Cooking for Vitality: Pilot Study of an Innovative Culinary Nutrition Intervention for Cancer-Related Fatigue in Cancer Survivors
Abstract
:1. Introduction
2. Methods
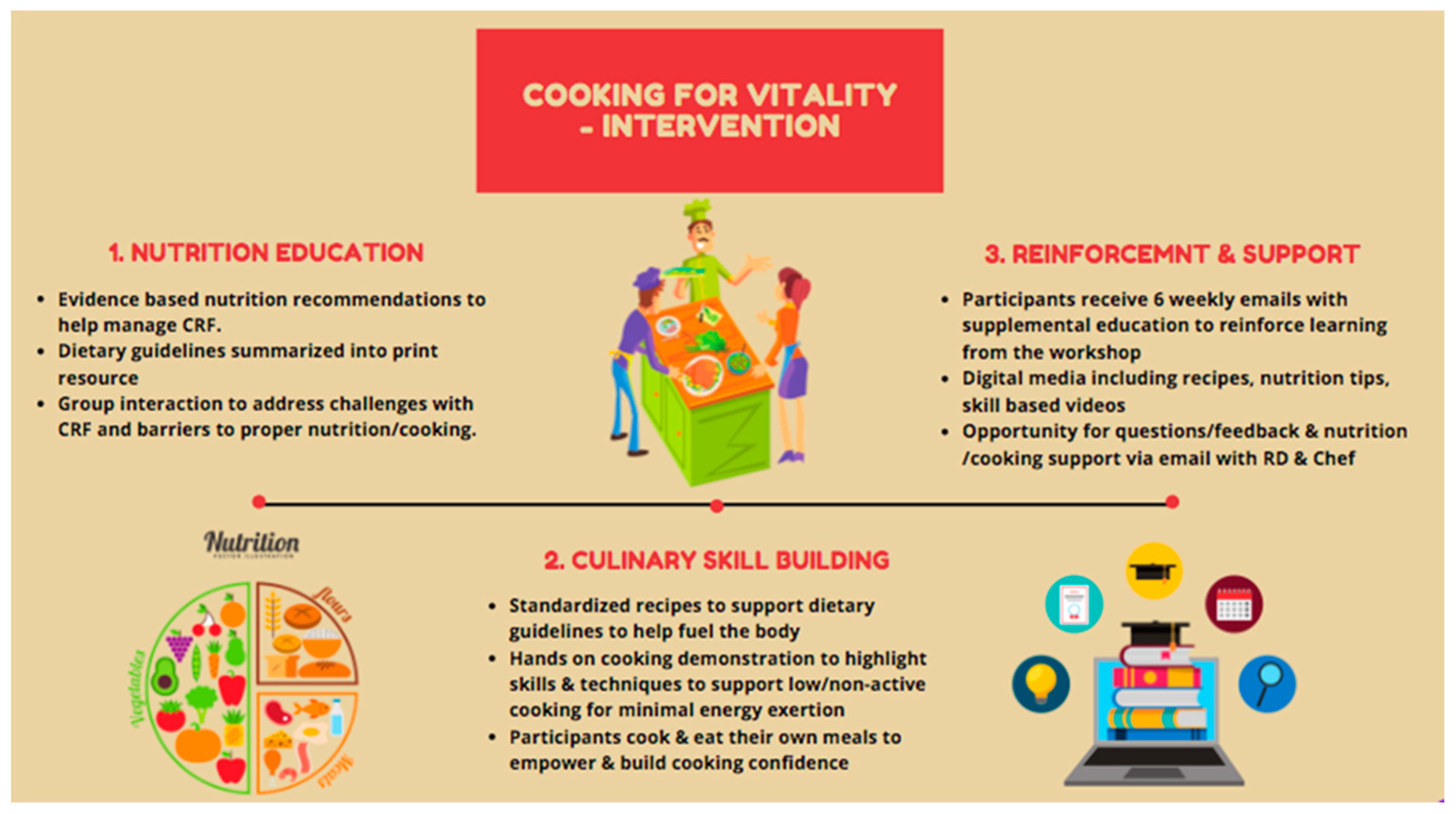
2.1. Intervention
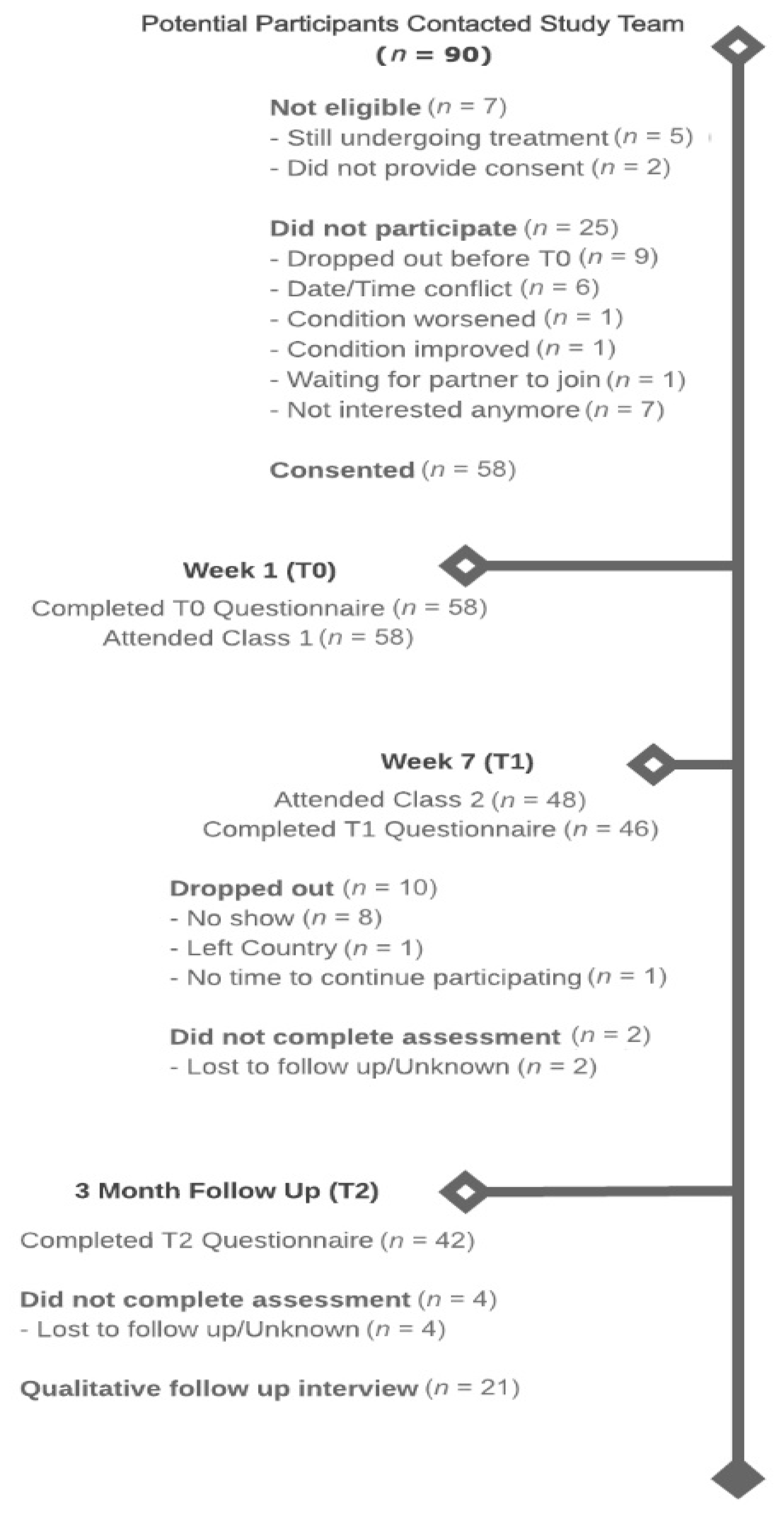
2.2. Participants and Procedure
2.3. Study Outcomes
2.3.1. Demographic and Clinical Data
2.3.2. Primary Outcome: Feasibility and Acceptability
2.3.3. Secondary Outcomes: Exploratory Clinical Outcomes
2.4. Data Analyses
3. Results
3.1. Feasibility
3.2. Acceptability
3.3. Satisfaction with the C4V Program
3.4. Areas for Program Improvement
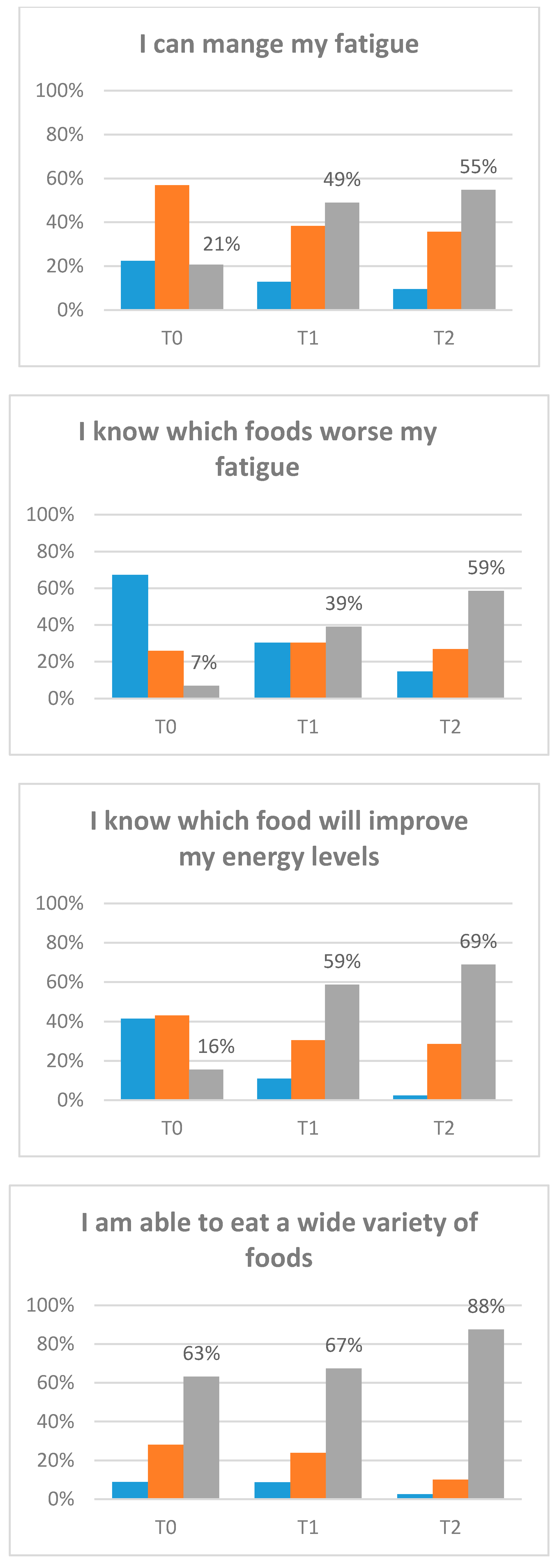
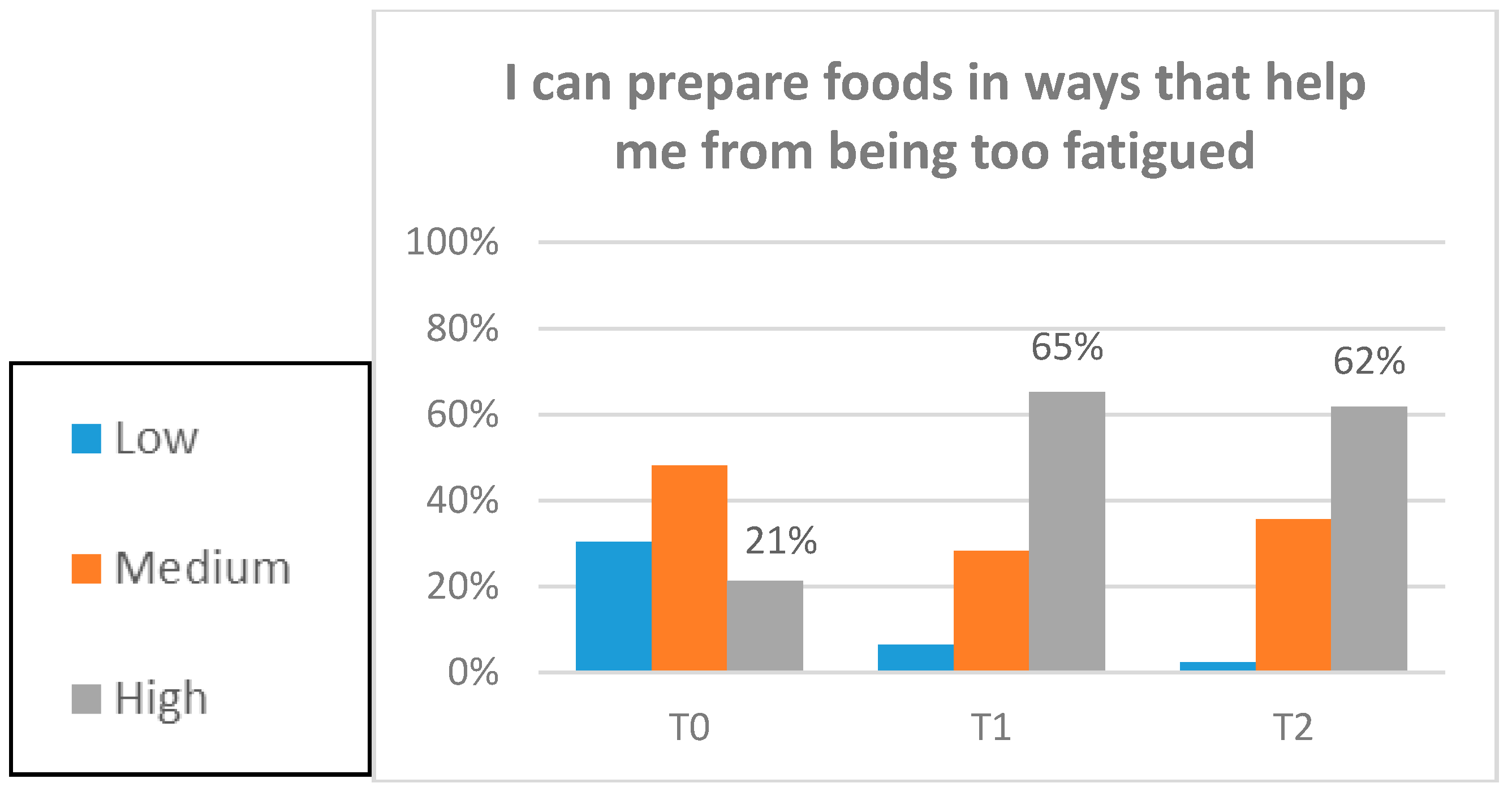
3.5. Exploratory Clinical Outcomes
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine; Board on Health Care Services; Committee on Psychosocial Services to Cancer Patients/Families in a Community Setting. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs; Adler, N., Page, A., Eds.; National Academies Press: Washington, DC, USA, 2008. [Google Scholar]
- Soothill, K.; Morris, S.M.; Thomas, C.; Harman, J.C.; Francis, B.; McIllmurray, M.B. The universal, situational, and personal needs of cancer patients and their main carers. Eur. J. Oncol. Nurs. 2003, 7, 5–13. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Guidelines Version 2. 2018 Cancer-Related Fatigue Plymouth Meeting, PA National Comprehensive Cancer Network. 2018. Available online: https://www.nccn.org/professionals/physician_gls (accessed on 22 June 2020).
