Use of Non-Pharmacological Supplementations in Children and Adolescents with Attention Deficit/Hyperactivity Disorder: A Critical Review
Abstract
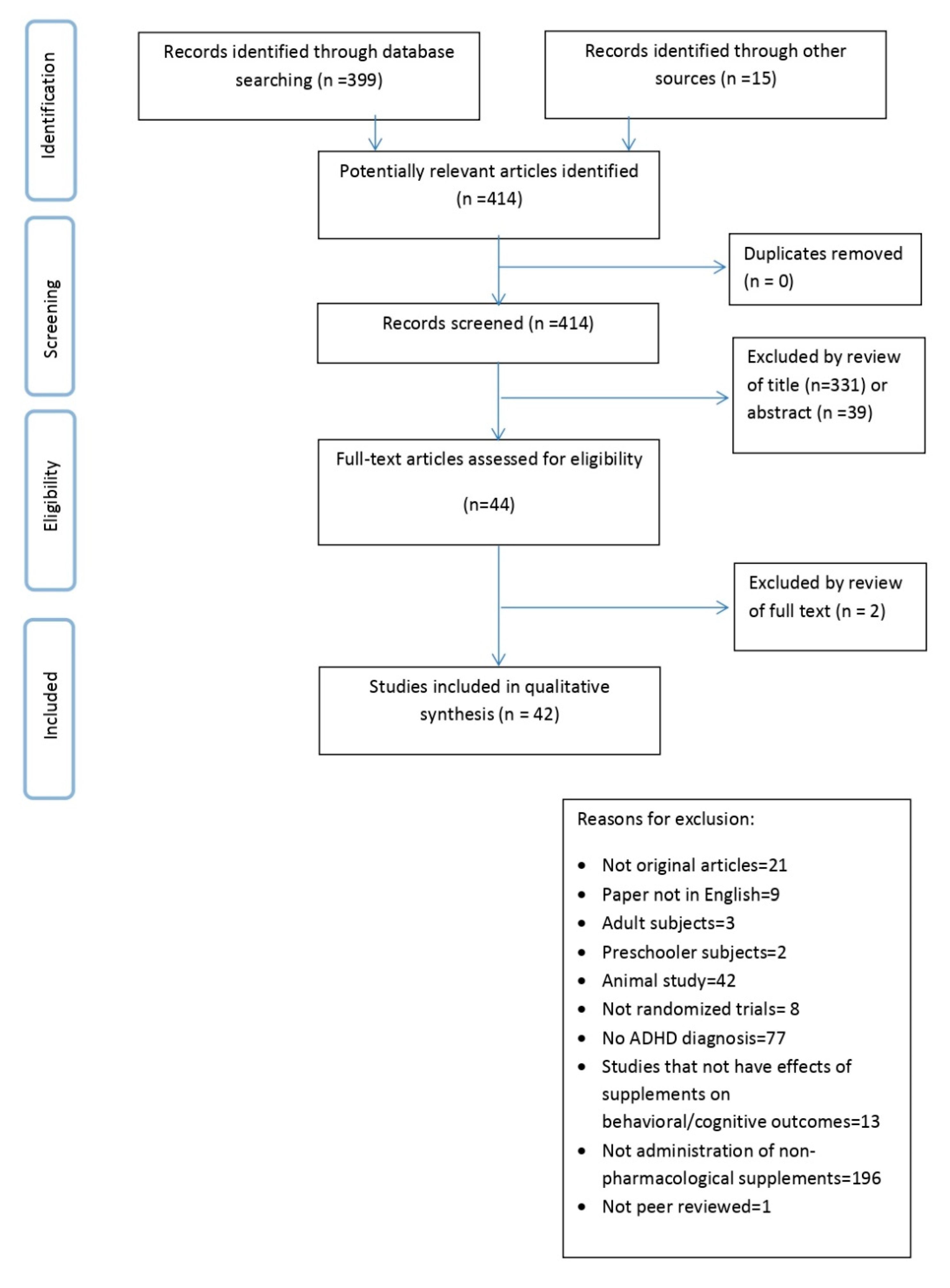
:1. Introduction
2. Materials and Methods
3. Results
- PUFAs;
- peptides and amino acids derivatives;
- single micronutrient (Zinc or Vitamin D);
- micronutrients mix;
- plant extracts or herbal supplementations;
- probiotics.
