Effects of l-Carnitine Supplementation in Patients Receiving Hemodialysis or Peritoneal Dialysis
Abstract
:1. Introduction
2. Materials and Methods
Study Design
3. Results
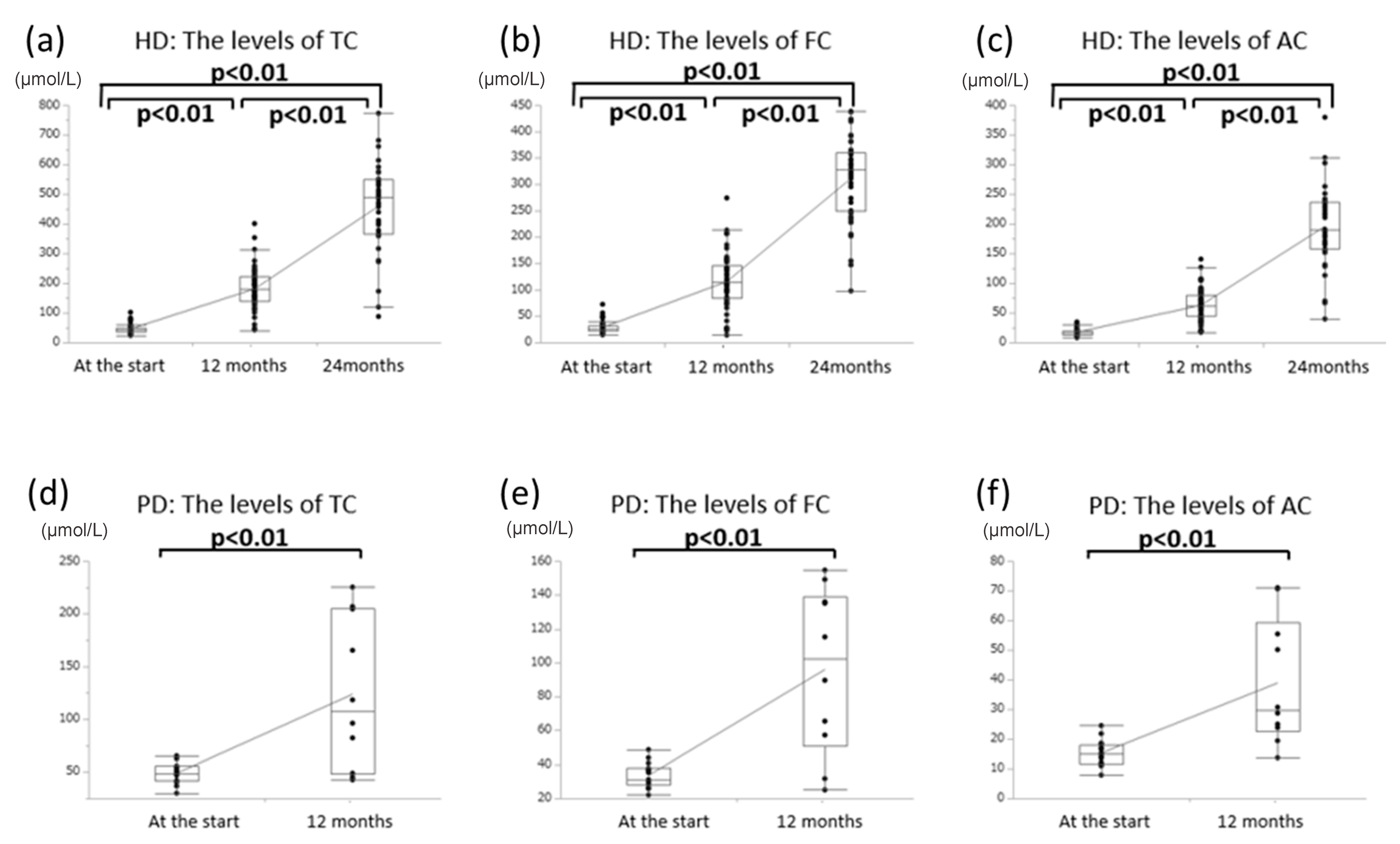
3.1. Effects of Oral l-Carnitine on Carnitine Concentrations
3.2. Effects of l-Carnitine on Muscle Symptoms
3.3. Effects of Oral l-Carnitine on Cardiac Function
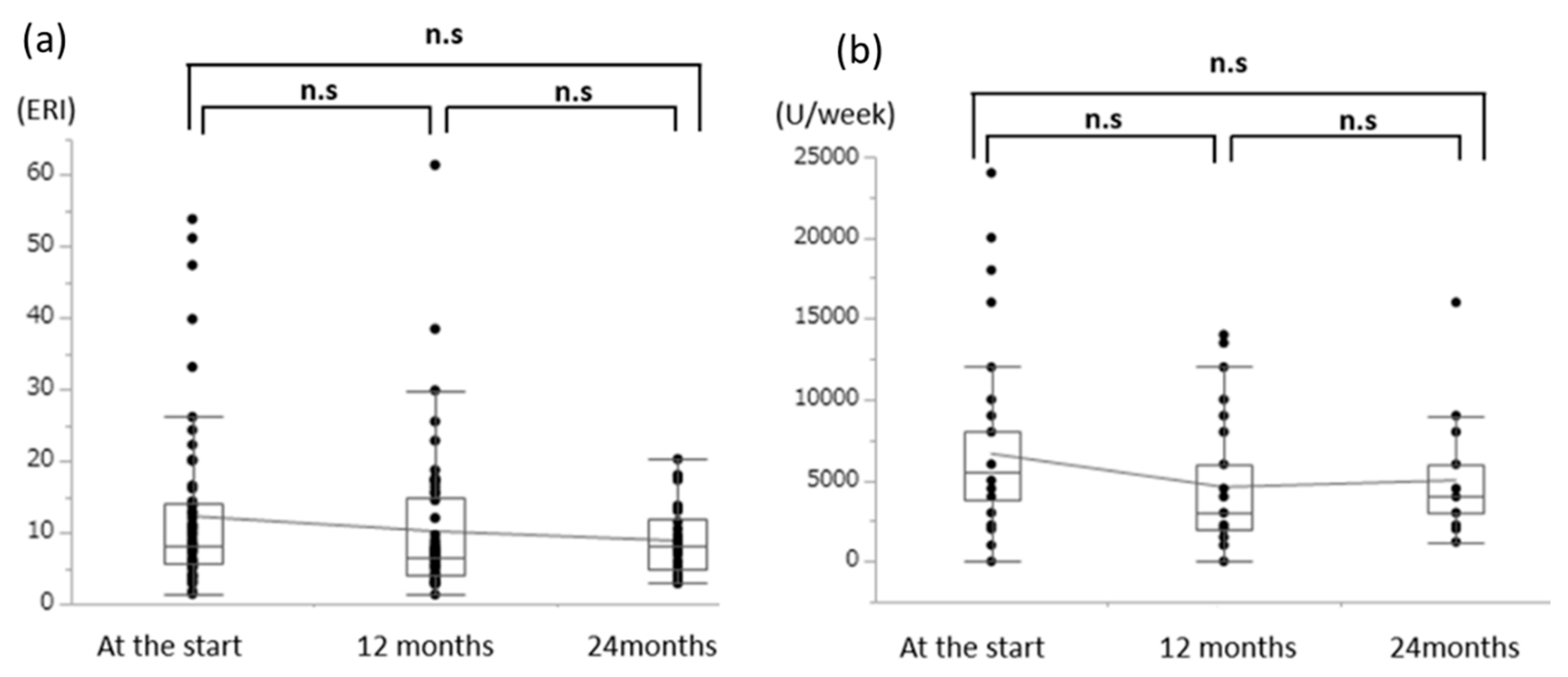
3.4. Effects of Oral l-Carnitine on Renal Anemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rizos, I. Three-year survival of patients with heart failure caused by dilated cardiomyopathy and l-carnitine administration. Am. Heart J. 2000, 139, S120–S123. [Google Scholar] [CrossRef] [PubMed]
- Evans, A. Dialysis-related carnitine disorder and levocarnitine pharmacology. Am. J. Kidney Dis. 2003, 41, S13–S26. [Google Scholar] [CrossRef]