- Behringer, K.; Goergen, H.; Müller, H.; Thielen, I.; Brillant, C.; Kreissl, S.; Halbsguth, T.V.; Meissner, J.; Greil, R.; Moosmann, P.; et al. Cancer-Related Fatigue in Patients with and Survivors of Hodgkin Lymphoma: The Impact on Treatment Outcome and Social Reintegration. J. Clin. Oncol. 2016, 34, 4329–4337. [Google Scholar] [CrossRef]
- Servaes, P.; Verhagen, C.; Bleijenberg, G. Fatigue in cancer patients during and after treatment: Prevalence, correlates and interventions. Eur. J. Cancer 2002, 38, 27–43. [Google Scholar] [CrossRef]
- Kuhnt, S.; Ernst, J.; Singer, S.; Stolzenburg, J.-U.; Schwarz, R.; Rüffer, J.; Kortmann, R.-D. Fatigue in Cancer Survivors—Prevalence and Correlates. Onkologie 2009, 32, 312–317. [Google Scholar] [CrossRef]
- Minton, O.; Stone, P. How common is fatigue in disease-free breast cancer survivors? A systematic review of the literature. Breast Cancer Res. Treat. 2007, 112, 5–13. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Desmond, K.A.; Bernaards, C.; Rowland, J.H.; Meyerowitz, B.E.; Belin, T.R. Fatigue in long-term breast carcinoma survivors. Cancer 2006, 106, 751–758. [Google Scholar] [CrossRef]
- Curt, G.A.; Breitbart, W.; Cella, D.; Groopman, J.E.; Horning, S.J.; Itri, L.M.; Johnson, D.H.; Miaskowski, C.; Scherr, S.L.; Portenoy, R.K.; et al. Impact of cancer-related fatigue on the lives of patients: New findings from the fatigue coalition. Oncologist 2000, 5, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Inglis, J.E.; Lin, P.-J.; Kerns, S.L.; Kleckner, I.R.; Kleckner, A.S.; Castillo, D.A.; Mustian, K.M.; Peppone, L.J. Nutritional Interventions for Treating Cancer-Related Fatigue: A Qualitative Review. Nutr. Cancer 2019, 71, 21–40. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Tao, M.L.; Hu, W.; Belin, T.R.; Sepah, S.; Cole, S.; Aziz, N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin. Cancer Res. 2009, 15, 5534–5540. [Google Scholar] [CrossRef] [Green Version]
- Bower, J.E. Cancer-related fatigue: Links with inflammation in cancer patients and survivors. Brain Behav. Immun. 2007, 21, 863–871. [Google Scholar] [CrossRef] [Green Version]
- National Cancer Institute. National Cancer Institute: Nutrition in Cancer Care (PDQ®)–Health Professional Version. 2017. Available online: https://www.cancer.gov/about-cancer/treatment/side-effects/appetite-loss/nutrition-hp-pdq (accessed on 24 June 2020).
- Demark-Wahnefried, W.; Campbell, K.L.; Hayes, S.C. Weight management and its role in breast cancer rehabilitation. Cancer 2012, 118, 2277–2287. [Google Scholar] [CrossRef] [Green Version]
- Ziętarska, M.; Krawczyk-Lipiec, J.; Kraj, L.; Zaucha, R.; Małgorzewicz, S. Chemotherapy-Related Toxicity, Nutritional Status and Quality of Life in Precachectic Oncologic Patients with, or without, High Protein Nutritional Support. A Prospective, Randomized Study. Nutrients 2017, 9, 1108. [Google Scholar] [CrossRef] [Green Version]
- Kilgour, R.D.; Viganò, A.; Trutschnigg, B.; Lucar, E.; Borod, M.; Morais, J.A. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support. Care Cancer 2013, 21, 3261–3270. [Google Scholar] [CrossRef]
- Kilgour, R.D.; Vigano, A.; Trutschnigg, B.; Hornby, L.; Lucar, E.; Bacon, S.L.; Morais, J.A. Cancer-related fatigue: The impact of skeletal muscle mass and strength in patients with advanced cancer. J. Cachexia Sarcopenia Muscle 2010, 1, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Ryan, A.M.; Power, D.G.; Daly, L.E.; Cushen, S.J.; Bhuachalla, Ē.N.; Prado, C.M. Cancer-associated malnutrition, cachexia and sarcopenia: The skeleton in the hospital closet 40 years later. Proc. Nutr. Soc. 2016, 75, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Segerstrom, S.C.; Miller, G.E. Psychological Stress and the Human Immune System: A Meta-Analytic Study of 30 Years of Inquiry. Psychol. Bull. 2004, 130, 601–630. [Google Scholar] [CrossRef] [Green Version]
- Collado-Hidalgo, A.; Bower, J.; Ganz, P.; Cole, S.; Irwin, M. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin. Cancer Res. 2006, 12, 2759–2766. [Google Scholar] [CrossRef] [Green Version]
- Robien, K.; Demark-Wahnefried, W.; Rock, C.L. Evidence-Based Nutrition Guidelines for Cancer Survivors: Current Guidelines, Knowledge Gaps, and Future Research Directions. J. Am. Diet. Assoc. 2011, 111, 368–375. [Google Scholar] [CrossRef]
- Rock, C.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.; Schwartz, A.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef] [Green Version]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2018. Available online: http://dietandcancerreport.org (accessed on 2 September 2020).
