Duration of Lactation and Maternal Risk of Metabolic Syndrome: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Quality Appraisal
2.2. Statistical Analysis
3. Results
3.1. Literature Search Results
3.2. Prospective Studies
3.3. Cross-Sectional Studies
3.4. Criteria for Duration of Lactation
3.5. The Definition of Metabolic Syndrome
3.6. Quality Appraisal
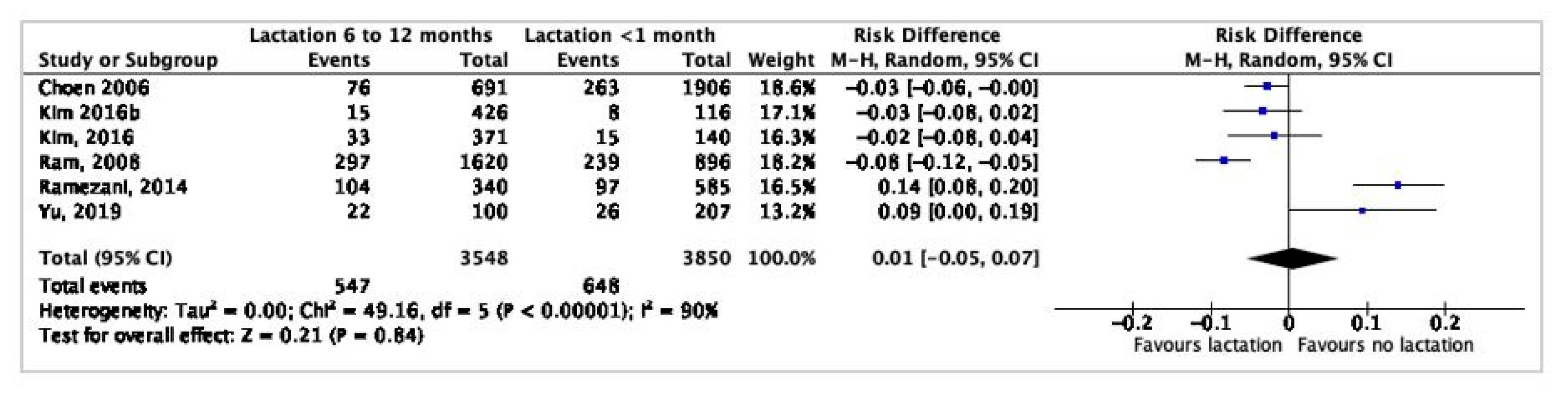
3.7. Meta-Analysis
4. Discussion
4.1. Summary of Main Results
4.2. Potential Mechanisms
4.3. Risk Assessment and Follow-Up Post-Pregnancy
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woodward, M. Cardiovascular disease and the female disadvantage. Int. J. Environ. Res. Public Health 2019, 16, 1165. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.S.; Lloyd-Jones, D.M.; O’Flaherty, M.; Capewell, S.; Kershaw, K.; Carnethon, M.; Khan, S.S. Trends in cardiometabolic mortality in the United States. Lett. JAMA 2019, 322, 780–782. [Google Scholar] [CrossRef]
- National Center for Health Statistics. Health, United States, 2011: With Special Feature on Socioeconomic Status and Health; National Center for Health Statistics: Hyattsville, MD, USA, 2012.
