The Role of Vitamin D in the Development of Diabetes Post Gestational Diabetes Mellitus: A Systematic Literature Review
Abstract
:1. Introduction
2. Method
2.1. Search Strategy
2.2. Study Selection
- -
- Population: This review considered studies where the population involved were pregnant women diagnosed with GDM, ≥18 years of age, and without a previous diagnosis of T2DM. There was no restriction regarding the methods to diagnose GDM.
- -
- Intervention: Vitamin D intervention or exposure was the target in this review. The considered sources of maternal vitamin D were vitamin D in food or supplements; and, maternal vitamin D concentration from blood or serum samples during and after pregnancy among women with a GDM diagnosis during their pregnancy. The included measurements of vitamin D in this review were dietary intake of D2-ergocalciferol and D3-cholecalciferol and blood/serum concentration of 25(OH)D2, 25(OH)D3, or 1.25(OH)D.
- -
- Comparator: Relevant comparisons to include in this review were against placebo in studies with vitamin D supplementation; high versus low doses of vitamin D supplementation; higher versus lower 25(OH)D concentrations in blood or serum; dose–response blood or serum concentrations; and no-treatment control group.
- -
- Outcome: The diagnosis of T2DM was the primary outcome. Diabetes markers such as (but not limited to) blood glucose, insulin resistance, insulin sensitivity, impaired beta-cell function, and glycated hemoglobin were the secondary outcomes. There were no restrictions regarding measurements methods or units used.
- -
- Study design: Observational prospective comparative cohort studies, controlled (non-randomized) clinical trials (CCTs), and randomized clinical trials (RCTs) were considered for this review. Cross-sectional and case–control studies were initially to be excluded, however, due to the paucity of available studies and to provide a more holistic overview of the current literature on the topic, results from these study designs were included. Case series and case reports were excluded. There were no restrictions based on length of follow-up, and animal studies were not included.
2.3. Data Extraction
2.4. Quality Assessment
2.5. Meta-Analysis
3. Results
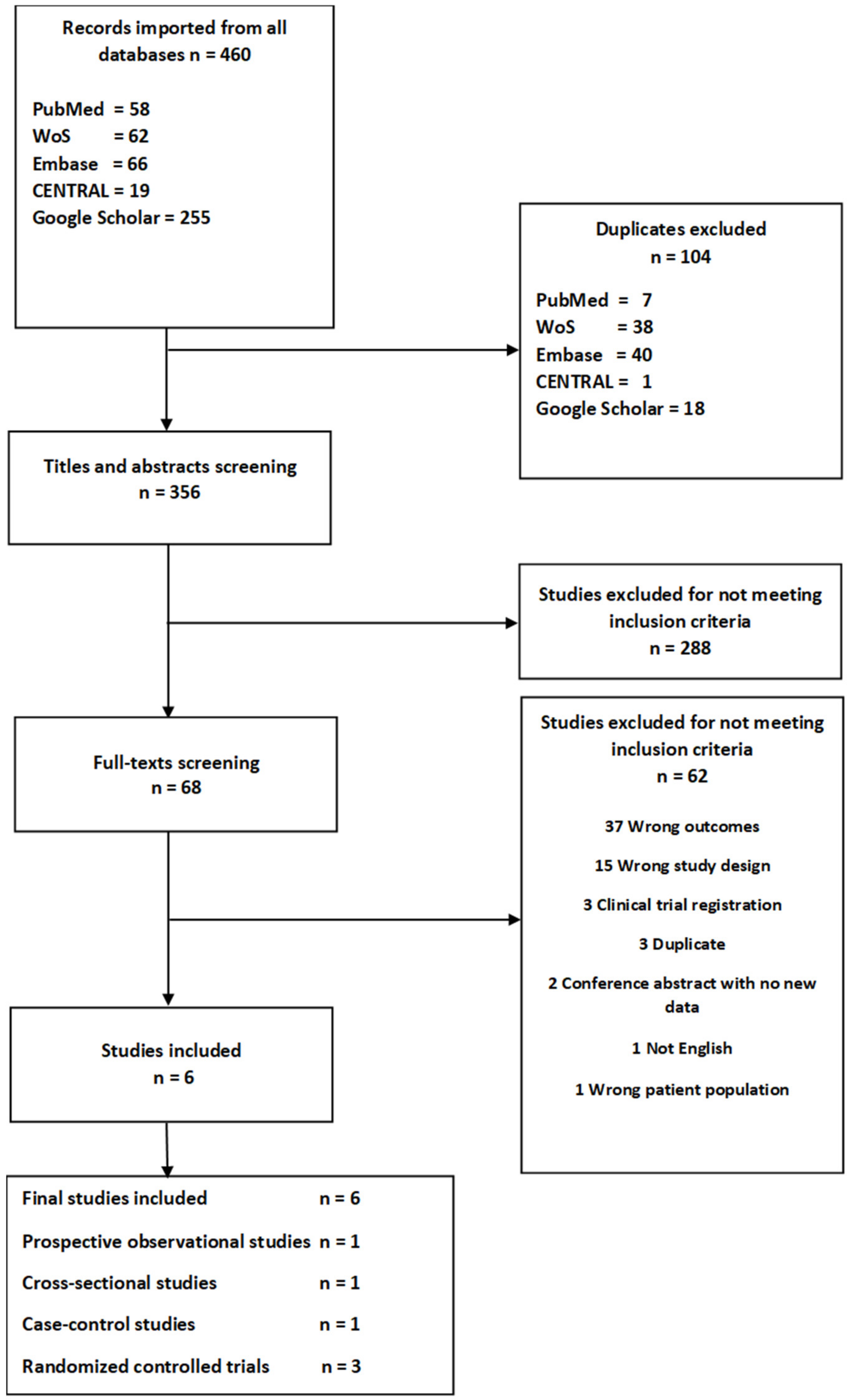
3.1. Literature Search
3.2. Description of the Studies
3.3. Description of Vitamin D Interventions or Exposure and Comparator Groups
3.4. Vitamin D Concentrations
3.5. Synthesis of Results
3.5.1. Insulin Sensitivity and Resistance
3.5.2. Beta-Cell Function
3.5.3. Glucose Measurements
3.5.4. Glycated Hemoglobin
3.5.5. Diabetes
3.6. Risk of Bias within the Six Included Studies
4. Discussion
4.1. Context with Previous Published Results
