Semi-Solid Nutrients for Prevention of Enteral Tube Feeding-Related Complications in Japanese Population: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Types of Studies
2.2. Types of Participants
2.3. Types of Interventions and Comparisons
2.4. Outcomes
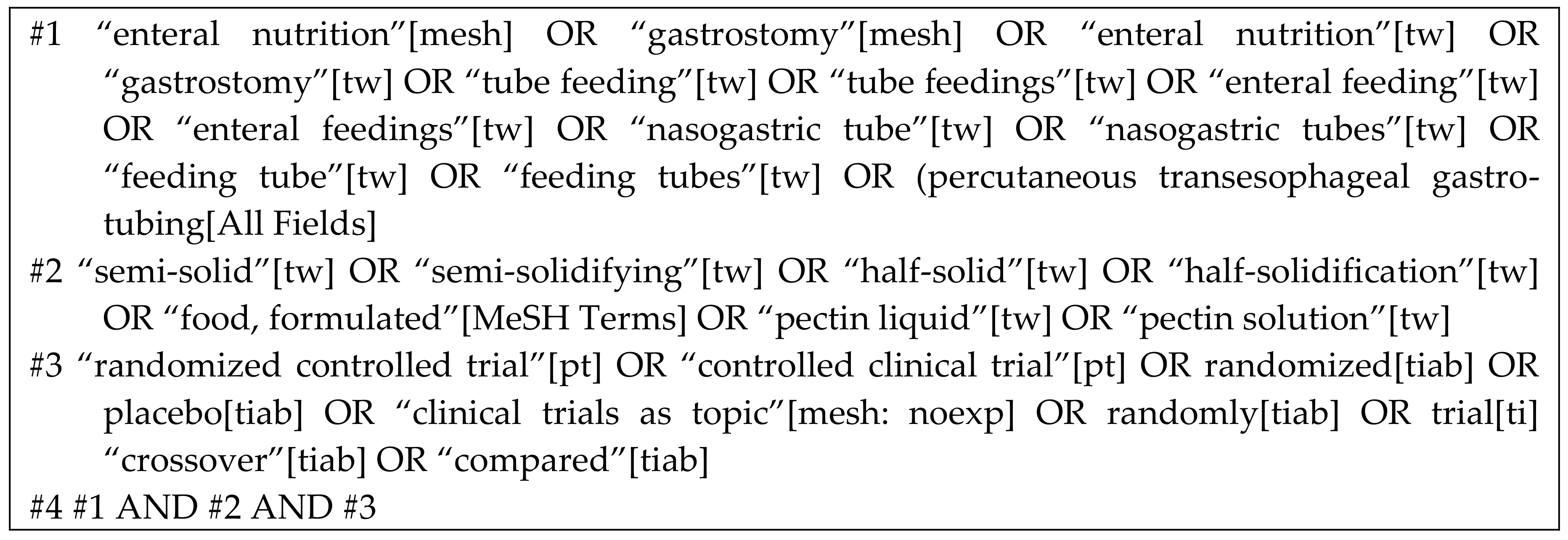
2.5. Search Strategy
2.6. Selection of Studies
2.7. Data Extraction and Management
2.8. Risk of Bias Assessment
2.9. Statistical Analysis
3. Results
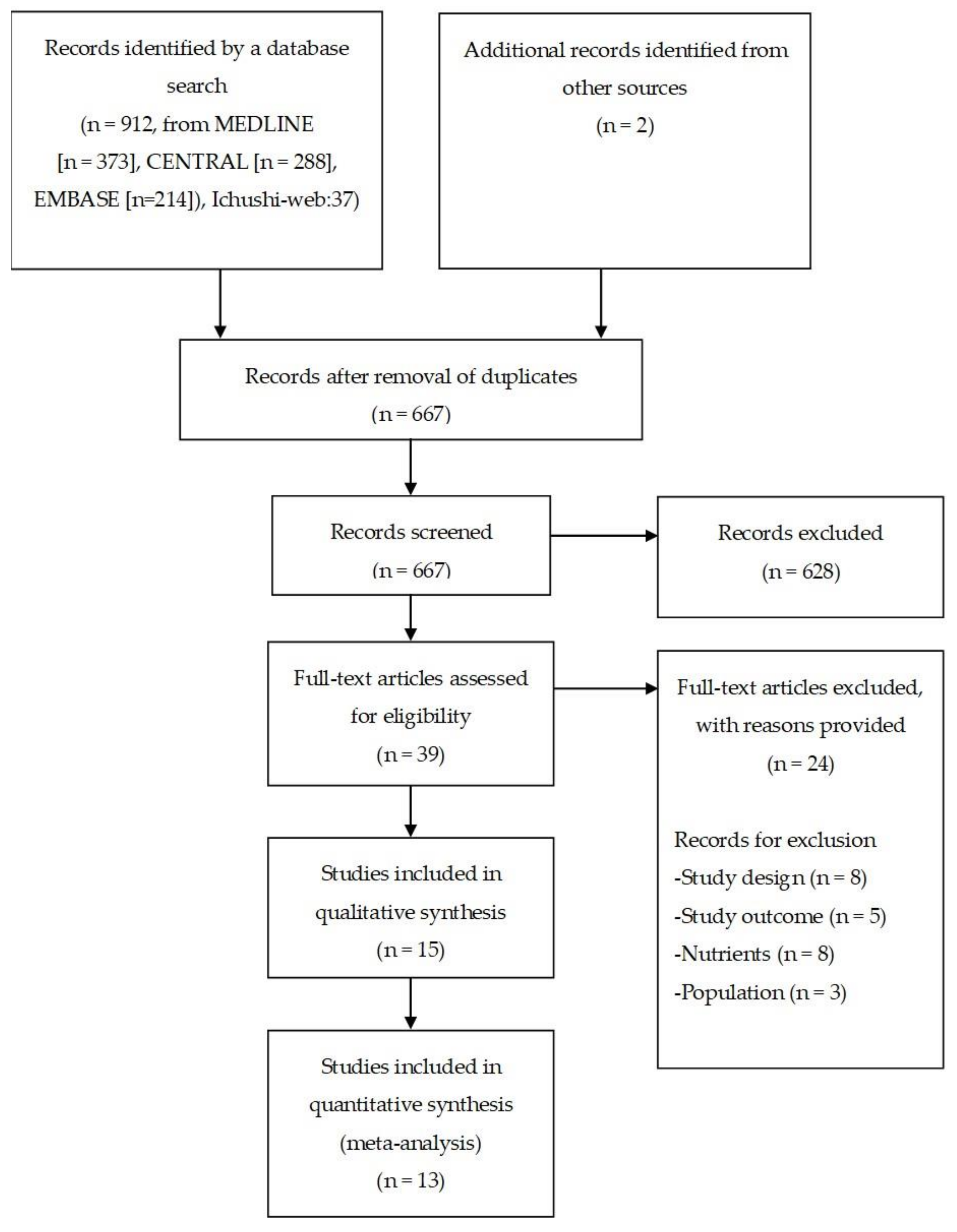
3.1. Study Selection
3.2. Patient Characteristics
3.3. Intervention
3.4. Outcomes
3.5. Risk of Bias Assessment
3.6. Quantitative Synthesis
3.6.1. Gastroesophageal Reflux
3.6.2. Pneumonia
3.6.3. Diarrhea
3.6.4. Constipation
3.6.5. Leak from Gastrostomy Site
3.6.6. Dwell Time in the Stomach
3.6.7. Care Time
3.6.8. Pressure Ulcer, Rehabilitation Time, Activities of Daily Living, and Medical Costs
4. Discussion
4.1. Summary of Results
4.2. Overall Completeness and Applicability of Evidence
4.3. Quality of Evidence
4.4. Potential Biases in the Review Process
4.5. Agreements and Disagreements with Other Studies or Reviews
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A



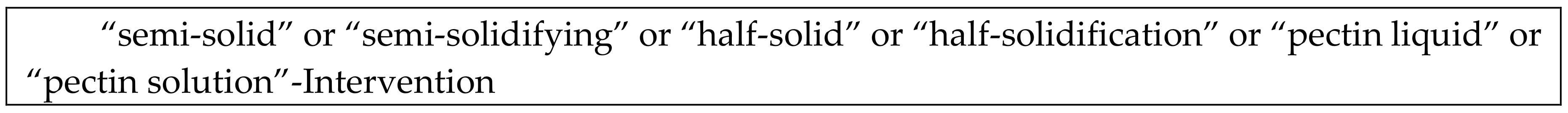
References
- Blumenstein, I.; Shastri, Y.M.; Stein, J. Gastroenteric tube feeding: Techniques, problems and solutions. World J. Gastroenterol. 2014, 20, 8505–8524. [Google Scholar] [CrossRef]