- Stobäus, N.; Müller, M.J.; Küpferling, S.; Schulzke, J.-D.; Norman, K. Low Recent Protein Intake Predicts Cancer-Related Fatigue and Increased Mortality in Patients with Advanced Tumor Disease Undergoing Chemotherapy. Nutr. Cancer 2015, 67, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Guest, D.D.; Evans, E.M.; Rogers, L.Q. Diet components associated with perceived fatigue in breast cancer survivors. Eur. J. Cancer Care 2012, 22, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Alfano, C.; Imayama, I.; Neuhouser, M.; Kiecolt-Glaser, J.; Smith, A.; Meeske, K.; McTiernan, A.; Bernstein, L.; Baumgartner, K.B.; Ulrich, C.M.; et al. Fatigue, inflammation, and omega-3 and omega-6 fatty acid intake among breast cancer survivors. J. Clin. Oncol. 2012, 30, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Sen, A.; Han-Markey, T.L.; Harris, R.E. Examination of the Association of Diet and Persistent Cancer-Related Fatigue: A Pilot Study. Oncol. Nurs. Forum 2012, 40, E41–E49. [Google Scholar] [CrossRef] [Green Version]
- George, S.M.; Alfano, C.M.; Neuhouser, M.L.; Smith, A.W.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L.; Ballard-Barbash, R. Better postdiagnosis diet quality is associated with less cancer-related fatigue in breast cancer survivors. J. Cancer Surviv. 2014, 8, 680–687. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Q.; Kang, X.-M.; Song, Y.; Zhao, W. Factors associated with cancer-related fatigue in breast cancer patients undergoing endocrine therapy in an urban setting: A cross-sectional study. BMC Cancer 2010, 10, 453. [Google Scholar] [CrossRef] [Green Version]
- Baguley, B.J.; Bolam, K.A.; Wright, O.R.L.; Skinner, T.L. The Effect of Nutrition Therapy and Exercise on Cancer-Related Fatigue and Quality of Life in Men with Prostate Cancer: A Systematic Review. Nutrients 2017, 9, 1003. [Google Scholar] [CrossRef] [Green Version]
- Zick, S.M.; Colacino, J.A.; Cornellier, M.; Khabir, T.; Surnow, K.; Djuric, Z. Fatigue reduction diet in breast cancer survivors: A pilot randomized clinical trial. Breast Cancer Res. Treat. 2016, 161, 299–310. [Google Scholar] [CrossRef]
- Blanchard, C.; Courneya, K.; Stein, K. Cancer Survivors’ Adherence to Lifestyle Behavior Recommendations and Associations with Health-Related Quality of Life: Results from the American Cancer Society’s SCS-II. J. Clin. Oncol. 2008, 26, 2198–2204. [Google Scholar] [CrossRef]
- Mayer, D.K.; Terrin, N.C.; Menon, U.; Kreps, G.L.; McCance, K.; Parsons, S.K.; Mooney, K.H. Health Behaviors in Cancer Survivors. Oncol. Nurs. Forum 2007, 34, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Gu, Q.; Dummer, T.B.J.; Spinelli, J.J.; Murphy, R.A. Diet Quality among Cancer Survivors and Participants without Cancer: A Population-Based, Cross-Sectional Study in the Atlantic Partnership for Tomorrow’s Health Project. Nutrients 2019, 11, 3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maschke, J.; Kruk, U.; Kastrati, K.; Kleeberg, J.; Buchholz, D.; Erickson, N.; Huebner, J. Nutritional care of cancer patients: A survey on patients’ needs and medical care in reality. Int. J. Clin. Oncol. 2016, 22, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Pearson, E.; Morris, M.; Di Stefano, M.; McKinstry, C. Interventions for cancer-related fatigue: A scoping review. Eur. J. Cancer Care 2016, 27, e12516. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.M.; Mooney, K.; Alvarez-Perez, A.; Breitbart, W.S.; Carpenter, K.M.; Cella, D.; Cleeland, C.; Dotan, E.; Eisenberger, M.A.; Escalante, C.P.; et al. Cancer-Related Fatigue, Version 2.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 1012–1039. [Google Scholar] [CrossRef]
- Bower, J.E.; Bak, K.; Berger, A.; Breitbart, W.; Escalante, C.P.; Ganz, P.A.; Schnipper, H.H.; Lacchetti, C.; Ligibel, J.A.; Lyman, G.H.; et al. Screening, Assessment, and Management of Fatigue in Adult Survivors of Cancer: An American Society of Clinical Oncology Clinical Practice Guideline Adaptation. J. Clin. Oncol. 2014, 32, 1840–1850. [Google Scholar] [CrossRef] [Green Version]
- Howell, D.; Keller-Olaman, S.; Oliver, T.; Hack, T.; Broadfield, L.; Biggs, K.; Chung, J.; Gravelle, D.; Green, E.; Hamel, M.; et al. A pan-Canadian practice guideline and algorithm: Screening, assessment, and supportive care of adults with cancer-related fatigue. Curr. Oncol. 2013, 20, e233–e246. [Google Scholar] [CrossRef] [Green Version]
- Baguley, B.J.; Skinner, T.L.; Wright, O.R.L. Nutrition therapy for the management of cancer-related fatigue and quality of life: A systematic review and meta-analysis. Br. J. Nutr. 2019, 122, 527–541. [Google Scholar] [CrossRef]
- Basen-Engquist, K.; Alfano, C.M.; Maitin-Shepard, M.; Thomson, C.A.; Schmitz, K.; Pinto, B.M.; Stein, K.; Zucker, D.S.; Syrjala, K.L.; Fallon, E.; et al. Agenda for Translating Physical Activity, Nutrition, and Weight Management Interventions for Cancer Survivors into Clinical and Community Practice. Obesity 2017, 25, S9–S22. [Google Scholar] [CrossRef] [Green Version]
- Michie, S.; Jochelson, K.; Markham, W.A.; Bridle, C. Low-income groups and behaviour change interventions: A review of intervention content, effectiveness and theoretical frameworks. J. Epidemiol. Community Health 2009, 63, 610–622. [Google Scholar] [CrossRef] [Green Version]
- Jerant, A.; Von Friederichs-Fitzwater, M.M.; Moore, M. Patients’ perceived barriers to active self-management of chronic conditions. Patient Educ. Couns. 2005, 57, 300–307. [Google Scholar] [CrossRef]
- Larson, N.I.; Perry, C.L.; Story, M.; Neumark-Sztainer, D. Food Preparation by Young Adults Is Associated with Better Diet Quality. J. Am. Diet. Assoc. 2006, 106, 2001–2007. [Google Scholar] [CrossRef]
- Garcia, A.L.; Reardon, R.; McDonald, M.; Vargas-Garcia, E.J. Community Interventions to Improve Cooking Skills and Their Effects on Confidence and Eating Behaviour. Curr. Nutr. Rep. 2016, 5, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Wrieden, W.L.; Anderson, A.S.; Longbottom, P.J.; Valentine, K.; Stead, M.; Caraher, M.; Lang, T.; Gray, B.; Dowler, E. The impact of a community-based food skills intervention on cooking confidence, food preparation methods and dietary choices—An exploratory trial. Public Health Nutr. 2007, 10, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Fulkerson, J.A.; Rydell, S.; Kubik, M.Y.; Lytle, L.; Boutelle, K.; Story, M.; Neumark-Sztainer, D.; Dudovitz, B.; Garwick, A. Healthy Home Offerings via the Mealtime Environment (HOME): Feasibility, Acceptability, and Outcomes of a Pilot Study. Obesity 2010, 18 (Suppl. 1), S69–S74. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.J.; Hermann, J.R. Cooking Classes Increase Fruit and Vegetable Intake and Food Safety Behaviors in Youth and Adults. J. Nutr. Educ. Behav. 2005, 37, 104–105. [Google Scholar] [CrossRef]
- Flego, A.; Herbert, J.; Waters, E.; Gibbs, L.; Swinburn, B.; Reynolds, J.; Moodie, M. Jamie’s Ministry of Food: Quasi-Experimental Evaluation of Immediate and Sustained Impacts of a Cooking Skills Program in Australia. PLoS ONE 2014, 9, e114673. [Google Scholar] [CrossRef]