- Bugiardini, R.; Manfrini, O.; Cenko, E. Female sex as a biological variable: A review on younger patients with acute coronary syndrome. Trends Cardiovas. Med. 2019, 29, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Rosamond, W.D.; Caughey, M.C. Response by arora et al. to letter regarding article, twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction: The ARIC community surveillance study. Circulation 2019, 139, 1047–1056. [Google Scholar] [CrossRef]
- D’Onofrio, G.; Safdar, B.; Lichtman, J.H.; Strait, K.M.; Dreyer, R.P.; Geda, M.; Spertus, J.A.; Krumholz, H.M. Sex differences in reperfusion in young patients with ST-segment-elevation myocardial infarction: Results from the VIRGO study. Circulation 2015, 131, 1324–1332. [Google Scholar] [CrossRef]
- Manrique-Acevedo, C.; Chinnakotla, B.; Padilla, J.; Martinez-Lemus, L.A.; Gozal, D. Obesity and cardiovascular disease in women. Int. J. Obes. 2020, 44, 1210–1226. [Google Scholar] [CrossRef]
- Isiadinso, I.; Wenger, N.K. Do we need a different approach to assess cardiovascular risk in women? US Cardiol. Rev. 2017, 11, 5–9. [Google Scholar] [CrossRef]
- Countouris, M.E.; Holzman, C.; Althouse, A.D.; Snyder, G.G.; Barinas-Mitchell, E.; Reis, S.E.; Catov, J.M. Lactation and maternal subclinical atherosclerosis among women with and without a history of hypertensive disorders of pregnancy. J. Womens Health 2020, 29, 789–798. [Google Scholar] [CrossRef]
- Schwarz, E.B.; McClure, C.K.; Tepper, P.G.; Thurston, R.; Janssen, I.; Matthews, K.A.; Sutton-Tyrrell, K. Lactation and maternal measures of subclinical cardiovascular disease. Obstet. Gynecol. 2010, 115, 41–48. [Google Scholar] [CrossRef]
- McClure, C.K.; Catov, J.M.; Ness, R.B.; Schwarz, E.B. Lactation and maternal subclinical cardiovascular disease among premenopausal women. Am. J. Obstet. Gynecol. 2012, 207, 46.e1–46.e8. [Google Scholar] [CrossRef]
- Schwarz, E.B.; Ray, R.M.; Stuebe, A.M.; Allison, M.A.; Ness, R.B.; Freiberg, M.S.; Cauley, J.A. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet. Gynecol. 2009, 113, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight: Factsheet. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 4 September 2020).
- Pacyga, D.C.; Henning, M.; Chiang, C.; Smith, R.L.; Flaws, J.A.; Strakovsky, R.S. Associations of pregnancy history with BMI and weight gain in 45–54-year-old women. Curr. Dev. Nutr. 2020, 4, nzz139. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Alberti, K.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.V.; Mechanick, J.I. The metabolic syndrome. Nutr. Clin. Pract. 2009, 24, 560–577. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef]
- Bentley-Lewis, R.; Koruda, K.; Seely, E.W. The metabolic syndrome in women. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 696–704. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Murtaugh, M.A.; Lewis, C.E.; Quesenberry, C.P.; West, D.S.; Sidney, S. Excess gains in weight and waist circumference associated with childbearing: The coronary artery risk development in young adults study (CARDIA). Int. J. Obes. Relat. Metab. Disord. 2004, 28, 525–535. [Google Scholar] [CrossRef]
- Cohen, A.; Pieper, C.F.; Brown, A.J.; Bastian, L.A. Number of children and risk of metabolic syndrome in women. J. Womens Health 2006, 15, 763–773. [Google Scholar] [CrossRef]
- Lao, X.Q.; Thomas, G.N.; Jiang, C.Q.; Zhang, W.S.; Yin, P.; Schooling, C.M.; Heys, M.; Leung, G.M.; Adab, P.; Cheng, K.K.; et al. Parity and the metabolic syndrome in older Chinese women: The guangzhou biobank cohort study. Clin. Endocrinol. 2006, 65, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P.; Jacobs, D.R., Jr.; Chiang, V.; Lewis, C.E.; Tsai, A.; Quesenberry, C.P., Jr.; Sidney, S. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: The CARDIA study. Am. J. Obstet. Gynecol. 2009, 201, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Jesmin, S.; Rahman, M.; Islam, M.; Khatun, M.T.; Yamaguchi, N.; Akashi, H.; Mizutani, T. Higher gravidity and parity are associated with increased prevalence of metabolic syndrome among rural Bangladeshi women. PLoS ONE 2013, 8, e68319. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, G.; Shen, L.; Zhang, Y.; Song, L.; Yang, S.; Yang, H.; Yuan, J.; Liang, Y.; Wang, Y.; et al. Parity and risk of metabolic syndrome among Chinese women. J. Womens Health 2015, 24, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Kramer, M.S.; Kakuma, R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst. Rev. 2012, 8, Cd003517. [Google Scholar] [CrossRef] [PubMed]