- Boullata, J.I.; Carrera, A.L.; Harvey, L.; Escuro, A.A.; Hudson, L.; Mays, A.; McGinnis, C.; Wessel, J.J.; Bajpai, S.; Beebe, M.L.; et al. ASPEN Safe Practices for Enteral Nutrition Therapy. J. Parenter. Enter. Nutr. 2017, 41, 15–103. [Google Scholar] [CrossRef] [Green Version]
- McClave, S.A.; Martindale, R.G.; Vanek, V.W.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J. Parenter. Enter. Nutr. 2009, 33, 277–316. [Google Scholar] [CrossRef]
- Windsor, A.C.; Kanwar, S.; Li, A.G.; Barnes, E.; Guthrie, J.A.; Spark, J.I.; Welsh, F.; Guillou, P.J.; Reynolds, J.V. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut 1998, 42, 431–435. [Google Scholar] [CrossRef]
- Ammori, B.J. Importance of the early increase in intestinal permeability in critically ill patients. Eur. J. Surg. 2002, 168, 660–661. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Bankhead, R.; Boullata, J.; Brantley, S.; Corkins, M.; Guenter, P.; Krenitsky, J.; Lyman, B.; Metheny, N.A.; Mueller, C.; Robbins, S.; et al. A.S.P.E.N. Enteral nutrition practice recommendations. J. Parenter. Enter. Nutr. 2009, 33, 122–167. [Google Scholar] [CrossRef] [Green Version]
- Montejo, J.C. Enteral nutrition-related gastrointestinal complications in critically ill patients: A multicenter study. The Nutritional and Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Crit. Care Med. 1999, 27, 1447–1453. [Google Scholar] [CrossRef]