- Mochizuki, T.; Takahashi, M.; Abe, R.; Oishi, T.; Shiga, N.; Kaneko, M.; Ishizuka, E.; Kunitake, T. Carnitine metabolism and its relation to nutritional parameters in patients with peritoneal dialysis. J. Jpn. Soc. Dial. Ther. 2002, 35, 165–170. [Google Scholar] [CrossRef]
- Arduini, A.; Gorbunov, N.; Arrigoni-Martelli, E.; Dottori, S.; Molajoni, M.; Russo, F.; Federici, G. Effects of l-carnitine and its acetate and propionate esters on the molecular dynamics of human erythrocyte membrane. Biochim. Biophys. Acta 1993, 1146, 229–235. [Google Scholar] [CrossRef]
- Rebouche, C.J. Carnitine. In Modern Nutrition in Health and Disease, 9th ed.; Shils, M.E., Olson, J.A., Shike, M., Ross, A.C., Eds.; Lippincott Williams and Wilkins: New York, NY, USA, 1999; pp. 505–512. [Google Scholar]
- Anonymous. Carnitine: Lessons from one hundred years of research. Ann. N.Y. Acad. Sci. 2004, 1033, ix–xi. [Google Scholar] [CrossRef]
- Fukami, K.; Sakai, K.; Kaida, Y.; Otsuka, A.; Wada, Y.; Sugi, K.; Okuda, S. Effects of oral or intravenous l-carnitine administration on serum carnitine levels and clinical parameters in hemodialysis patients. J. Jpn. Soc. Dial. Ther. 2014, 47, 367–374. [Google Scholar] [CrossRef]