- Stuebe, A.M.; Rich-Edwards, J.W. The reset hypothesis: Lactation and maternal metabolism. Am. J. Perinatol. 2009, 26, 81–88. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Lewis, C.E.; Wei, G.S.; Whitmer, R.A.; Quesenberry, C.P.; Sidney, S. Lactation and changes in maternal metabolic risk factors. Obstet. Gynecol. 2007, 109, 729–738. [Google Scholar] [CrossRef]
- Chowdhury, R.; Sinha, B.; Sankar, M.J.; Taneja, S.; Bhandari, N.; Rollins, N.; Bahl, R.; Martines, J. Breastfeeding and maternal health outcomes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 96–113. [Google Scholar] [CrossRef]
- Gunderson, E.; Hurston, S.; Ning, X.; Lo, J.; Crites, Y.; Walton, D.; Dewey, K.; Azevedo, R.; Young, S.; Fox, G. Lactation and progression to type 2 diabetes after gestational diabetes. Ann. Intern. Med. 2015, 163, 1–36. [Google Scholar] [CrossRef]
- Zachou, G.; Armeni, E.; Lambrinoudaki, I.V. Lactation and maternal cardiovascular disease risk in later life. Maturitas 2019, 122, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- The Joanna Briggs Institute. The Joanna Briggs Institute Critical Appraisal Tools for Use in JBI Systematic Reviews; The Joanna Briggs Institute: North Adelaide, Australia, 2017. [Google Scholar]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Wiley- Blackwell, 2019. [Google Scholar]
- Gunderson, E.P.; Jacobs, D.R., Jr.; Chiang, V.; Lewis, C.E.; Feng, J.; Quesenberry, C.P., Jr.; Sidney, S. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: A 20-Year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults). Diabetes 2010, 59, 495–504. [Google Scholar] [CrossRef]
- Ramezani Tehrani, F.; Momenan, A.A.; Khomami, M.B.; Azizi, F. Does lactation protect mothers against metabolic syndrome? findings from the tehran lipid and glucose study. J. Obstet. Gynaecol. Res. 2014, 40, 736–742. [Google Scholar] [CrossRef]
- Choi, S.R.; Kim, Y.M.; Cho, M.S.; Kim, S.H.; Shim, Y.S. Association between duration of breast feeding and metabolic syndrome: The Korean national health and nutrition examination surveys. J. Women’s Health 2017, 26, 361–367. [Google Scholar] [CrossRef]
- Ki, E.Y.; Han, K.D.; Park, Y.G. Relationship between duration of breast-feeding and obesity in korean women: The Korea national health and nutrition examination survey (KNHANES) 2010–2012. Maturitas 2017, 102, 41–45. [Google Scholar] [CrossRef]
- Ram, K.T.; Bobby, P.; Hailpern, S.M.; Lo, J.C.; Schocken, M.; Skurnick, J.; Santoro, N. Duration of lactation is associated with lower prevalence of the metabolic syndrome in midlife—SWAN, the study of women’s health across the nation. Am. J. Obstet. Gynecol. 2008, 198, 268.e1–268.e6. [Google Scholar] [CrossRef]
- Yu, J.; Pudwell, J.; Dayan, N.; Smith, G. Postpartum breastfeeding and cardiovascular risk assessment in women following pregnancy complications. J. Womens Health 2019, 41, 737. [Google Scholar] [CrossRef]
- Cho, G.J.; Park, H.T.; Shin, J.-H.; Kim, T.; Hur, J.Y.; Kim, Y.T.; Lee, K.W.; Kim, S.H. The relationship between reproductive factors and metabolic syndrome in Korean postmenopausal women: Korea national health and nutrition survey 2005. Menopause 2009, 16, 998–1003. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.S. Differences in prevalence of metabolic syndrome by breastfeeding experience of women in their 30s and 40s. Asian Nurs. Resour. (Korean Soc. Nurs. Sci.) 2016, 10, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Zamani, F.; Pishgar, F.; Ordookhani, S.; Nateghi, N.; Salehi, F. Parity, duration of lactation and prevalence of maternal metabolic syndrome: A cross-sectional study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 201, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Azizi, F.; Hadaegh, F.; Khalili, D.; Esteghamati, A.; Hosseinpanah, F.; Delavari, A.; Larijani, B.; Mirmiran, P.; Zabetian, A.; Mehrabi, Y.; et al. Appropriate definition of metabolic syndrome among Iranian adults: Report of the Iranian national committee of obesity. Arch. Iran. Med. 2010, 13. [Google Scholar]
- Lee, S.Y.; Park, H.S.; Kim, D.J.; Han, J.H.; Kim, S.M.; Cho, G.J.; Kim, D.Y.; Kwon, H.S.; Kim, S.R.; Lee, C.B.; et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res. Clin. Pract. 2007, 75, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- National cholesterol education program expert panel on detection E, treatment of high blood cholesterol in A: Third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report. Circulation 2002, 106, 3143–3421.
