 | −0.058 | 0.012 | −0.058 | -0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Lu, J.; Wickens, K.; Stanley, T.; Maude, R.; Stone, P.; Barthow, C.; Crane, J.; Mitchell, E.A.; Merien, F.; et al. Effect of Lactobacillus rhamnosus Probiotic in Early Pregnancy on Plasma Conjugated Bile Acids in a Randomised Controlled Trial. Nutrients 2021, 13, 209. https://doi.org/10.3390/nu13010209
Chen Y, Lu J, Wickens K, Stanley T, Maude R, Stone P, Barthow C, Crane J, Mitchell EA, Merien F, et al. Effect of Lactobacillus rhamnosus Probiotic in Early Pregnancy on Plasma Conjugated Bile Acids in a Randomised Controlled Trial. Nutrients. 2021; 13(1):209. https://doi.org/10.3390/nu13010209
Chicago/Turabian StyleChen, Yutao, Jun Lu, Kristin Wickens, Thorsten Stanley, Robyn Maude, Peter Stone, Christine Barthow, Julian Crane, Edwin A. Mitchell, Fabrice Merien, and et al. 2021. "Effect of Lactobacillus rhamnosus Probiotic in Early Pregnancy on Plasma Conjugated Bile Acids in a Randomised Controlled Trial" Nutrients 13, no. 1: 209. https://doi.org/10.3390/nu13010209