- Reicks, M.; Trofholz, A.C.; Stang, J.S.; Laska, M.N. Impact of cooking and home food preparation interventions among adults: Outcomes and implications for future programs. J. Nutr. Educ. Behav. 2014, 46, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Herbert, J.; Flego, A.; Gibbs, L.; Waters, E.; Swinburn, B.; Reynolds, J.; Moodie, M. Wider impacts of a 10-week community cooking skills program—Jamie’s Ministry of Food, Australia. BMC Public Health 2014, 14, 1161. [Google Scholar] [CrossRef] [Green Version]
- Borek, A.J.; Abraham, C. How do Small Groups Promote Behaviour Change? An Integrative Conceptual Review of Explanatory Mechanisms. Appl. Psychol. Health Well-Being 2018, 10, 30–61. [Google Scholar] [CrossRef] [Green Version]
- Greaves, C.; Campbell, J.L. Supporting self-care in general practice. Br. J. Gen. Pract. 2007, 57, 814–821. [Google Scholar]
- West, R.; Walia, A.; Hyder, N.; Shahab, L.; Michie, S. Behavior change techniques used by the English Stop Smoking Services and their associations with short-term quit outcomes. Nicotine Tob. Res. 2010, 12, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, U.; Haaland-Øverby, M.; Fredriksen, K.; Westermann, K.F.; Kvisvik, T. A scoping review of the literature on benefits and challenges of participating in patient education programs aimed at promoting self-management for people living with chronic illness. Patient Educ. Couns. 2016, 99, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A. Social Learning Theory; Prentice Hall: Englewood Cliffs, NJ, USA, 1977. [Google Scholar]
- Abraham, C.; Gardner, B. What psychological and behaviour changes are initiated by ‘expert patient’ training and what training techniques are most helpful? Psychol. Health 2009, 24, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Pearson, E.; Morris, M.; McKinstry, C. Cancer related fatigue: Implementing guidelines for optimal management. BMC Health Serv. Res. 2017, 17, 496. [Google Scholar] [CrossRef] [Green Version]
- Cancer Care Ontario. CCO Guidelines for Managing Fatigue. Available online: https://www.cancercareontario.ca/en/symptom-management/3991 (accessed on 28 June 2020).
- Bandura, A. Self-Efficacy: The Exercise of Control; Freeman: New York, NY, USA, 1997. [Google Scholar]
- Morris, T. Experiential learning—A systematic review and revision of Kolb’s model. Interact. Learn. Environ. 2019, 1–14. [Google Scholar] [CrossRef]
- Hollywood, L.E.; Surgenor, D.; Reicks, M.; McGowan, L.; Lavelle, F.; Spence, M.; Raats, M.M.; McCloat, A.; Mooney, E.; Caraher, M.; et al. Critical review of behaviour change techniques applied in intervention studies to improve cooking skills and food skills among adults. Crit. Rev. Food Sci. Nutr. 2017, 58, 2882–2895. [Google Scholar] [CrossRef] [Green Version]
- Michie, S.; Ashford, S.; Sniehotta, F.F.; Dombrowski, S.U.; Bishop, A.; French, D.P.; Williams, S.L. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: The CALO-RE taxonomy. Psychol. Health 2011, 26, 1479–1498. [Google Scholar] [CrossRef]
- Azzolino, D.; Arosio, B.; Marzetti, E.; Calvani, R.; Cesari, M. Nutritional Status as a Mediator of Fatigue and Its Underlying Mechanisms in Older People. Nutrients 2020, 12, 444. [Google Scholar] [CrossRef] [Green Version]
- Saunders, B.; Sim, J.; Kingstone, T.; Baker, S.; Waterfield, J.; Bartlam, B.; Burroughs, H.; Jinks, C. Saturation in qualitative research: Exploring its conceptualization and operationalization. Qual. Quant. 2018, 52, 1893–1907. [Google Scholar] [CrossRef]
- Cella, D.; Lai, J.-S.; Chang, C.-H.; Peterman, A.; Slavin, M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer 2002, 94, 528–538. [Google Scholar] [CrossRef]
- Yellen, S.B.; Cella, D.; Webster, K.; Blendowski, C.; Kaplan, E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J. Pain Symptom Manag. 1997, 13, 63–74. [Google Scholar] [CrossRef]
- Van Belle, S.; Paridaens, R.; Evers, G.; Kerger, J.; Bron, D.; Foubert, J.; Ponnet, G.; Steichel, D.V.; Heremans, C.; Rosillon, D. Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: Proposal for use as a screening tool. Support. Care Cancer 2004, 13, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Curran, S.L.; Andrykowski, M.A.; Studts, J.L. Short form of the profile of mood states (POMS-SF): Psychometric information. Psychol. Assess. 1995, 7, 80–83. [Google Scholar] [CrossRef]
- Baker, F.; Denniston, M.; Zabora, J.; Polland, A.; Dudley, W.N. A POMS short form for cancer patients: Psychometric and structural evaluation. Psycho-Oncology 2002, 11, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Üstün, T.B.; Chatterji, S.; Kostanjsek, N.; Rehm, J.; Kennedy, C.; Epping-Jordan, J.; Saxena, S.; Von Korff, M.; Pull, C. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull. World Health Organ. 2010, 88, 815–823. [Google Scholar] [CrossRef]
- Devis, J.V.L.; Ayuso-Mateos, J.L.; Aguado, J.; Fernández, A.; Serrano-Blanco, A.; Roca, M.; Haro, J.M. The 12-item World Health Organization Disability Assessment Schedule II (WHO-DAS II): A nonparametric item response analysis. BMC Med. Res. Methodol. 2010, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Yates, P.; Aranda, S.; Hargraves, M.; Mirolo, B.; Clavarino, A.; McLachlan, S.; Skerman, H.; Thuret, R.; Renaudin, K.; Leclère, J.; et al. Randomized Controlled Trial of an Educational Intervention for Managing Fatigue in Women Receiving Adjuvant Chemotherapy for Early-Stage Breast Cancer. J. Clin. Oncol. 2005, 23, 6027–6036. [Google Scholar] [CrossRef] [Green Version]
- Barton, K.L.; Wrieden, W.L.; Anderson, A.S. Validity and reliability of a short questionnaire for assessing the impact of cooking skills interventions. J. Hum. Nutr. Diet. 2011, 24, 588–595. [Google Scholar] [CrossRef]
- Lancaster, G.A.; Dodd, S.; Williamson, P.R. Design and analysis of pilot studies: Recommendations for good practice. J. Eval. Clin. Pr. 2004, 10, 307–312. [Google Scholar] [CrossRef]
- Boeije, H. A Purposeful Approach to the Constant Comparative Method in the Analysis of Qualitative Interviews. Qual. Quant. 2002, 36, 391–409. [Google Scholar] [CrossRef]
- Cella, D.; Eton, D.T.; Lai, J.-S.; Peterman, A.H.; Merkel, D.E. Combining Anchor and Distribution-Based Methods to Derive Minimal Clinically Important Differences on the Functional Assessment of Cancer Therapy (FACT) Anemia and Fatigue Scales. J. Pain Symptom Manag. 2002, 24, 547–561. [Google Scholar] [CrossRef]
- Jarpe-Ratner, E.; Folkens, S.; Sharma, S.; Daro, D.; Edens, N.K. An Experiential Cooking and Nutrition Education Program Increases Cooking Self-Efficacy and Vegetable Consumption in Children in Grades 3–8. J. Nutr. Educ. Behav. 2016, 48, 697–705.e1. [Google Scholar] [CrossRef] [Green Version]
- Farmer, N.; Touchton-Leonard, K.; Ross, A. Psychosocial Benefits of Cooking Interventions: A Systematic Review. Health Educ. Behav. 2017, 45, 167–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, H. A Pilot Study: Evaluation of the Effectiveness of a Cooking Demonstration and Nutrition Education Class on Cancer Patients’ Attitude and Self-Perception Change. Master’s Thesis, Baylor University, Waco, TX, USA, 2016. [Google Scholar]