- Kahn, R.; Buse, J.B.; Ferrannini, E.; Stern, M. The Metabolic Syndrome: Time for a Critical Appraisal: Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2005, 28, 2289–2304. [Google Scholar] [CrossRef]
- Natland, S.T.; Nilsen, T.I.; Midthjell, K.; Andersen, L.F.; Forsmo, S. Lactation and cardiovascular risk factors in mothers in a population-based study: The HUNT-study. Int. Breastfeed. J. 2012, 7, 8. [Google Scholar] [CrossRef]
- Stuebe, A.M.; Mantzoros, C.; Kleinman, K.; Gillman, M.W.; Rifas-Shiman, S.; Gunderson, E.P.; Rich-Edwards, J. Duration of lactation and maternal adipokines at 3 years postpartum. Diabetes 2011, 60, 1277–1285. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Romundstad, P.R.; Vatten, L. Breastfeeding and the maternal risk of type 2 diabetes: A systematic review and dose–response meta-analysis of cohort studies. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 107–115. [Google Scholar] [CrossRef]
- Jäger, S.; Jacobs, S.; Kröger, J.; Fritsche, A.; Schienkiewitz, A.; Rubin, D.; Boeing, H.; Schulze, M.B. Breast-feeding and maternal risk of type 2 diabetes: A prospective study and meta-analysis. Diabetologia 2014, 57, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P.; Lewis, C.E.; Lin, Y.; Sorel, M.; Gross, M.; Sidney, S.; Jacobs, D.R.; Shikany, J.M.; Quesenberry, C.P. Lactation duration and progression to diabetes in women across the childbearing years: The 30-year CARDIA Study. JAMA Intern. Med. 2018, 178, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Ellison, P.T. On Fertile Ground; Harvard University Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Chu, S.Y.; Callaghan, W.M.; Bish, C.L.; D’Angelo, D. Gestational weight gain by body mass index among US women delivering live births, 2004–2005: Fueling future obesity. Am. J. Obstet. Gynecol. 2009, 200, 271.e1–271.e7. [Google Scholar] [CrossRef]
- Butte, N.F.; King, J.C. Energy requirements during pregnancy and lactation. Public Health Nutr. 2005, 8, 1010–1027. [Google Scholar] [CrossRef]
- Tørris, C.; Thune, I.; Emaus, A.; Finstad, S.E.; Bye, A.; Furberg, A.-S.; Barrett, E.; Jasienska, G.; Ellison, P.; Hjartåker, A. Duration of lactation, maternal metabolic profile, and body composition in the Norwegian EBBA I-study. Breastfeed. Med. 2013, 8, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Janney, C.A.; Zhang, D.; Sowers, M. Lactation and weight retention. Am. J. Clin. Nutr. 1997, 66, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G.; Heinig, M.J.; Nommsen, L.A. Maternal weight-loss patterns during prolonged lactation. Am. J. Clin. Nutr. 1993, 58, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Lelis, D.F.; Freitas, D.F.; Machado, A.S.; Crespo, T.S.; Santos, S.H.S. Angiotensin-(1-7), adipokines and inflammation. Metabolism 2019, 95, 36–45. [Google Scholar] [CrossRef]
- Blüher, M.; Mantzoros, C.S. From leptin to other adipokines in health and disease: Facts and expectations at the beginning of the 21st century. Metabolism 2015, 64, 131–145. [Google Scholar] [CrossRef]
- Deng, Y.; Scherer, P.E. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann. N. Y. Acad. Sci. 2010, 1212, E1–E19. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; Boyko, E.J.; Sicree, R.A.; Zimmet, P.Z.; Soderberg, S.; Alberti, K.G.M.; Tuomilehto, J.; Chitson, P.; Shaw, J.E. Central obesity as a precursor to the metabolic syndrome in the ausdiab study and mauritius. Obesity 2008, 16, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Laclaustra, M.; Corella, D.; Ordovas, J.M. Metabolic syndrome pathophysiology: The role of adipose tissue. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; Kahn, C.R. Tissue–specific insulin signaling, metabolic syndrome, and cardiovascular disease. Arter. Thromb. Vasc. Biol. 2012, 32, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Kizaki, T.; Maegawa, T.; Sakurai, T.; Ogasawara, J.; Ookawara, T.; Oh-Ishi, S.; Izawa, T.; Haga, S.; Ohno, H. Voluntary exercise attenuates obesity-associated inflammation through ghrelin expressed in macrophages. Biochem. Biophys. Res. Commun. 2011, 413, 454–459. [Google Scholar] [CrossRef]