3.1. PUFAs
3.1.1. Methodologies
3.1.2. Results
3.2. Peptides and Amino Acids Derivatives
3.2.1. Methodologies
3.2.2. Results
3.3. Single Micronutrient (Zinc or Vitamin D)
3.3.1. Methodologies
3.3.2. Results
3.4. Micronutrients Mix
3.4.1. Methodologies
3.4.2. Results
3.5. Plant Extracts or Herbal Supplementations
3.5.1. Methodologies
3.5.2. Results
3.6. Probiotics: Methodologies and Results
4. Discussion
4.1. Discussion of Methodologies
4.2. Discussion of Results
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Arnsten, A.F.; Pliszka, S.R. Catecholamine influences on prefrontal cortical function: Relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol. Biochem. Behav. 2011, 99, 211–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, S.M. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications, 3rd ed.; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Storebø, O.J.; Krogh, H.B.; Ramstad, E.; Moreira-Maia, C.R.; Holmskov, M.; Skoog, M.; Nilausen, T.D.; Magnusson, F.L.; Zwi, M.; Gillies, D.; et al. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ 2015, 351, h5203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punja, S.; Shamseer, L.; Hartling, L.; Urichuk, L.; Vandermeer, B.; Nikles, J.; Vohra, S. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst. Rev. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.R.; Akhondzadeh, S. Pharmacotherapy of attention-deficit/hyperactivity disorder: Nonstimulant medication approaches. Exp. Rev. Neurother. 2007, 7, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.; Jensen, P.S.; Quinn, D.M. Remission versus response as the goal of therapy in ADHD: A new standard for the field? Clin. Ther. 2006, 28, 1892–1908. [Google Scholar] [CrossRef]
- Groenman, A.P.; Schweren, L.J.; Dietrich, A.; Hoekstra, P.J. An update on the safety of psychostimulants for the treatment of attention-deficit/hyperactivity disorder. Expert Opin. Drug Saf. 2017, 16, 455–464. [Google Scholar] [CrossRef]
- Bangs, M.E.; Wietecha, L.A.; Wang, S.; Buchanan, A.S.; Kelsey, D.K. Meta-analysis of suicide-related behavior or ideation in child, adolescent, and adult patients treated with atomoxetine. J. Child Adolesc. Psychopharmacol. 2014, 24, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Thapar, A.; Cooper, M.; Jefferies, R.; Stergiakouli, E. What causes attention deficit hyperactivity disorder? Arch. Dis. Child. 2012, 97, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Janssen, C.I.; Kiliaan, A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: The influence of LCPUFA on neural development, aging, and neurodegeneration. Progr. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef]
- Agostoni, C.; Nobile, M.; Ciappolino, V.; Delvecchio, G.; Tesei, A.; Turolo, S.; Crippa, A.; Mazzocchi, A.; Altamura, C.A.; Brambilla, P. The role of omega-3 fatty acids in developmental psychopathology: A systematic review on early psychosis, autism, and ADHD. Int. J. Mol. Sci. 2017, 18, 2608. [Google Scholar] [CrossRef] [Green Version]
- Tesei, A.; Crippa, A.; Busti Ceccarelli, S.; Mauri, M.; Molteni, M.; Agostoni, C.; Nobile, M. The potential relevance of docosahexaenoic acid and eicosapentaenoic acid to the etiopathogenesis of childhood neuropsychiatric disorders. Eur. Child Adolesc. Psychiatry 2017, 26, 1011–1030. [Google Scholar] [CrossRef] [PubMed]
- Adriani, W.; Rea, M.; Baviera, M.; Invernizzi, W.; Carli, M.; Ghirardi, O.; Caprioli, A.; Laviola, G. Acetyl-L-carnitine reduces impulsive behaviour in adolescent rats. Psychopharmacology 2004, 176, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.-H.; Heidari, S.; Mohammadi, M.-R.; Tabrizi, M.; Ghaleiha, A.; Akhondzadeh, S. Acetyl-L-Carnitine as an Adjunctive Therapy in the Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents: A Placebo-Controlled Trial. Child Psychiatry Hum. Dev. 2011, 42, 367–375. [Google Scholar] [CrossRef]
- Prokopieva, V.; Yarygina, E.; Bokhan, N.; Ivanova, S. Use of carnosine for oxidative stress reduction in different pathologies. Oxid. Med. Cell. Longev. 2016, 2939087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghajar, A.; Aghajan-Nashtaei, F.; Afarideh, M.; Mohammadi, M.-R.; Akhondzadeh, S. L-Carnosine as Adjunctive Therapy in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Child Adolesc. Psychopharmacol. 2018, 5, 331–337. [Google Scholar] [CrossRef]
- Chez, M.G.; Buchanan, C.P.; Aimonovitch, M.C.; Becker, M.; Schaefer, K.; Black, C.; Komen, J. Double-blind, placebo-controlled study of l-carnosine supplementation in children with autistic spectrum disorders. J. Child Neurol. 2002, 17, 833–837. [Google Scholar] [CrossRef]