4.2. Strengths and Weaknesses of This Systematic Literature Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jager, N.; Schöpe, J.; Wagenpfeil, S.; Bocionek, P.; Saternus, R.; Vogt, T.; Reichrath, J. The Impact of UV-dose, Body Surface Area Exposed and Other Factors on Cutaneous Vitamin D Synthesis Measured as Serum 25(OH)D Concentration: Systematic Review and Meta-analysis. Anticancer Res. 2018, 38, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.H.; Camargo, C.A. Vitamin D and gestational diabetes mellitus. Curr. Diab. Rep. 2014, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ 2013, 346, f1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.K. Gestational Diabetes Mellitus, Fetal Growth and Vitamin D. J. Bone Metab. 2017, 24, 155. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.M.; Mølsted-Pedersen, L.; Beck-Nielsen, H.; Westergaard, J.G.; Ovesen, P.; Damm, P. Screening for gestational diabetes mellitus by a model based on risk indicators: A prospective study. Am. J. Obstet. Gynecol. 2003, 189, 1383–1388. [Google Scholar] [CrossRef]
- Arora, G.P.; Thaman, R.G.; Prasad, R.B.; Almgren, P.; Brøns, C.; Groop, L.C.; Vaag, A.A. Prevalence and risk factors of gestational diabetes in Punjab, North India: Results from a population screening program. Eur. J. Endocrinol. 2015, 173, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Getahun, D.; Fassett, M.J.; Jacobsen, S.J. Gestational diabetes: Risk of recurrence in subsequent pregnancies. Am. J. Obstet. Gynecol. 2010, 203, 467.e1–467.e6. [Google Scholar] [CrossRef]
- MacNeill, S.; Dodds, L.; Hamilton, D.C.; Armson, B.A.; VandenHof, M. Rates and Risk Factors for Recurrence of Gestational Diabetes. Diabetes Care 2001, 24, 659–662. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Zhang, Y.; Wang, X.; You, L.; Xu, P.; Cui, X.; Zhu, L.; Ji, C.; Guo, X.; Wen, J. Maternal Vitamin D Status and Risk of Gestational Diabetes: A Meta-Analysis. Cell. Physiol. Biochem. 2018, 45, 291–300. [Google Scholar] [CrossRef]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Shakeri, H.; Esmaillzadeh, A. Vitamin D Supplementation Affects Serum High-Sensitivity C-Reactive Protein, Insulin Resistance, and Biomarkers of Oxidative Stress in Pregnant Women. J. Nutr. 2013, 143, 1432–1438. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberatî, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRIZMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlan, A.D.; Malmivaara, A.; Chou, R.; Maher, C.G.; Deyo, R.A.; Schoene, M.; Bronfort, G.; Van Tulder, M.W. 2015 updated method guideline for systematic reviews in the Cochrane Back and Neck Group. Spine (Phila. Pa. 1976) 2015, 40, 1660–1673. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Schünemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari-Khosravi, H.; Hosseinzadeh-Shamsi-Anar, M.; Salami, M.-A.; Hadinedoushan, H.; Mozayan, M.R. Effects of a single post-partum injection of a high dose of vitamin D on glucose tolerance and insulin resistance in mothers with first-time gestational diabetes mellitus. Diabet. Med. 2012, 29, 36–42. [Google Scholar] [CrossRef]