- Jacobs, S.; Chang, R.W.; Lee, B.; Bartlett, F.W. Continuous enteral feeding: A major cause of pneumonia among ventilated intensive care unit patients. J. Parenter. Enter. Nutr. 1990, 14, 353–356. [Google Scholar] [CrossRef]
- Olivares, L.; Segovia, A.; Revuelta, R. Tube feeding and lethal aspiration in neurological patients: A review of 720 autopsy cases. Stroke 1974, 5, 654–657. [Google Scholar] [CrossRef] [Green Version]
- Kanie, J.; Suzuki, Y.; Iguchi, A.; Akatsu, H.; Yamamoto, T.; Shimokata, H. Prevention of gastroesophageal reflux using an application of half-solid nutrients in patients with percutaneous endoscopic gastrostomy feeding. J. Am. Geriatr. Soc. 2004, 52, 466–467. [Google Scholar] [CrossRef]
- Nishiwaki, S.; Araki, H.; Shirakami, Y.; Kawaguchi, J.; Kawade, N.; Iwashita, M.; Tagami, A.; Hatakeyama, H.; Hayashi, T.; Maeda, T.; et al. Inhibition of gastroesophageal reflux by semi-solid nutrients in patients with percutaneous endoscopic gastrostomy. J. Parenter. Enter. Nutr. 2009, 33, 513–519. [Google Scholar] [CrossRef]
- Shimizu, A.; Muramatsu, H.; Kura, T.; Sakata, T. Incidence of gastroesophageal reflux associated with percutaneous endoscopic gastrostomy contrast agent viscosity: A randomized controlled crossover trial. Eur. J. Clin. Nutr. 2016, 70, 1057–1061. [Google Scholar] [CrossRef]
- Current Status and Problems of “Semi-Solidification”. Available online: http://www.peg.or.jp/paper/article/semi-solid/30.html (accessed on 25 April 2020). (In Japanese).