- Greenlee, H.; Gaffney, A.; Aycinena, A.; Koch, P.; Contento, I.; Karmally, W.; Richardson, J.M.; Lim, E.; Tsai, W.-Y.; Crew, K.; et al. ¡Cocinar Para Su Salud!: Randomized controlled trial of a culturally based dietary intervention among Hispanic breast cancer survivors. J. Acad. Nutr. Diet. 2015, 115, 709–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aycinena, A.C.; Jennings, K.-A.; Gaffney, A.O.; Koch, P.A.; Contento, I.; Gonzalez, M.; Guidon, E.; Karmally, W.; Hershman, D.; Greenlee, H. ¡Cocinar Para Su Salud! Development of a Culturally Based Nutrition Education Curriculum for Hispanic Breast Cancer Survivors Using a Theory-Driven Procedural Model. Health Educ. Behav. 2016, 44, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Newman, V.A.; Thomson, C.A.; Rock, C.L.; Flatt, S.W.; Kealey, S.; Bardwell, W.A.; Caan, B.J.; Pierce, J.P. Achieving substantial changes in eating behavior among women previously treated for breast cancer—An overview of the intervention. J. Am. Diet. Assoc. 2005, 105, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.; Rock, C.L.; Caan, B.; Flatt, S.W.; Al-Delaimy, W.; Newman, V.A.; Hajek, R.A.; Chilton, J.A.; Pierce, J.P. Increase in Cruciferous Vegetable Intake in Women Previously Treated for Breast Cancer Participating in a Dietary Intervention Trial. Nutr. Cancer 2007, 57, 11–19. [Google Scholar] [CrossRef]
- Goldfield, G.; Epstein, L.; Kilanowski, C.; Paluch, R.; Kogut-Bossler, B. Cost-effectiveness of group and mixed family-based treatment for childhood obesity. Int. J. Obes. 2001, 25, 1843–1849. [Google Scholar] [CrossRef] [Green Version]
- Singer, S.; Kuhnt, S.; Zwerenz, R.; Eckert, K.; Hofmeister, D.; Dietz, A.; Giesinger, J.M.; Hauss, J.; Papsdorf, K.; Briest, S.; et al. Age- and sex-standardised prevalence rates of fatigue in a large hospital-based sample of cancer patients. Br. J. Cancer 2011, 105, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Bezanson, K.; Luxton, M. Social Reproduction: Feminist Political Economy Challenges Neo-Liberalism; McGill-Queen’s University Press: Montreal, QC, Canada; Kingston, ON, Canada, 2006. [Google Scholar]
- Palinkas, L.A.; Horwitz, S.M.; Green, C.A.; Wisdom, J.P.; Duan, N.; Hoagwood, K. Purposeful Sampling for Qualitative Data Collection and Analysis in Mixed Method Implementation Research. Adm. Policy Ment. Health Ment. Health Serv. Res. 2015, 42, 533–544. [Google Scholar] [CrossRef] [Green Version]
- Alexander, S.; Minton, O.; Andrews, P.; Stone, P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur. J. Cancer 2009, 45, 384–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Age (Years) mean (SD), range | 58 (± 12.3), 25–86 |
| Sex n (%) | |
| Male | 7 (12%) |
| Female | 51 (88%) |
| Marital Statusn (%) | |
| Married/Common Law | 23 (40%) |
| Divorced/Separated | 12 (21%) |
| Widowed | 1 (2%) |
| Single | 21 (37%) |
| Cancer Siten (%) | |
| Breast | 35 (61%) |
| Gynecological | 7 (12%) |
| Gastrointestinal | 2 (3.5%) |
| Genitourinary | 3 (5%) |
| Endocrinology | 3 (5%) |
| Hematology | 4 (7%) |
| Head and Neck | 1 (2%) |
| Central Nervous System | 1 (2%) |
| Skin | 1 (2%) |
| Time Since Diagnosis (months) | |
| mean (SD), range | 24 (± 24.4), 0–176 |
| Cancer Treatmentsn (%) | |
| Surgery | 46 (81%) |
| Chemotherapy | 33 (58%) |
| Radiation Therapy | 40 (70%) |
| Themes | Analytic Note | Example Quote |
|---|---|---|
| Program length and frequency | Most participants felt that the program length and frequency of in-class sessions were appropriate, particularly in light of restrictions on energy posed by cancer-related fatigue (CRF). Although, some explained that they would have liked more in-class sessions, they appreciated that this could pose a barrier to those with more severe CRF. | “This (program length and frequency) was perfect for what we were trying to do and I think especially for the people who really were quite overwhelmed by their sickened stances I think they found it fun and positive and not purely demanding, which is important.” “I don’t think I have any suggestions (for program improvements). The program takes into consideration our fatigue and so it’s a good balance of learning, but not overdoing it in terms of time (commitment). I would have liked more in-person classes, but know that this could deter some people whose fatigue is really bad (from taking part in the program). So, I think it (Cooking for Vitality) is very balanced.” |
| Ease of implementation | All participants explained that recipes and culinary strategies acquired through the program were easy to understand and most described ease of implementing this knowledge and skill at home. Participants found that the positive environment in which recipes were acquired provided a “halo” effect to the content that further helped to motivate participants to implement during and beyond intervention. | “So I’m sure that, well I can only speak for myself, but I went away feeling “gosh, we made some interesting recipes there, I can do that!” and I will do that, because it’s, and probably the recipes acquired a halo from the context in which I first saw them.” “There is a mental barrier for people who don’t cook. We don’t like cooking, just to do it. But I attended that session, and it came together so easily, and it tasted so good. So what are you waiting for, just do it! So I started making baked fish with vegetables [at home] just like the way he [wellness chef] taught us. It was so simple and so healthy too.” |
| Program flexibility | Participants appreciated flexibility to attend other in-class sessions when timing conflicts and or restrictions posed by fatigue prevented them from attending their scheduled group sessions. Without this flexibility, some participants explained that they would not have been able to fully adhere to the intervention by attending both in-class sessions. | “One of the classes I had to miss because I just didn’t have the energy that day. Well, Geremy reached out to see how I was doing and let me know that if I wanted, and I did, I could sit in with another group so that I didn’t miss out on anything, so that was helpful because, well at least my fatigue isn’t predictable, so that flexibility is really important for program like this.” |
| Theme: Satisfaction with the C4V Program | ||
| Sub-Themes: | Analytic Note | Example Quote |