- Shaat, N.; Ignell, C.; Katsarou, A.; Berntorp, K. Glucose homeostasis, beta cell function, and insulin resistance in relation to vitamin D status after gestational diabetes mellitus. Acta Obstet. Gynecol. Scand. 2017, 96, 821–827. [Google Scholar] [CrossRef]
- Tänczer, T.; Magenheim, R.; Fürst, Á.; Domján, B.; Janicsek, Z.; Szabó, E.; Ferencz, V.; Tabák, Á.G. The Relationship between 25-hydroxyvitamin D Levels, Insulin Sensitivity and Insulin Secretion in Women 3 Years after Delivery. Can. J. Diabetes 2017. [Google Scholar] [CrossRef] [Green Version]
- Valizadeh, M.; Piri, Z.; Mohammadian, F.; Kamali, K.; Amir Moghadami, H.R. The Impact of Vitamin D Supplementation on Post-Partum Glucose Tolerance and Insulin Resistance in Gestational Diabetes: A Randomized Controlled Trial. Int. J. Endocrinol. Metab. 2016, 14, e34312. [Google Scholar] [CrossRef] [Green Version]
- Yeow, T.P.; Lim, S.L.; Hor, C.P.; Khir, A.S.; Wan Mohamud, W.N.; Pacini, G. Impact of Vitamin D Replacement on Markers of Glucose Metabolism and Cardio-Metabolic Risk in Women with Former Gestational Diabetes—A Double-Blind, Randomized Controlled Trial. PLoS ONE 2015, 10, e0129017. [Google Scholar] [CrossRef] [Green Version]
- Kramer, C.K.; Swaminathan, B.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B.; Retnakaran, R. Prospective associations of vitamin D status with β-Cell function, insulin sensitivity, and glycemia: The impact of parathyroid hormone status. Diabetes 2014, 63, 3868–3879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Gong, Y.; Xue, H.; Xiong, J.; Cheng, G. Vitamin D and gestational diabetes mellitus: A systematic review based on data free of Hawthorne effect. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 784–793. [Google Scholar] [CrossRef] [Green Version]
- Amraei, M.; Mohamadpour, S.; Sayehmiri, K.; Mousavi, S.F.; Shirzadpour, E.; Moayeri, A. Effects of vitamin D deficiency on incidence risk of gestational diabetes mellitus: A systematic review and meta-analysis. Front. Endocrinol. (Lausanne) 2018, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Corcoy, R.; Mendoza, L.C.; Simmons, D.; Desoye, G.; Adelantado, J.M.; Chico, A.; Devlieger, R.; van Assche, A.; Galjaard, S.; Timmerman, D.; et al. The DALI vitamin D randomized controlled trial for gestational diabetes mellitus prevention: No major benefit shown besides vitamin D sufficiency. Clin. Nutr. 2020, 39, 976–984. [Google Scholar] [CrossRef] [Green Version]