- Lundh, A.; Gotzsche, P.C. Recommendations by Cochrane Review Groups for assessment of the risk of bias in studies. BMC Med. Res. Methodol. 2008, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Kanda, Y. Investigation of the freely available easy- to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Muramatsu, H.; Tanaka, I.; Ihara, H.; Mikami, L.; Nakaya, R.; Kusakabe, T.; Watanabe, H.; Kitagawa, K.; Tsuchida, S.; Hisai, H.; et al. Addition of gelling agent (pectin-calcium) to a fluid intragastric diet decreased pneumonia and diarrhea inpatients with percutaneous endoscopic gastrostomy, a multi-center randomized control study. J. JSPEN 2018, 33, 611–616. [Google Scholar] [CrossRef]
- Ishii, A.; Ono, K.; Nishijima, A.; Hasegawa, T.; Kamiguchi, S.; Hamamura, M.; Tsuchiya, T. Trial of semi-solid nutrients to reduce the side effects of enteral nutrition. J. JSPEN 2006, 21, 99. (In Japanese) [Google Scholar]
- Togashi, A.; Paku, H. Examination of administration method and time schedule of semi-solid nutrients and liquid nutrients early after percutaneous endoscopic gastrostomy in hospital NST management. J. JSPEN 2012, 27, 496. (In Japanese) [Google Scholar]
- Paku, H.; Togashi, A.; Ikeda, T.; Kikuchi, T.; Furuya, T.; Koshinaga, S. Examination of semi-solid nutrients and liquid nutrients at the early stage after percutaneous endoscopic gastrostomy. J. JSPEN 2012, 27, 440. (In Japanese) [Google Scholar]
- Nakahori, M.; Miyashita, Y.; Abe, Y.; Takahashi, A.; Segawa, Y.; Obata, Y. Investigation of usefulness of semi-solidified nutrient in early phase postoperative period of gastrostomy. J. JSPEN 2011, 26, 581. (In Japanese) [Google Scholar]
- Abe, Y.; Obata, Y.; Segawa, Y.; Takahashi, A.; Miyashita, Y.; Nakahori, M. Usefulness of semi-solidified nutrient after percutaneous endoscopic gastrostomy. J. JSPEN 2011, 26, 396. (In Japanese) [Google Scholar]
- Muramatsu, H.; Ihara, H.; Yano, Y. Effects of semi-solid nutrients on shape of stool improvement in patients with gastrostomy. J. JSPEN 2010, 25, 690. (In Japanese) [Google Scholar]
- Shizuku, T.; Adachi, K.; Furuta, K.; Niigaki, M.; Miyaoka, M.; Katoh, S.; Kobayashi, K.; Otani, M.; Kawashima, K.; Otani, J.; et al. Efficacy of half-solid nutrient for the elderly patients with percutaneous endoscopic gastrostomy. J. Clin. Biochem. Nutr. 2011, 48, 226–269. [Google Scholar] [CrossRef] [Green Version]
- Nagasawa, K. Infiuence of Semi-Solid Nutrient on Gastric Emptying in Patients with Gastrostomy. Jpn. J. Clin. Physiol. 2009, 39, 297–302. [Google Scholar]
- Yoon, E.W.T.; Yoneda, K.; Nishihara, K. Semi-solid feeds may reduce the risk of aspiration pneumonia and shorten postoperative length of stay after percutaneous endoscopic gastrostomy (PEG). Endosc. Int. Open 2016, 4, E1247–E1251. [Google Scholar] [CrossRef] [Green Version]
- Tabei, I.; Tsuchida, S.; Akashi, T.; Ookubo, K.; Hosodae, S.; Furukawa, Y.; Tanabe, Y.; Tamura, Y. Effects of a novel method for enteral nutrition infusion involving a viscosity-regulating pectin solution: A multicenter randomized controlled trial. Clin. Nutr. ESPEN 2018, 23, 34–40. [Google Scholar] [CrossRef]
- Higashiguchi, T.; Suzuki, Y.; Maruyama, M.; Shintani, S.; Tominaga, T.; Akanuma, J.; Imamura, A.; Kondo, Y.; Seki, Y.; Okabe, S.; et al. Clinical trial of enteral nutrient P0201 for patients with gastrostomy (phase III). J. New Rem. Clin. 2014, 63, 837–843. (In Japanese) [Google Scholar]
 = low risk of bias;
= low risk of bias;  = unclear;
= unclear;  = high risk of bias.