| Expert information and personalized support from a chef and dietitian | All of the participants reinforced the value of having a central and credible source of information specific to the needs and limitations of those living with CRF. The delivery of this information from professionals with specific expertise in the area of food/nutrition and cancer was particularly important to reinforcing the credibility of the program. Participants further stressed the importance of a personalized approach, adjusting recipes to meet their dietary restrictions and preferences | “It was really helpful; it is like having an expert in your pocket. If you put in the effort and make this recipes and you come up with this road block there is someone there that will help you to figure it out. Because I want the recipes, at the time I was trying to avoid white flour, white rice, all sugars, and at the end of the recipe he (C4V Chef) would tell me how to adapt.” |
| The provision of culinary tips, tricks, and tools to facilitate cooking while experiencing fatigue | Participants in the program were advised to work with or around their fatigue, rather than attempting to re-establish their pre-cancer culinary practices. Participants described a number of energy conservation strategies learned through the program that they found to be of value. Those discussed most frequently included batch cooking and freezing, the use of parchment paper/one pot meals to reduce clean-up, and recipes that used non-perishable or frozen ingredients to limit the need for multiple visits to the grocery store. | “I can make a batch of food and I can divide it into portions and freeze it and then re-heat it afterwards I realized that the amount of work that I put in for let’s say five portions, it pays off. If I was doing individual portions, I would keep doing it and keep doing it, and doing it. I don’t have to waste my time and my energy, so I save [the frozen meals] for days that I don’t have any energy. So that is very useful.” “Pros (of the C4V program) are the tips and tricks and techniques and the kind of flair of making something that is really healthy and nutritious relatively easily, expending a little energy and it’s also fun. You feel supported and have good results. It was overall positive.” |
| Experiential learning | The opportunity to apply nutritional information and culinary strategies in a hands-on, class-based setting was crucial to participants’ capacity to retain nutritional information as well as practice and refine newly acquired culinary techniques and skills. Access to this kind of experiential learning helped participants to more easily transition these skills from the classroom to the home. | “Being able to do things hands-on, even if only part of the recipe we did ourselves, for me it makes it much more real and more plausible to do it at home. If I just watch, I understand it and see that it is possible but it doesn’t really penetrate, but it is the hands on portion that brings it to life. So I did come home and prepared some food in parchment packages.” |
| Social support | The group-based environment permitted cancer survivors to interact with as well as learn from each other. This was perceived as valuable for two key reasons. First, it helped to enhance the overall educational experience by fostering group-based question and answer periods. Second, the sharing of experiences between program participants provided opportunities for cancer survivors to normalize and validate their experiences with Cancer-related fatigue (CRF) which can be challenging given the invisibility of this side effect. | “I think connecting with other people who are also going through this struggle; I think connecting with them helps too. It’s not just like a cooking show, when we are meeting together, we kind of share our struggle, even though some of theirs were different than mine. But connecting with them kind of helps you, gives you encouragement. If they are trying, then maybe I should try too.” |
| Theme: Areas for program improvement | ||
| Sub-themes: | Analytic Note | Example quote |
| One-on-one consultation | Some participants explained that initial one-on-one consultations with each program participant could help to further tailor the content of the class to the unique circumstances and needs of those in the group. This was particularly true for those who felt that their life circumstances or needs were somewhat different from those most typically diagnosed with cancer. | “Because everybody in the class, well, you know (are) older, you have a room of more mature (people). So their metabolism and their goals and expectations are different than mine (as a younger person). So yeah, those one-on-one sessions in the beginning would be nice and helpful I guess, to understand what our expectations and objectives are for attending the session. That and how they can tailor the sessions for us and to help us address our concerns. Or help us get started on our goals.” |
| A graduated, multi-tiered program | Participants entered the program with different nutritional and culinary backgrounds. It was suggested that taking a multi-tiered approach (e.g., beginner, intermediate, advanced) to delivery would allow participants to enter the program at a level they felt most comfortable with and confident in. This approach was also suggested by those who sought to build upon and advance their knowledge base by graduating to different levels of the program. | P: “I would say it (the C4V Program) is a very positive experience. I was just disappointed that they didn’t have a phase two or phase three. They told me he would run the classes with the same recipes, but I told him that if he was to run the course with different recipes I would definitely go.” I: “In addition to learning new recipes, is there anything else you would hope to get from phase 2 and 3 (of the program)?” P: “Not so much the recipes, but the skills I think, and the experience of actually doing things. The more we do, even though the majority of the stuff is already prepared, the more that we would do ourselves, the more we would start to feel that we are better capable of doing it.” |
| More in-person cooking sessions | Participants explained that what made this program truly unique was the opportunity to execute recipes in real-time, with the guidance and support of a chef and registered dietitian. Being able to prepare recipes in this context was described as motivating, fostered greater uptake and retention of the recipes and skills, and made meal preparation at home feel more feasible. This was particularly important for those who entered the program with less culinary experience, as they felt additional in-class time was needed to refine and hone their newly-acquired culinary skills. | P: “I think the only (recommendation) is like, we wish there could have been more sessions. More interactive sessions.” I: “Why would that have been beneficial?” P: “… I think that you learn more when you are… you just learn more [in-class] than when you’re given a paper because you get to actually see it, cook it yourself and smell it and taste it. You get to see the finished product. That has a stronger impact and can make it more…it gives me more inspiration.” |