- Corcoy, R.; Mendoza, L.C.; Simmons, D.; Desoye, G.; Mathiesen, E.R.; Kautzky-Willer, A.; Damm, P.; Dunne, F.P.; Wender-Ozegowska, E.; Lapolla, A.; et al. Re: Vitamin D and gestational diabetes mellitus: A systematic review based on data free of Hawthorne effect. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1338–1339. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, Y.; Zheng, Y.; Wang, P.; Zhang, Y. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: A systematic review and meta-analysis. Nutrients 2018, 10, 375. [Google Scholar]
- George, P.S.; Pearson, E.R.; Witham, M.D. Effect of vitamin D supplementation on glycaemic control and insulin resistance: A systematic review and meta-analysis. Diabet. Med. 2012, 29, e142–e150. [Google Scholar] [CrossRef]
- Lee, C.J.; Iyer, G.; Liu, Y.; Kalyani, R.R.; Bamba, N.; Ligon, C.B.; Varma, S.; Mathioudakis, N. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: A systematic review and meta-analysis of intervention studies. J. Diabetes Complicat. 2017, 31, 1115–1126. [Google Scholar] [CrossRef]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study’s First Author, Year, Country | Study Design, N, Follow-Up, Retention | Age (Mean ± SD) (Median 25th, 75th Pct) | Ethnicity | Intervention/Exposure | Comparison/Control Group | Reported Outcomes | Results | Conflict of Interest |
|---|---|---|---|---|---|---|---|---|
| [22] Kramer et al., 2014, Canada | Prospective cohort study, 494, 3–12 months | 34.8 ± 4.3 | 60.2% Caucasian 39.8% Other | 25(OH)D serum concentration measured after pregnancy by competitive electro chemiluminescent immunoassay Classified in three groups: (a) Deficiency (25(OH)D < 50 nmol/L) (b) Insufficiency (25(OH)D ≥ 50 < 75 nmol/L) (c) Sufficiency (25(OH)D ≥ 75 nmol/L | Comparisons between 25(OH)D status groups with vitamin D sufficiency as reference | Insulin sensitivity | No potential conflicts of interest relevant to this article were reported | |
| Matsuda index | 1 Deficient −0.099, p = 0.08 1 Insufficient −0.013, p = 0.79 | |||||||
| β-cell function | ||||||||
| ISSI-2 | 1Deficient −0.062, p = 0.16 1 Insufficient −0.111, p = 0.006 | |||||||
| Glucose measurements | ||||||||
| FBG on OGTT (nmol/L) | 1 Deficient 0.026, p = 0.008 1 Insufficient 0.011, p = 0.20 | |||||||
| 2 hour 75 g OGTT (mg/dL) | 1 Deficient 0.070, p = 0.01 1 Insufficient 0.051, p = 0.04 | |||||||
| [19] Tänczer et al., 2017, Hungary | Nested case–control, 132, 3.2 (±0.6) years | Cases 34.8 ± 4.4 Controls 33.8 ± 3.6 | 100% Caucasian | * 25(OH)D concentration measured after pregnancy by chemiluminescent immunoassay (CLIA) method, used continuously (ng/mL) | Comparisons between women with GDM and a randomly selected control group of women with normal glucose tolerance during pregnancy | Insulin sensitivity | No potential conflicts of interest relevant to this article were reported | |