- Harvey, R.E.; Howard, V.G.; Lemus, M.B.; Jois, T.; Andrews, Z.B.; Sleeman, M.W. The Ghrelin/GOAT system regulates obesity-induced inflammation in male mice. Endocrinology 2017, 158, 2179–2189. [Google Scholar] [CrossRef]
- Ukkola, O.; Pöykkö, S.; Päivänsalo, M.; Kesäniemi, Y.A. Interactions between ghrelin, leptin and IGF-I affect metabolic syndrome and early atherosclerosis. Ann. Med. 2008, 40, 465–473. [Google Scholar] [CrossRef]
- Holzer, P.; Reichmann, F.; Farzi, A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 2012, 46, 261–274. [Google Scholar] [CrossRef]
- McGowan, B.; Bloom, S.R. Peptide YY and appetite control. Curr. Opin. Pharmacol. 2004, 4, 583–588. [Google Scholar] [CrossRef]
- Knudsen, N.; Laurberg, P.; Rasmussen, L.B.; Bülow, I.; Perrild, H.; Ovesen, L.; Jørgensen, T. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J. Clin. Endocrinol. Metab. 2005, 90, 4019–4024. [Google Scholar] [CrossRef] [PubMed]
- Panuganti, P.L.; Hinkle, S.N.; Rawal, S.; Grunnet, L.G.; Lin, Y.; Liu, A.; Thuesen, A.C.B.; Ley, S.H.; Olsen, S.F.; Zhang, C. Lactation duration and long-term thyroid function: A study among women with gestational diabetes. Nutrients 2018, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.I.; Nam, J.Y.; Shin, S.K.; Kang, E.W.; Chang, T.I. Low triiodothyronine syndrome and long-term cardiovascular outcome in incident peritoneal dialysis patients. Clin. J. Am. Soc. Nephrol. 2015, 10, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Roef, G.L.; Rietzschel, E.R.; Van Daele, C.M.; Taes, Y.E.; De Buyzere, M.L.; Gillebert, T.; Kaufman, J.M. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid 2014, 24, 223–231. [Google Scholar] [CrossRef]
- Ladyman, S.R.; Aung, Z.K.; Grattan, D.R. Impact of pregnancy and lactation on the long-term regulation of energy balance in female mice. Endocrinology 2018, 159, 2324–2336. [Google Scholar] [CrossRef]
- Karnik, S.K.; Chen, H.; McLean, G.W.; Heit, J.J.; Gu, X.; Zhang, A.Y.; Fontaine, M.; Yen, M.H.; Kim, S.K. Menin controls growth of pancreatic -cells in pregnant mice and promotes gestational diabetes mellitus. Science 2007, 318, 806–809. [Google Scholar] [CrossRef]
- Stuenkel, C.A. Do we have new preventive strategies for optimizing cardiovascular health in women? Climacteric 2019, 22, 133–139. [Google Scholar] [CrossRef]
- Millington, S.; Magarey, J.; Dekker, G.A.; Clark, R.A. Cardiac conditions in pregnancy and the role of midwives: A discussion paper. Nurs. Open 2019, 6, 722–732. [Google Scholar] [CrossRef]
- Renfrew, M.J.; Rm, A.M.; Bastos, M.H.; Campbell, J.; Channon, A.A.; Cheung, N.F.; Silva, D.R.A.D.; Downe, S.; Kennedy, H.P.; Malata, A.; et al. Midwifery and quality care: Findings from a new evidence-informed framework for maternal and newborn care. Lancet 2014, 384, 1129–1145. [Google Scholar] [CrossRef]
- Cusimano, M.C.; Pudwell, J.; Roddy, M.; Cho, C.-K.J.; Smith, G.N. The maternal health clinic: An initiative for cardiovascular risk identification in women with pregnancy-related complications. Am. J. Obstet. Gynecol. 2014, 210, 438.e1–438.e9. [Google Scholar] [CrossRef]