= high risk of bias.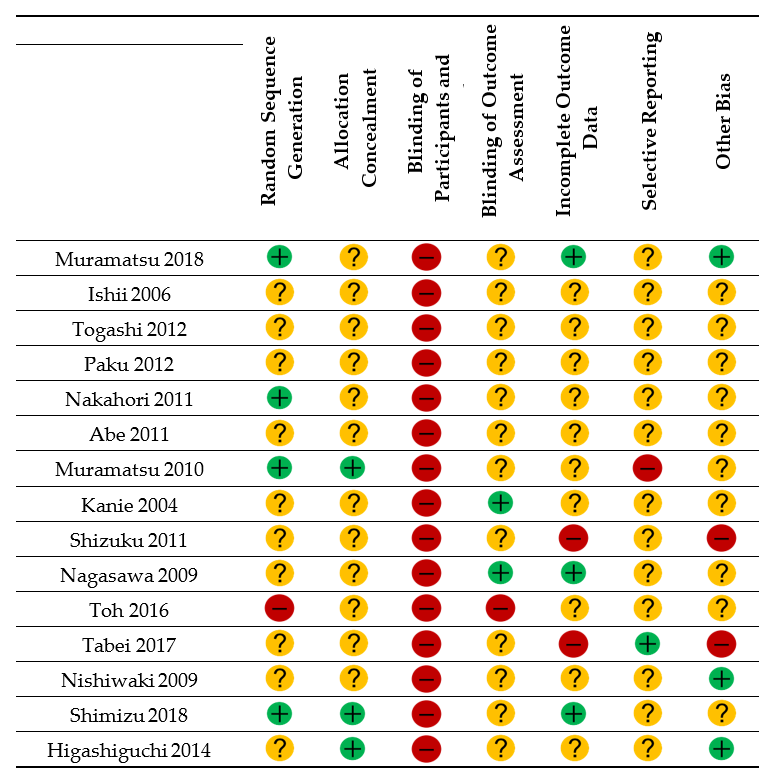
| Study | Country | Participants | n (I/C) | Intervention | Control | Outcomes | Conclusion |
|---|---|---|---|---|---|---|---|
| Muramatsu 2018 | Japan | Patients who agreed to study participation before gastrostomy | 151 (75/76) | Semi-solid enteral nutrients prepared by adding pectin and calcium with a viscosity of 20,000 cP | Liquid enteral nutrients | Pneumonia | Semi-solid enteral nutrients decreased the risk of pneumonia |
| Ishii 2006 | Japan | Inpatients with gastrostomy | 14 (7/7) | Semi-solid enteral nutrients | Liquid enteral nutrients | GER 1 Diarrhea Leak from the gastrostomy site | Diarrhea and leak from the gastrostomy site were less common with semi-solid nutrients than with liquid enteral nutrients |
| Togashi 2012 | Japan | Inpatients with gastrostomy | 94 (50/44) | Semi-solid enteral nutrients with viscosity of 20,000 cP | Liquid enteral nutrients | Pneumonia Diarrhea | Patients on semi-solid enteral nutrients had a lower incidence of pneumonia and diarrhea |
| Paku 2012 | Japan | Inpatients with gastrostomy | 94 (50/44) | Semi-solid enteral nutrients with viscosity of 20,000 cP | Liquid enteral nutrients | Pneumonia Diarrhea | Patients on semi-solid enteral nutrients had a lower incidence of pneumonia and diarrhea |
| Nakahori 2011 | Japan | Inpatients with gastrostomy | 20 (10/10) | Semi-solid enteral nutrients | Liquid enteral nutrients | Pneumonia Diarrhea | Pneumonia and diarrhea were difficult to evaluate because of the small number of cases |
| Abe 2011 | Japan | Inpatients with gastrostomy | 15 (8/7) | Semi-solid enteral nutrients | Liquid enteral nutrients | Diarrhea Care time | Semi-solid enteral nutrients were associated with decreased occurrence of diarrhea and shorter care time |
| Muramatsu 2010 | Japan | Inpatients with PEG 2 | 22 (11/11) | Semi-solid enteral nutrients | Liquid enteral nutrients | Consistency of stools | Consistency of stools improved from watery to solid in the semi-solid nutrient group |
| Kanie 2004 | Japan | Patients being fed by PEG | 34 (17/17) | Half-solid enteral nutrients were prepared by mixing with 5 g of agarose | Liquid enteral nutrients | GER | The rate of GER was lower in the half-solid nutrient group |
| Shizuku 2011 | Japan | Elderly patients undergoing PEG feeding | 64 (32/32) | Half-solid enteral nutrients MEDI-F Pushcare® (Nestle, Kobe) with viscosity of about 2000 mPa·s | Liquid enteral nutrients | Diarrhea Care time needed | The care time needed was significantly less in the half-solid enteral nutrient group; the numbers of patients who developed diarrhea were similar between the groups |
| Nagasawa 2009 | Japan | Patients more than 1 week after gastrostomy | 20 (10/10) | Semi-solid nutrients prepared by adding Easy gel to RACOL® (Otsuka, Tokyo) | Liquid enteral nutrients (RACOL®, Otsuka, Tokyo) | Dwell time in the stomach | Semi-solid enteral nutrients accelerate gastric emptying during the early phase when compared with liquid enteral nutrients |
| Toh 2016 | Japan | Patients who received gastrostomy for enteral nutrition Tube feeding for 2 weeks prior to gastrostomy | 117 (45/72) | Semi-solid enteral feed with a dynamic viscosity of 20,000 cP | Liquid feed with dynamic viscosity of 5–10 mPa s | Pneumonia Diarrhea | Using semi-solid enteral feeds may reduce the risk of pneumonia No statistically significant difference in the rates of diarrhea between the two groups |