| HOMA2-S | 2 β 0.017 (95%CI 0.003, 0.031), p = 0.02 | |||||||
| β-cell function | ||||||||
| HOMA2-B | 2 β –0.009 (95%CI 0.001, 0.018), p = 0.085 | |||||||
| [18] Shaat et al., 2017, Sweden | Cross-sectional, 376, 12–24 months | 34.3 ± 4.8 | 79% Caucasian 10% Asian 9% Arab 2% Other | 25(OH)D serum concentration measured by liquid chromatography mass spectrophotometry after pregnancy. Classified in three groups: (a) Deficiency (25(OH)D < 50 nmol/L) (b) Insufficiency (25(OH)D ≥ 50 < 75 nmol/L) (c) Sufficiency (25(OH)D ≥ 75 nmol/L) | Comparisons between 25(OH)D status groups | Insulin resistance | The authors have stated explicitly that there are no conflicts of interest in connection with this article | |
| HOMA-IR (median, IQR) | 3 Deficient: 1.8 (1.1–2.7) Insufficient: 1.6 (1.0–2.3) Sufficient: 1.1 (0.8–1.8) p = 0.001 | |||||||
| β-cell function (median, IQR) | ||||||||
| Insulinogenic index | 3 Deficient: 12.1 (7.7–20.4) Insufficient: 12.4 (8.5–18.9) Sufficient: 11.1 (8.4–18.2) p = 0.730 | |||||||
| £ Disposition index (I/G30)/HOMA-IR | 3 Deficient: 8.1 (4.9–12.3) Insufficient: 8.5 (5.4–14.0) Sufficient: 10.1 (5.4–14.6) p = 0.035 | |||||||
| ¥ T2DM after GDM (OR, 95% CI) | 4 1.0 (1.0, 1.1), p = 0.130 | |||||||
| [17] Mozaffari-Khosravi et al., 2012, Iran | RCT, 45, 3 months, 45/45 (100%) | Intervention group 30.7 ± 6.2 Control group 29.5 ± 4.0 | Not mentioned | One intramuscular injection of 300.000 IU 3-10 days after pregnancy. 25(OH)D concentration measured by immunoassay method (NycoCard; Nyco Corporation, Oslo, Norway) | No-treatment control group | Insulin sensitivity | The authors have stated that they had nothing to declare in connection with this article | |
| QUICKI (mean ± SD) | 5 Intervention: 0.38 ± 0.02 Control: 0.36 ± 0.02, p = 0.006 | |||||||
| HOMA-S (mean ± SD) | 5 Intervention: 169.68 ± 53.48 Control: 122.84 ± 41.15, p = 0.002 | |||||||
| Insulin resistance | ||||||||
| HOMA-IR (25th, 50th, 75th pct) | 6 Intervention: 0.4, 0.5, 0.8 Control: 0.7, 0.9, 1.0, p = 0.004 | |||||||
| β-cell function HOMA-B (mean ± SD) | 5 Intervention: 69.97 ± 28.13 Control: 77.68 ± 34.44, p = 0.40 | |||||||
| Glucose measurements | ||||||||
| FBG (mg/dL) (mean ± SD) | 5 Intervention: 92.9 ± 10.6 Control: 104.7 ± 33.5, p = 0.11 | |||||||
| 2 h 75 g OGTT (mg/dL) (mean ± SD) | 5 Intervention: 123 ± 69.04 Control: 117 ± 56.3, p = 0.8 | |||||||
| Glycated hemoglobin | ||||||||
| HbA1c (nmol/mol) (mean ± SD) | 5 Intervention: 37 ± 13 Control: 34 ± 6, p = 0.22 | |||||||
| HbA1c (%) (mean ± SD) | 5 Intervention: 5.58 ± 1.2 Control: 5.21 ± 0.52, p = 0.2 | |||||||