- Fukami, K.; Yamagishi, S.; Sakai, K.; Nasu, M.; Okuda, S. Effects of switching from oral administration to intravenous injection of l-carnitine on lipid metabolism in hemodialysis patients. Clin. Kidney J. 2014, 7, 470–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, M.; Ueda, S.; Misaki, H.; Sugiyama, N.; Matsumoto, K.; Matsuo, N.; Murao, S. Carnitine determination by an enzymatic cycling method with carnitine dehydrogenase. Clin. Chem. 1994, 40, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Painter, P.L.; Messer-Rehak, D.; Hanson, P.; Zimmerman, S.W.; Glass, N.R. Exercise capacity in hemodialysis, CAPD, and renal transplant patients. Nephron 1986, 42, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, B. Levocarnitine and dialysis. Nutr. Clin. Pract. 2005, 20, 218–243. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Merli, E.; Cicchitelli, G.; Mele, D.; Fucili, A.; Ceconi, C. Therapeutic effects of l-carnitine and propionyl-l-carnitine on cardiovascular diseases: A review. Ann. N. Y. Acad. Sci. 2004, 1033, 79–91. [Google Scholar] [CrossRef] [PubMed]
- United States Renal Data System. Annual Data Report. 2000, p. 139. Available online: http://www.USRDS.org (accessed on 22 March 2015).
- Vescovo, G.; Ravara, B.; Gobbo, V.; Sandri, M.; Angelini, A.; Della Barbera, M.; Dona, M.; Peluso, G.; Calvani, M.; Mosconi, L.; et al. l-carnitine: A potential treatment for blocking apoptosis and preventing skeletal muscle myopathy in heart failure. Am. J. Physiol.-Cell Physiol. 2002, 283, C802–C810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocchi, L.; Feola, I.; Calvani, M.; D’Iddio, S.; Alfarone, C.; Frascarelli, M. Effects of carnitine administration in patients with chronic renal failure undergoing periodic dialysis, evaluated by computerized electromyography. Drugs. Exp. Clin. Res. 1986, 12, 707–711. [Google Scholar] [PubMed]