- Smyth, D.; Hyde, A. Discourses and critiques of breastfeeding and their implications for midwives and health professionals. Nurs. Inq. 2020, 27. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.; Clowes, M.; Preston, L.; Booth, A. Meeting the review family: Exploring review types and associated information retrieval requirements. Health Inf. Libr. J. 2019, 36, 202–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zilov, A.; Soewondo, P.; Bech, O.M.; Sekkal, F.; Home, P. Observational studies: Going beyond the boundaries of randomized controlled trials. Diabetes Res. Clin. Pract. 2010, 88, S3–S9. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.R.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Djulbegovic, B.; Glasziou, P.; Chalmers, I. The importance of randomised vs non-randomised trials. Lancet 2019, 394, 634–635. [Google Scholar] [CrossRef]


| PICOS | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Adult women | Animal studies |
| Intervention | Breastfeeding | Lab studies Not breastfeeding |
| Comparison | Duration of breastfeeding | No duration of breastfeeding |
| Outcome | MetS (prevalence or incidence) | No established definition of MetS |
| Study design | Cross-sectional, prospective cohort, and intervention studies | Abstracts and protocols |
| Study | Participants | Adjustments | Results |
|---|---|---|---|
| Gunderson et al., 2010 [38], 20-year follow-up, CARDIA (1986–2006) | n = 1399 18–30 years, nulliparous and free of MetS at baseline, NCEP ATP III, 0–1 month, >1–5 months, 6–9 months, and >9 months, cumulative lactation duration for all births within intervals | Unadjusted Fully adjusted (study center, race, age, education, smoking, parity, BMI, MetS components, physical activity) | Longer duration of lactation inversely associated with the incidence of MetS (>1–5 months, 6–9 months, >9 months) compared with <1 month (relative hazard range non-GDM 0.40–0.49 and GDM groups 0.11–0.28) all p < 0.001 The stronger association among GDM in a fully adjusted model (relative hazard range 0.14–0.33) than non-GDM (relative hazard range 0.44–0.61), all p = 0.03 |
| Ramezani Tehrani et al., 2014 [39], 9-year follow-up, TLGS | n = 925 15–50 years without MetS at baseline, the lifetime duration of lactation: none, 1–6, 7–12, 13–23, or ≥24 months | Age, physical activity, daily caloric intake, BMI, and parity | 13–23 months lifetime duration of lactation associated with a higher incidence of MetS compared with 24 months or more RR 1.8 (95% CI 1.0–3.4) (adjusted) |
| Reference | Population | Adjustments | Results |
|---|---|---|---|
| Cho et al., 2009 [44] | n = 892 postmenopausal women KNHANES (2005) | Age, BMI, demographic, socioeconomic, lifestyle factors | No association |
| Choi et al., 2017 [40] | n = 4724 aged 19–50 years KNHANES (2010–2013) | Age, BMI, household income, educational level, marriage status, smoking, alcohol, physical activity, age at menarche, menopause, parity, oral contraceptives | Lactation ≥12 months inversely associated with MetS 6–11 months OR 0.91 (95% CI 0.67–1.24) 12–23 months OR 0.73 (95% CI 0.56–0.95) ≥24 months OR 0.70 (95% CI 0.53–0.92) compared with ≤5 months (adjusted) |
| Cohen et al., 2006 [22] | n = 4699 NHANES III | Age, ethnicity, education, income, parity, employment, physical inactivity, alcohol use, smoking, oral contraceptives, postmenopausal hormone therapy | Lactation ≥1 month inversely associated with MetS OR 0.78 (95% CI 0.61–0.99) (adjusted) |
| Ki et al., 2017 [41] | n = 6621 postmenopausal women KNHANES 2010–2012 | Lower prevalence of MetS in women who breastfed ≥6 months compared to those who had not breastfed (29.5 (2.1) vs. 88.4 (4.6), p = 0.056) | |