| A varied approach to the provision of support materials | While some participants enjoyed the convenience of recipe emails and videos, others explained that technology posed a barrier to being able to fully engage with and benefit from the program. It was suggested that a more varied approach to the provision of support materials, designed specifically to the needs and preferences of participants, would help to enhance compliance with the program while at home. | “It would be very nice to have like, a paper copy for example because I don’t have a printer at home. So I have to go back and check the online part. For me, it’s easier to have the paper in front of me because again, we’re talking about fatigue and mental fatigue. What I see is that if I have to go to the computer, turn it on, and look for the recipe, my energy is low there is not motivation. Having my paper recipe in front of me is easier.” |
| Covariate | Time Point | N | Mean (SE) | 95% CI | Difference in the Estimates(SE) | p-Value |
|---|---|---|---|---|---|---|
| Fatigue: FACT-F Total Score | T0 | 56 | 23.45 (1.26) | 20.94–25.97 | - | - |
| T1 | 46 | 28.37 (1.34) | 25.71–31.05 | 4.91 (1.19) | <0.0001 | |
| T2 | 42 | 31.21 (1.38) | 28.47–33.95 | 7.75 (1.24) | <0.0001 | |
| Disability: WHODAS 2.0 Total Score | T0 | 56 | 16.12 (0.96) | 14.22–18.02 | - | - |
| T1 | 44 | 14.57 (1.02) | 12.54–16.61 | −1.55 (0.85) | 0.072 | |
| T2 | 42 | 13.69 (1.03) | 11.63–15.74 | −2.43 (0.86) | 0.006 | |
| Energy: Profile of Mood State (POMS)-Vigor Total Score | T0 | 57 | 9.41 (0.61) | 8.21–10.62 | - | - |
| T1 | 46 | 10.87 (0.65) | 9.59–12.16 | 1.46 (0.60) | 0.018 | |
| T2 | 41 | 11.02 (0.67) | 9.68–12.37 | 1.61 (0.64) | 0.013 | |
| Confidence in Managing Fatigue Total Score | T0 | 56 | 23.37 (0.93) | 21.52–25.23 | - | - |
| T1 | 45 | 31.11 (1.09) | 28.91–33.31 | 7.74 (1.28) | <0.0001 | |
| T2 | 39 | 34.44 (0.96) | 32.51–36.36 | 11.07 (1.30) | <0.0001 |
| Theme: Impact of the C4V Program | ||
|---|---|---|
| Sub-Themes: | Analytic Note | Example Quote |
| Improved motivation | Many described losing their motivation to cook because of the limitations posed by fatigue. The thought of cooking as they once had became overwhelming, with many participants opting for fast food instead. Recipes and culinary strategies provided through the C4V program made meal preparation feel more attainable, enhancing feelings of motivation. | “When you finish the program, you are so eager that you come home and prepare the dish the next day. Yes, you get more motivated, I found that I was more motivated after… It also got me excited and interested to make the dishes. And I like cooking and trying new things, so it got me motivated and excited to try the new recipes.” |
| Improved self-efficacy | Motivation paired with culinary strategies that considered the limitations of fatigue helped many participants to push through their fatigue to make “healthier choices” that they felt more confident with. Participants described feeling better able to apply the skills they learned to implement dietary behavior changes within their daily lives, employing energy conservation techniques to cook while experiencing fatigue. | “(The C4V program was) the game changer. I just picked up so many helpful tips that changed the way my husband and I are now eating. We’re making healthier choices, better choices. It just gave me that boost that I needed to get over the hump of the extraordinary fatigue that happens as a result of chemotherapy.” “There was definitely one day that I clearly remember thinking that I am so tired, and there is no way I’m eating anything healthy. (But then) I thought that I could just buy the fish and cook it. (…) Normally to shop and cook in the same day even if I wasn’t tired would seem impossible (…) So C4V is empowering and skill building in terms of dealing with the fatigue.” |
| Improved control | Many participants explained that they emerged from the C4V program with important knowledge and skills that enhanced their capacity to eat well. Establishing a sense of control over ones diet was one way participants began to return to normal and gain an improved sense of control over their lives. | “Instead of just being depressed that I don’t know how to feed myself and eating something from take out, now even if I’m tired, I know that I can stop at a grocery store, even stopping at a grocery store would be impossible, I stop at a grocery store and cook it on the same day. Like fish packs, I can go to the grocery store, come home, and within 20 min have something healthy to eat. Even when I am really tired, it still seems like a feasible idea. What this means is a possibility to eat better.” |
| Improvements to overall fatigue | A few participants explained that the nutritional knowledge and culinary strategies learned through C4V helped them to work around their fatigue to cook more often and felt that they were eating healthier as a result, which directly impacted their fatigue. Others felt that this helped to enhance their energy levels, facilitating engagement in other activities (e.g., physical activity) that were believed to collectively promote reduced fatigue. | “I’ll tell you up front, I think that my energy levels turned around as a result of the (C4V) program... I was eating better, cause I was shown some of the shortcuts, my body was absorbing nutrients that it hadn’t had before, and so I had more energy to go for a walk. I had more energy to prepare a nutritious meal. To me this program was just so significant it should be required (both laugh) for everybody in their cancer treatment. I mean what’s more important than what we put in our bodies?” “Managing fatigue is really about, for me, planning your day… (because of the C4V program) I have new preparation skills that help me to plan ahead, or have things ready frozen and ready to eat. I think that definitely… there are days where I am too tired to cook and (now) I have things available that aren’t a peanut butter sandwich.” “The granola has become my snack instead of the stuff that I am not supposed to eat. And I have more nutrition for my breakfast. So I think that if I have a more nutritious meal, that helps me to fight my fatigue and my tiredness as well.” |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pritlove, C.; Capone, G.; Kita, H.; Gladman, S.; Maganti, M.; Jones, J.M. Cooking for Vitality: Pilot Study of an Innovative Culinary Nutrition Intervention for Cancer-Related Fatigue in Cancer Survivors. Nutrients 2020, 12, 2760. https://doi.org/10.3390/nu12092760
Pritlove C, Capone G, Kita H, Gladman S, Maganti M, Jones JM. Cooking for Vitality: Pilot Study of an Innovative Culinary Nutrition Intervention for Cancer-Related Fatigue in Cancer Survivors. Nutrients. 2020; 12(9):2760. https://doi.org/10.3390/nu12092760
Chicago/Turabian StylePritlove, Cheryl, Geremy Capone, Helena Kita, Stephanie Gladman, Manjula Maganti, and Jennifer M. Jones. 2020. "Cooking for Vitality: Pilot Study of an Innovative Culinary Nutrition Intervention for Cancer-Related Fatigue in Cancer Survivors" Nutrients 12, no. 9: 2760. https://doi.org/10.3390/nu12092760