| [20] Valizadeh et al., 2016, Iran | RCT, 96, 6–12 weeks, 84/96 (87.5%) | Intervention group 32.0 ± 5.5 Control group 32.4 ± 4.7 | Not mentioned | Oral vitamin D supplementation of 700.000 IU from 12 gestational weeks until delivery. 25(OH)D concentration measured by ELISA (enzyme-linked immunosorbent assay) method (Immunodiagnostic Systems Ltd., Tyne and Wear, UK) | No-treatment control group | Insulin resistance | This study was supported by a studentship fund from Zanjan University of Medical Sciences. Farir-Teb Company supported this study by providing glucometers (Glucocard 0-1) for the parents | |
| HOMA-IR (mean ± SD) | 7 Intervention: 2.0 ± 1.3 Control: 1.8 ± 1.9, p = 0.58 | |||||||
| β-cell function | ||||||||
| Serum insulin level (µu/mL) (mean±SD) | 7 Intervention: 8.7 ± 4.4 Control: 8.8 ± 9.7, p = 0.99 | |||||||
| Glucose measurements | ||||||||
| FPG (mg/dL) (mean±SD) | 7 Intervention: 94 ± 16 Control: 89 ± 13, p = 0.12 | |||||||
| 2 h 75 g OGTT (mg/dL) (mean ± SD) | 7 Intervention: 115 ± 48 Control: 110 ± 36, p = 0.56 | |||||||
| Glycated hemoglobin | ||||||||
| HbA1c (%) (mean ± SD) | 7 Intervention: 5.6 ± 0.5 Control: 5.5± 0.5, p = 0.24 | |||||||
| HbA1c (nmol/mol) (mean ± SD) | 7 Intervention: 38 ± 5 Control: 37 ± 5, p = 0.24 | |||||||
| ɕ Dysglycemia (OR, 95% CI) | 8 1.02 (0.98, 1.06), p = 0.337 | |||||||
| [21] Yeow et al., 2015, Malaysia | RCT, 26, 6 months, 22/26 (84.6%) | Intervention group 36 (32, 38) Control group 35 (30, 40) | 100% Asian | Oral Vitamin D supplementation (capsules) of 4000 IU per day for 6 months, 6–48 months after pregnancy. 25(OH)D concentration measured the Elecsys Vitamin D Total assay (Roche Diagnostics GmbH, Sandhofer Strasse 116, D-68305 Mannheim, Germany) | Placebo capsules for 6 months, 6–48 months post-partum | Insulin sensitivity | The vitamin D and placebo capsules were supplied by Blackmore Ltd. without charge. This does not alter the author’s adherence to PLOS ONE policies on sharing data and materials. The authors declare no competing interest between all study investigators and Blackmore Ltd. in terms of employment, consultancy, and patents of the product or its development | |
| QUICKI (Δ Median (25th, 75th pct)) | 6 Intervention: −0.01 (−0.029, 0.01) Control: 0.01 (−0.005, 0.021), p = 0.047 | |||||||
| OGIS (mL/min/m2) (Δ Median (25th, 75th pct)) | 6 Intervention: −10 (−47.0, 55.0) Control: −1 (−82.0, 42.0), p = 1.00 | |||||||
| BIGTT-S (10–5 × (min × pmol/L)−1) (Δ Median (25th, 75th pct)) | 6 Intervention: −0.6 (−1.15, 0.60) Control: −0.3 (−1.29, 1.04), p = 0.699 | |||||||
| Insulin resistance | ||||||||
| Fasting insulin (pmol/L) (Δ Median (25th, 75th pct)) | 6 Intervention: 15.6 (−13.80, 51.00) Control: −14.4 (−46.20, 0), p = 0.034 | |||||||
| Fasting C-peptide (ng/mL) (Δ Median (25th, 75th pct)) | 6 Intervention: 0.4 (0.03, 0.54) Control: 0.3 (−0.09, 0.48), p = 0.365 | |||||||
| Glycated hemoglobin HbA1c (nmol/mol) (Δ Median (25th, 75th pct)) | 6 Intervention: −2 (−3, −1) Control: −2 (−4, 0), p = 0.847 | |||||||