| Kim et al., 2016 [45] | n = 1053 parous women 30–49 years KNHANES V-1 (2010) | Income, education level, exercise, the last childbirth age | No association Women in their 30s OR 0.99 (95% CI 0.95–1.02); Women in their 40s OR 0.98 (95% CI 0.94–1.02) (all adjusted) |
| Moradi et al., 2016 [46] | n = 978 reporting live birth | Age, age at first pregnancy, number of pregnancies, DM, hypertension | No association OR 0.99 (95% CI 0.99–1.00) (adjusted) |
| Ram et al., 2008 [42] | n = 2516 reporting live birth | Age, smoking, parity, ethnicity, socioeconomic status, study site, physical activity, caloric intake, high-school BMI | Duration of lactation inversely associated with MetS OR per each additional year of lactation 0.88 (95% CI: 0.77, 0.99) Ever having breastfeed associated with decreased risk of MetS, adjusted OR 0.77 (95% CI 0.62, 0.96) compared to not having breastfeed (all adjusted) |
| Yu et al., 2019 [43] | n = 622 Parous women ≥ child with pregnancy complications, 6–12 months postpartum last-child | Age, ethnicity, education, income, smoking, parity, physical activity, time postpartum, BMI, gestational weight gain | Increased breastfeeding duration decreased the likelihood of MetS (adjusted) OR 0.89 (95% CI 0.79–0.99) |
| Reference | Criterion |
|---|---|
| Gunderson et al., 2010 [38] | Total (i.e., 0–1 month, >1–5 months, 6–9 months, and >9 months) Nulliparous 18–30 years and free of MetS at baseline (20y follow-up) |
| Ramezani Tehrani et al., 2014 [39] | Total (none, 1–6 months, 7–12 months, 13–23 months, and ≥24 months) 15–50 years at baseline. Among the 340 non-lactating women, 311 were nulliparous |
| Cho et al., 2009 [44] | Total < or ≥ 1 month |
| Choi et al., 2017 [40] | Total (≤5, 6–11, 12–23, or ≥24 months) |
| Cohen et al., 2006 [22] | Total < or ≥ 1 month |
| Ki et al., 2017 [41] | Total per child (<1, 1–6, 7–12, 13–18, and >18 months) |
| Kim et al., 2016 [45] | Breastfeeding experience: yes/no |
| Moradi et al., 2016 [46] | Lifetime lactation duration after all deliveries (month) |
| Ram et al., 2008 [42] | Number of months per child |
| Yu et al., 2019 [43] | Last child (none, < 6 or ≥ 6 months) |
| Criteria | Study | Sample Population | |
|---|---|---|---|
| Waist | >80 cm | Choi et al., 2017 [40] | Korean |
| Ki et al., 2017 [41] | Korean Asian women | ||
| Ram et al., 2008 [42] | Chinese/Japanese | ||
| ≥85 cm | Cho et al., 2009 [44] | Korean | |
| Kim et al., 2016 [45] | Korean | ||
| >88 cm | Cohen et al., 2006 [22] | US, multi-ethnic | |
| Gunderson et al., 2010 [38] | US, multi-ethnic | ||
| Moradi et al., 2016 [46] | Iran | ||
| Ram et al., 2008 [42] | US/Canada, multi-ethnic | ||
| Yu et al., 2019 [43] | Canada | ||
| ≥95 cm | Ramezani Tehrani et al., 2014 [39] | Iran |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tørris, C.; Bjørnnes, A.K. Duration of Lactation and Maternal Risk of Metabolic Syndrome: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2718. https://doi.org/10.3390/nu12092718
Tørris C, Bjørnnes AK. Duration of Lactation and Maternal Risk of Metabolic Syndrome: A Systematic Review and Meta-Analysis. Nutrients. 2020; 12(9):2718. https://doi.org/10.3390/nu12092718
Chicago/Turabian StyleTørris, Christine, and Ann Kristin Bjørnnes. 2020. "Duration of Lactation and Maternal Risk of Metabolic Syndrome: A Systematic Review and Meta-Analysis" Nutrients 12, no. 9: 2718. https://doi.org/10.3390/nu12092718
APA StyleTørris, C., & Bjørnnes, A. K. (2020). Duration of Lactation and Maternal Risk of Metabolic Syndrome: A Systematic Review and Meta-Analysis. Nutrients, 12(9), 2718. https://doi.org/10.3390/nu12092718