- Matsumoto, Y.; Sato, M.; Ohashi, H.; Araki, H.; Tadokoro, M.; Osumi, Y.; Ito, H.; Morita, H.; Amano, I. Effects of l-carnitine supplementation on cardiac morbidity in hemodialyzed patients. Am. J. Nephrol. 2000, 20, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Van Es, A.; Henny, F.C.; Kooistra, M.P.; Lobatto, S.; Scholte, H.R. Amelioration of cardiac function by l-carnitine administration in patients on haemodialysis. Contrib. Nephrol. 1992, 98, 28–35. [Google Scholar] [PubMed]
- Reddan, D.N.; Frankenfield, D.L.; Klassen, P.S.; Coladonato, J.A.; Szczech, L.; Johnson, C.A.; Besarab, A.; Rocco, M.; McClellan, W.; Wish, J.; et al. Regional variability in anemia management and hemoglobin in the US. Nephrol. Dial. Transplant. 2003, 18, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ifudu, O.; Uribarri, J.; Rajwani, I.; Vlacich, V.; Reydel, K.; Delosreyes, G.; Friendman, E.A. Low hematocrit may connote a malnutrition/ inflammation syndrome in hemodialysis patients. Dial. Transplant 2002, 31, 845–848. [Google Scholar]
- Labonia, W.D. l-carnitine effects on anemia in hemodialyzed patients treated with erythropoietin. Am. J. Kidney Dis. 1995, 26, 757–764. [Google Scholar] [CrossRef]
- Zhu, Y.; Jameson, E.; Crosatti, M.; Schäfer, H.; Rajakumar, K.; Bugg, T.D.; Chen, Y. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc. Natl. Acad. Sci. USA 2014, 111, 4268–4273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
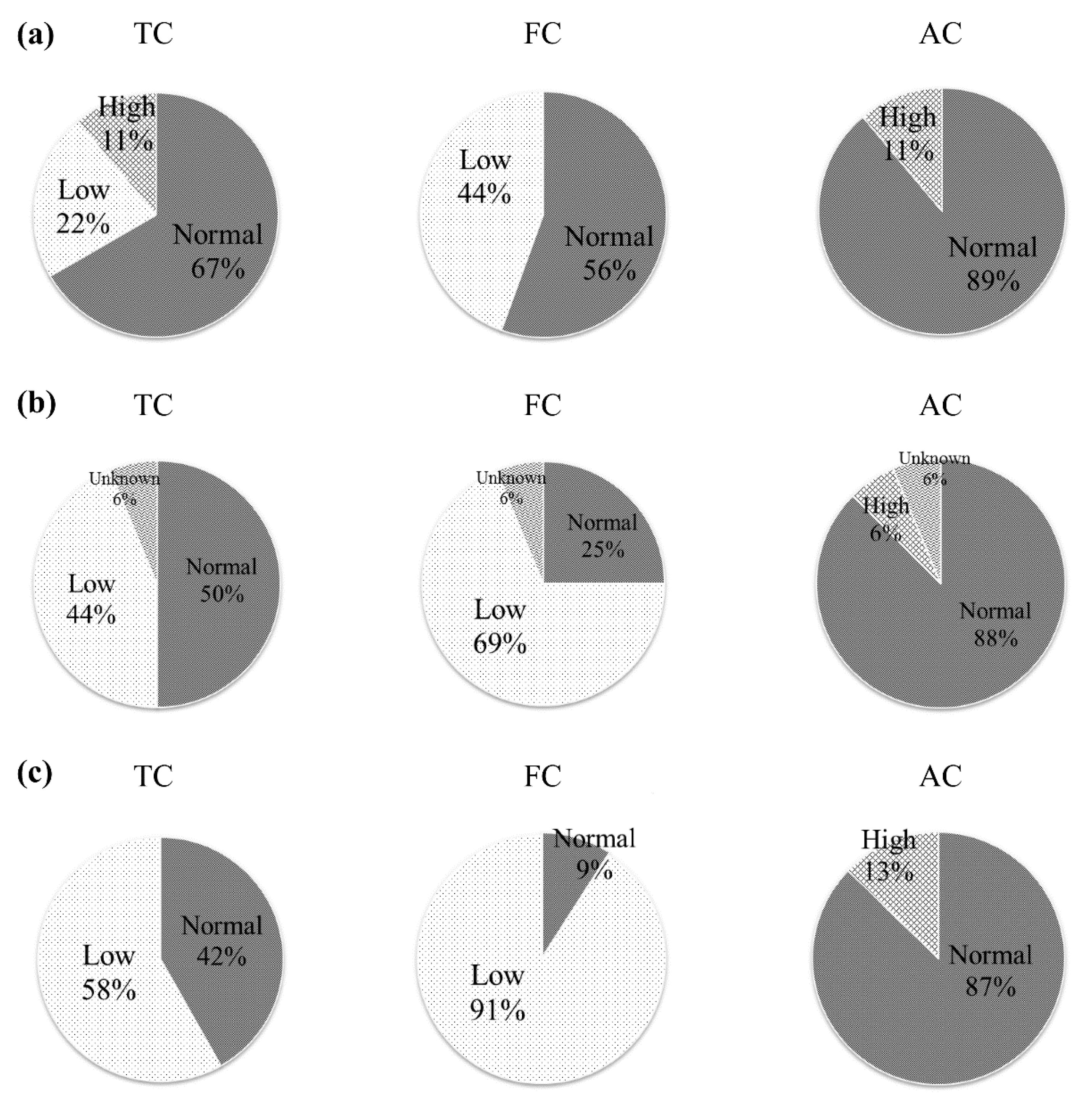
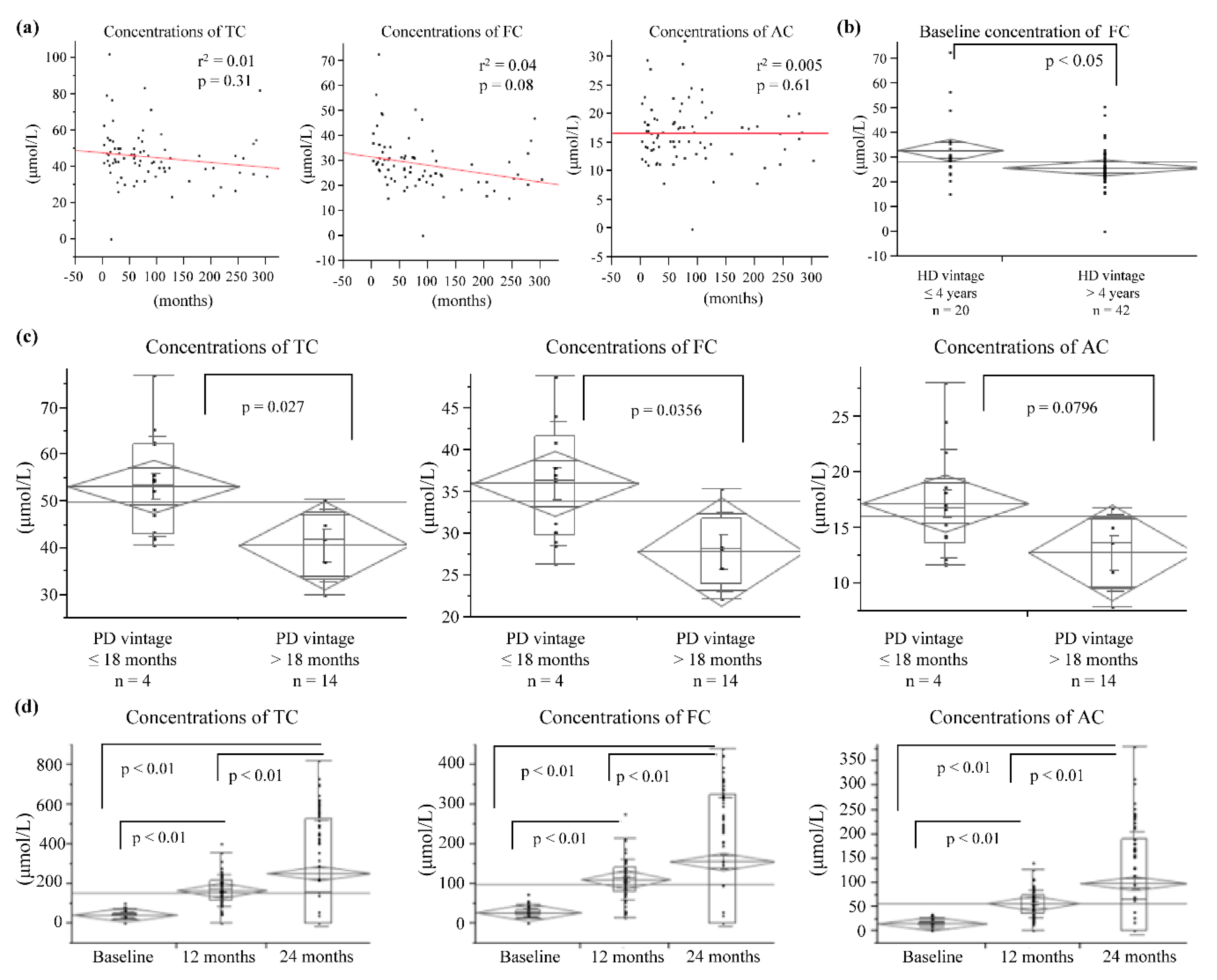
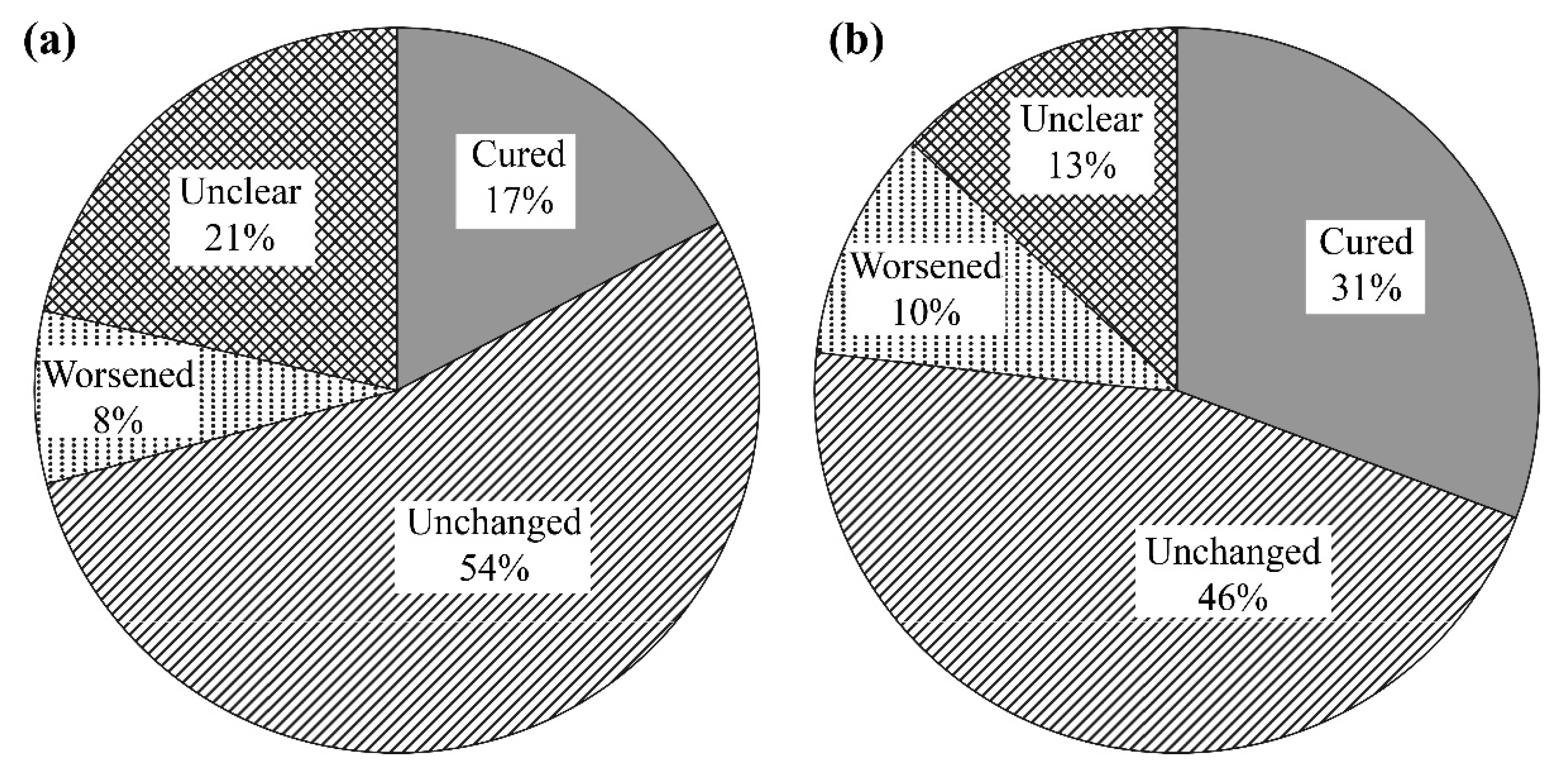
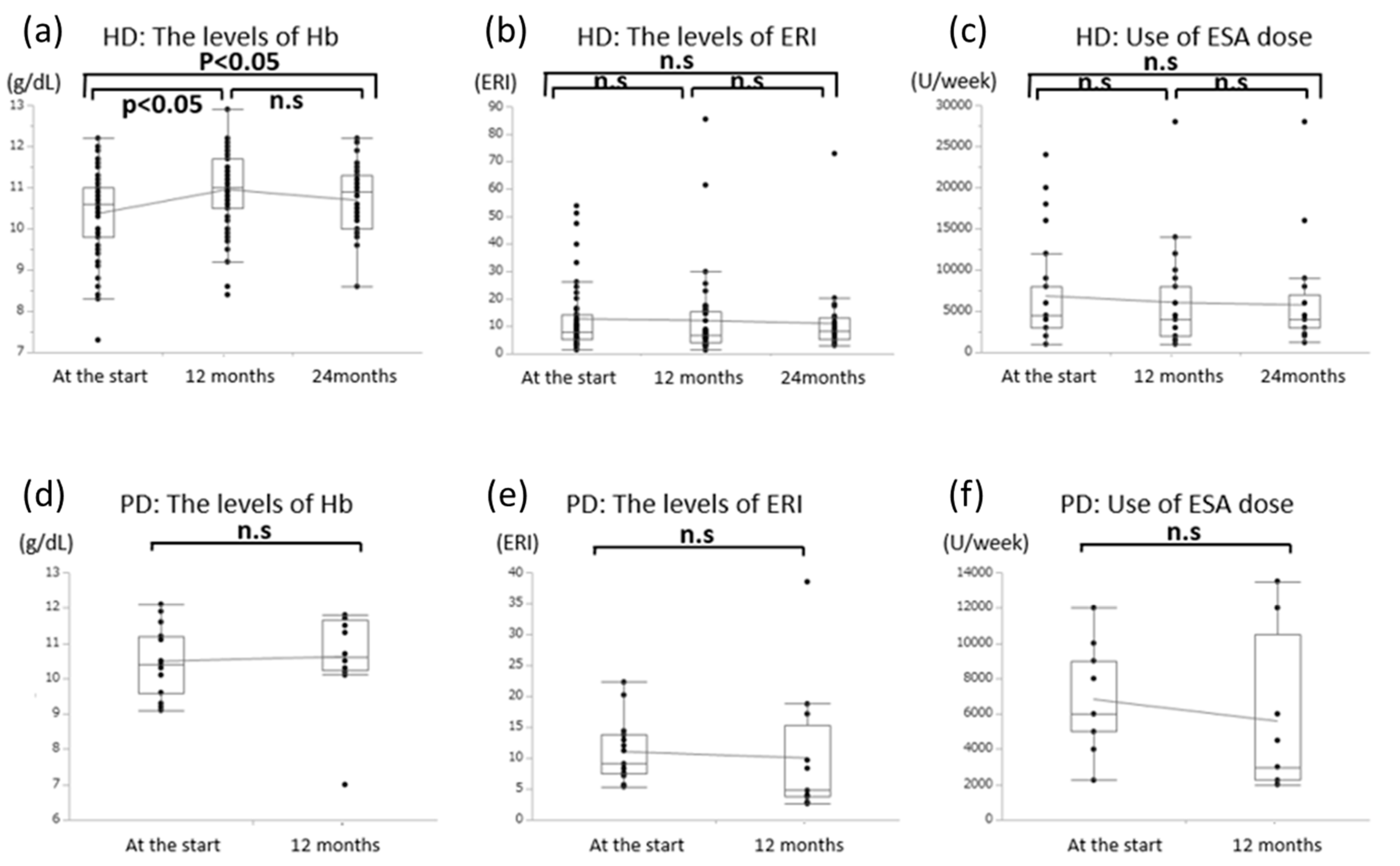
| All Patients (n = 80) | PD (n = 18) | HD (n = 62) | |
|---|---|---|---|
| Age (years) | 62.4 ± 14.8 | 56.7 ± 11.7 | 64.0 ± 15.3 |
| Dialysis duration (months) | 88.1 ± 85.4 | 14.8 ± 11.1 | 112.5 ± 85.4 |
| Diabetes mellitus | 18/80 | 0/18 | 18/62 |
| Male sex (%) | 78.8 | 66.1 | 66.7 |