| Glucose measurements | ||||||||
| FPG (Δ Median (25th, 75th pct)) | 6 Intervention: 0 (−0.20, 0) Control: 0.1 (−0.20, 0.50), p = 0.270 | |||||||
| 30 min 75 g OGTT (mmol/L) (Δ Median (25th, 75th pct)) | 6 Intervention: −0.4 (−2.40, 1.70) Control: −0.1 (−1.93, 0.48), p = 1.00 | |||||||
| 2 h 75 g OGTT (mmol/L) (Δ Median (25th, 75th pct)) | 6 Intervention: −2.6 (−1.50, −0.40) Control: 0.4 (−1.20, 1.00), p = 0.061 | |||||||
| AUCglucose (mmol/L) (Δ Median (25th, 75th pct)) | 6 Intervention: −28.5 (−199.50, 70.5) Control: −56.6 (−130.5, 54.0), p = 1.00 | |||||||
| β-cell function | ||||||||
| AUCinsulin (pmol/L) (Δ Median (25th, 75th pct)) | 6 Intervention: 17,376 (−8574, 41,514) Control: 3894 (−10,242, 17,524) p = 0.365 | |||||||
| AUCcp (ng/mL) (Δ Median (25th, 75th pct)) | 6 Intervention: 157 (79.0, 210.0) Control: 134 (86.0, 269.0), p = 1.00 | |||||||
| IGI60 (pmol/mmol) (Δ Median (25th, 75th pct)) | 6 Intervention: 31.8 (−30.05, 297.33) Control: 82.66 (−2.00, 203.87), p = 0.863 | |||||||
| BIGTT-AIR (min × pmol/L) (Δ Median (25th, 75th pct)) | 6 Intervention: 1241.2 (−299.48, 2260.43) Control: −144.8 (−1893.53, 916.62), p = 0.133 | |||||||
| Disposition index (OGIS * ratio of total AUCinsulin over AUCglucose) (Δ Median (25th, 75th pct)) | 6 Intervention: 7.7 × 103 (2.94 × 103, 17.52 × 103) Control: 4.5 × 103 (−3.88 × 103, 10.70 × 103), p = 0.171 |
| Study (Design) | N (%) | Median (25th, 75th Percentiles) or Mean ± SD | p-Value Difference within Group | N | Median (25th, 75th Percentiles) or Mean ± SD | p-Value Difference within Group | Between Group p-Value | |
|---|---|---|---|---|---|---|---|---|
| [22] Kramer et al. | Deficiency | 161 (33) | 35.7 ± 10.2 | - | - | - | ||
| (cohort) | Insufficiency | 178 (36) | 64.4 ± 7.4 | - | - | - | ||
| Sufficiency | 155 (31) | 91.2 ± 12.5 | - | - | - | <0.001 | ||
| [18] Shaat et al. | Deficiency | 198 (53) | 32.9 ± 11.2 | - | - | - | ||
| (cross-sectional) | Insufficiency | 125 (33) | 60.8 ± 7.1 | - | - | - | ||
| Sufficiency | 53 (13) | 88.1 ± 11.2 | - | - | - | <0.001 | ||
| Cases | Controls | |||||||
| [19] * Tänczer et al. | 87 | 68 ± 32.75 | 45 | 67.25 ± 24 | 0.888 | |||
| (case-control) | ||||||||
| Intervention group | Control group | |||||||
| [21] Yeow et al. | Baseline | 13 | 35.6 (25.60, 43.95) | 0.003 | 13 | 35.1 (21.63, 40.75) | 0.859 | |
| (RCT) | End | 92.4 (79.00, 102.34) | 28.5 (20.87, 42.43) | <0.001 | ||||
| [17] Mozaffari-Khosravi et al. | Baseline | 24 | 24.25 (17.05, 28.2) | <0.001 | 21 | 25.3 (20.0, 32.35) | 0.02 | |
| (RCT) | End | 62.10 (55.47, 71.70) | 24.1 (21.70, 48.60) | <0.001 | ||||
| [20] * Valizadeh et al. | Baseline | 42 | 36.5 ± 15.75 | 42 | 44.25 ± 15.25 | 0.04 | ||
| (RCT) | End | 81 ± 36 | 48.25 ± 24 | <0.001 | ||||
| Outcomes | Study | Direction of Associations |