| Tabei 2018 | Japan | Age ≥20 years Patients who needed nutritional therapy via a percutaneous endoscopic gastrostomy, percutaneous transesophageal gastric tube, or a nasogastric tube | 27 (15/12) | Pectin solution with viscosity of 1000–2000 mPa·s | Liquid enteral nutrition diet of K-LEC® (Kewpie Corporation, Tokyo) with viscosity of 5 mPa·s | Pneumonia Diarrhea Care time | No cases of pneumonia in either group No between-group difference in incidence of diarrhea Pectin solution was able to be administered in a significantly shorter time than the liquid enteral nutrition diet |
| Nishiwaki 2009 | Japan | Patients more than 1 month after gastrostomy | 30 (15/15) | Semi-solid enteral nutrients prepared by adding agar to RACOL® (Otsuka, Tokyo) | Liquid enteral nutrients (RACOL® (Otsuka, Tokyo) | GER Dwell time in the stomach | GER was significantly inhibited by semi-solid enteral nutrients No between-group difference in gastric emptying time |
| Shimizu 2016 | Japan | Patients who planned to undergo PEG for the first time | 132 (66/66) | Semi-solid contrast agent with viscosity of 6000 mPa·s | Liquid contrast agent (3 mPa·s) | GER | Semi-solid contrast agents reduced the incidence of GER after PEG |
| Higashiguchi 2014 | Japan | Aged >20 years Patients undergoing PEG or had plans for PEG | 112 (56/56) | Semi-solid enteral nutrients with viscosity of 6500–12,500 mPa·s prepared using alginic acid and agar powder | Liquid enteral nutrients with viscosity of 5.51–6.52 mPa·s | Pneumonia Diarrhea Constipation Care time | Semi-solid enteral nutrients were able to be administered in a significantly shorter time than liquid enteral nutrition No statistically significant difference in the rates of pneumonia, diarrhea, and constipation between the two groups |
| Outcome | Studies, n | Participants (Intervention/Control), n | Effect Size | 95% CI 1 | Inconsistency, I2 (%) |
|---|---|---|---|---|---|
| Gastroesophageal reflux (present or absent) | 3 | 180 (90/90) | RR: 0.39 | (0.21, 0.73) | 0 |
| Gastroesophageal reflux (GER 2 index) | 1 | 30 (15/15) | MD 3: −2.93 | (−5.18, −0.68) | - |
| Pneumonia | 7 | 615 (328/287) | RR: 0.99 | (0.51, 1.93) | 58.0 |
| Diarrhea | 8 | 541 (292/249) | RR: 0.82 | (0.57, 1.18) | 47.4 |
| Constipation | 1 | 112 (56/56) | RR: 0.25 | (0.03, 2.17) | - |
| Leak from gastrostomy site | 1 | 14 (7/7) | 5 RR: 0.20 | (0.01, 3.50) | - |
| Dwell time in the stomach | 2 | 70 (35/35) | 4 SMD: −0.50 | (−0.99, −0.02) | 50.0 |
| Care time | 3 | 369 (189/180) | SMD: −8.02 | (−10.94, −5.10) | 95.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokura, Y.; Suzuki, C.; Wakabayashi, H.; Maeda, K.; Sakai, K.; Momosaki, R. Semi-Solid Nutrients for Prevention of Enteral Tube Feeding-Related Complications in Japanese Population: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1687. https://doi.org/10.3390/nu12061687
Kokura Y, Suzuki C, Wakabayashi H, Maeda K, Sakai K, Momosaki R. Semi-Solid Nutrients for Prevention of Enteral Tube Feeding-Related Complications in Japanese Population: A Systematic Review and Meta-Analysis. Nutrients. 2020; 12(6):1687. https://doi.org/10.3390/nu12061687
Chicago/Turabian StyleKokura, Yoji, Chieko Suzuki, Hidetaka Wakabayashi, Keisuke Maeda, Kotomi Sakai, and Ryo Momosaki. 2020. "Semi-Solid Nutrients for Prevention of Enteral Tube Feeding-Related Complications in Japanese Population: A Systematic Review and Meta-Analysis" Nutrients 12, no. 6: 1687. https://doi.org/10.3390/nu12061687