| Peritoneal Dialysis | Hemodialysis | ||||
|---|---|---|---|---|---|
| 0 Months | 12 Months | 0 Months | 12 Months | 24 Months | |
| Hb (g/dL) | 10.6 ± 1.1 | 10.6 ± 1.3 | 10.2 ± 1.2 * | 10.9 ± 0.9 * | 10.7 ± 0.8 * |
| LVMI 1 (g/m 2) | 142.4 ± 46.8 | 133.6 ± 37.4 | 152.5 ± 37.8 | 151.2 ± 36.6 | 153.3 ± 36.8 |
| E/A 2 | 0.83 ± 0.35 | 0.70 ± 0.37 | 0.77 ± 0.28 | 0.75 ± 0.20 | 0.75 ± 0.19 |
| E/e’3 | 10.0 ± 3.6 | 10.4 ± 5.0 | 13.9 ± 5.5 | 15.1 ± 7.2 | 16.2 ± 7.3 |
| BNP 4 (pg/mL) | 121.5 ± 119 | 130.7 ± 140 | 455.0 ± 40.3 | 446.8 ± 57.8 | 449 ± 56.7 |
| ANP 5 (pg/mL) | 54.3 ± 41.45 | 52.3 ± 38.3 | 84.6 ± 66.5 | 74.8 ± 58.4 | 79.2 ± 59.3 |
| LDL 6 (mg/dL) | 95.0 ± 22.3 | 92.0 ± 21.4 | 83.0 ± 28.4 | 89.0 ± 24.5 | 89.7 ± 24.8 |
| HDL 7 (mg/dL) | 48.0 ± 10.2 | 58.0 ± 18.1 | 44.0 ± 12.2 | 45.0 ± 11.5 | 44.9 ± 12.8 |
| TG 8 (mg/dL) | 104.0 ± 33.6 | 101.0 ± 36.7 | 101.0 ± 39.8 | 122.0 ± 55.8 | 125.6 ± 56.7 |