|---|---|---|
| Insulin sensitivity | ||
| Matsuda index | Kramer et al. [22] | * Deficiency: no |
| * Insufficiency: no | ||
| HOMA2-S | Tänczer et al. [19] | (+) |
| HOMA-S | Mozaffari-Khosravi et al. [17] | (+) |
| QUICKI | Mozaffari-Khosravi et al. [17] | (+) |
| Yeow et al. [21] | (−) | |
| OGIS | Yeow et al. [21] | No |
| BIGTT-S | Yeow et al. [21] | No |
| Insulin resistance | ||
| HOMA-IR | Shaat et al. [18] | (−) |
| Mozaffari-Khosravi et al. [17] | (−) | |
| Valizadeh et al. [20] | No | |
| Fasting insulin | Valizadeh et al. [20] | No |
| Yeow et al. [21] | (+) | |
| Fasting C-peptide | Yeow et al. [21] | No |
| Beta-cell function | ||
| HOMA2-B | Tänczer et al. [19] | No |
| HOMA-B | Mozaffari-Khosravi et al. [17] | No |
| ISSI-2 | Kramer et al. [22] | * Deficiency: no |
| * Insufficiency: (+) | ||
| Insulinogenic index | Shaat et al. [18] | No |
| Disposition index | Shaat et al. [18] | (+) |
| Yeow et al. [21] | No | |
| AUCinsulin (pmol/L) | Yeow et al. [21] | No |
| AUCcp (ng/mL) | Yeow et al. [21] | No |
| IGI60 (pmol/mmol) | Yeow et al. [21] | No |
| BIGTT-AIR | Yeow et al. [21] | No |
| Glucose measurements | ||
| FBG | Kramer et al. [22] | * Deficiency: (−) |
| * Insufficiency: no | ||
| Mozaffari-Khosravi et al. [17] | No | |
| FPG | Valizadeh et al. [20] | No |
| Yeow et al. [21] | No | |
| 30 min 75 g OGTT | Yeow et al. [21] | No |
| 2 h 75 g OGTT | Kramer et al. [22] | * Deficiency: (−) |
| * Insufficiency: (−) | ||
| Mozaffari-Khosravi et al. [17] | No | |
| Valizadeh et al. [20] | No | |
| AUCglucose (mmol/L) | Yeow et al. [21] | No |
| Glycated hemoglobin | ||
| HbA1c | Mozaffari-Khosravi et al. [17] | No |
| Valizadeh et al. [20] | No | |
| Yeow et al. [21] | No | |
| % HbA1c | Mozaffari-Khosravi et al. [17] | No |
| Valizadeh et al. [20] | No | |
| Diabetes | ** Shaat et al. [18] | No |
| *** Valizadeh et al. [20] | No |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, A.; Varela Vazquez, C.; Dangol, R.; Damm, P.; Heitmann, B.L.; Händel, M.N. The Role of Vitamin D in the Development of Diabetes Post Gestational Diabetes Mellitus: A Systematic Literature Review. Nutrients 2020, 12, 1733. https://doi.org/10.3390/nu12061733
Keller A, Varela Vazquez C, Dangol R, Damm P, Heitmann BL, Händel MN. The Role of Vitamin D in the Development of Diabetes Post Gestational Diabetes Mellitus: A Systematic Literature Review. Nutrients. 2020; 12(6):1733. https://doi.org/10.3390/nu12061733
Chicago/Turabian StyleKeller, Amélie, Carmen Varela Vazquez, Rojina Dangol, Peter Damm, Berit Lilienthal Heitmann, and Mina Nicole Händel. 2020. "The Role of Vitamin D in the Development of Diabetes Post Gestational Diabetes Mellitus: A Systematic Literature Review" Nutrients 12, no. 6: 1733. https://doi.org/10.3390/nu12061733