| TSAT 9 (%) | 35.8 ± 8.8 | 27.6 ± 11.4 | 21.8 ± 11.5 | 24.4 ± 12.1 | 25.6 ± 12.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuwasawa-Iwasaki, M.; Io, H.; Muto, M.; Ichikawa, S.; Wakabayashi, K.; Kanda, R.; Nakata, J.; Nohara, N.; Tomino, Y.; Suzuki, Y. Effects of l-Carnitine Supplementation in Patients Receiving Hemodialysis or Peritoneal Dialysis. Nutrients 2020, 12, 3371. https://doi.org/10.3390/nu12113371
Kuwasawa-Iwasaki M, Io H, Muto M, Ichikawa S, Wakabayashi K, Kanda R, Nakata J, Nohara N, Tomino Y, Suzuki Y. Effects of l-Carnitine Supplementation in Patients Receiving Hemodialysis or Peritoneal Dialysis. Nutrients. 2020; 12(11):3371. https://doi.org/10.3390/nu12113371
Chicago/Turabian StyleKuwasawa-Iwasaki, Masako, Hiroaki Io, Masahiro Muto, Saki Ichikawa, Keiichi Wakabayashi, Reo Kanda, Junichiro Nakata, Nao Nohara, Yasuhiko Tomino, and Yusuke Suzuki. 2020. "Effects of l-Carnitine Supplementation in Patients Receiving Hemodialysis or Peritoneal Dialysis" Nutrients 12, no. 11: 3371. https://doi.org/10.3390/nu12113371