- Pepeu, G.; Pepeu, I.M.; Amaducci, L. A review of phosphatidylserine pharmacological and clinical effects. Is phosphatidylserine a drug for the ageing brain? Pharmacol. Res. 1996, 33, 73–80. [Google Scholar] [CrossRef]
- Starks, M.A.; Starks, S.L.; Kingsley, M.; Purpura, M.; Jäger, R. The effects of phosphatidylserine on endocrine response to moderate intensity exercise. J. Int. Soc. Sports Nutr. 2008, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Hirayama, S.; Terasawa, K.; Rabeler, R.; Hirayama, T.; Inoue, T.; Tatsumi, Y.; Purpura, M.; Jager, R. The effect of phosphatidylserine administration on memory and symptoms of attention-deficit hyperactivity disorder: A randomised, double-blind, placebo-controlled clinical. J. Hum. Nutr. Diet. 2013, 27, 284–291. [Google Scholar] [CrossRef]
- Bener, A.; Khattab, A.O.; Al-Dabbagh, M.M. Is high prevalence of Vitamin D deficiency evidence for autism disorder? In a highly endogamous population. J. Pediatr. Neurosci. 2014, 9, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Chiang, M.; Natarajan, R.; Fan, X. Vitamin D in schizophrenia: A clinical review. Evid. Based Ment. Health 2016, 19, 6–9. [Google Scholar] [CrossRef]
- Meyer, T.; Becker, A.; Sundermann, J.; Rothenberger, A.; Herrmann-Lingen, C. Attention deficit-hyperactivity disorder is associated with reduced blood pressure and serum vitamin D levels: Results from the nationwide German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Eur. Child Adolesc. Psychiatry 2017, 26, 165–175. [Google Scholar] [CrossRef]
- Mohammadpour, N.; Jazayeri, S.; Tehrani-Doost, M.; Djalali, M.; Hosseini, M.; Effatpanah, M.; Davari-Ashtiani, R.; Karami, E. Effect of vitamin D supplementation as adjunctive therapy to methylphenidate on ADHD symptoms: A randomized, double blind, placebo-controlled trial. Nutr. Neurosci. 2018, 21, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Toren, P.; Eldar, S.; Sela, B.A.; Wolmer, L.; Weitz, R.; Inbar, D.; Koren, S.; Reiss, A.; Weizman, R.; Laor, N. Zinc deficiency in attention-deficit hyperactivity disorder. Biol. Psychiatry 1996, 40, 1308–1310. [Google Scholar] [CrossRef]
- Sandstead, H.H.; Fosmire, G.J.; Halas, E.S.; Jacob, R.A.; Strobel, D.A.; Marks, E.O. Zinc deficiency: Effects on brain and behavior of rats and rhesus monkeys. Teratology 1977, 16, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Aggett, P.J.; Harries, J.T. Current status of zinc in health and disease states. Arch. Dis. Child. 1979, 54, 909–917. [Google Scholar] [CrossRef] [Green Version]
- Rucklidge, J.J.; Kaplan, B.J. Broad-spectrum micronutrient treatment for attention-deficit/hyperactivity disorder: Rationale and evidence to date. CNS Drugs 2014, 28, 775–785. [Google Scholar] [CrossRef]
- Sinha, D.; Efron, D. Complementary and alternative medicine use in children with attention deficit hyperactivity disorder. J. Paediatr. Child Health 2005, 41, 23–26. [Google Scholar] [CrossRef]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain-Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144, 1394–1401. [Google Scholar] [CrossRef] [Green Version]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Phys. Ther. 2009, 89, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Sachdeva, A. Effect of Poly Unsaturated Fatty Acids Administration on Children with Attention Deficit Hyperactivity Disorder: A Randomized Controlled Trial. J. Clin. Diagn. Res. 2016, 10, 1–5. [Google Scholar] [CrossRef]
- Assareh, M.; Ashtiani, R.D.; Khademi, M.; Jazayeri, S.; Rai, A.; Nikoo, M. Efficacy of Polyunsaturated Fatty Acids (PUFA) in the Treatment of Attention Deficit Hyperactivity Disorder: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Atten. Disord. 2017, 21, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Barragán, E.; Breuer, D.; Döpfner, M. Efficacy and Safety of Omega-3/6 Fatty Acids, Methylphenidate, and a Combined Treatment in Children with ADHD. J. Atten. Disord. 2017, 21, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Behdani, F.; Hebrani, P.; Naseraee, A.; Haghighi, M.B.; Akhavanrezayat, A. Does omega-3 supplement enhance the therapeutic results of methylphenidate in attention deficit hyperactivity disorder patients? J. Res. Med. Sci. 2013, 18, 653–658. [Google Scholar] [PubMed]
- Bos, D.J.; Oranje, B.; Veerhoek, E.S.; Van Diepen, R.M.; Weusten, J.M.; Demmelmair, H.; Koletzko, B.; GM de Sain-van der Velden, M.; Eilander, A.; Hoeksma, M.; et al. Reduced symptoms of inattention after dietary omega-3 fatty acid supplementation in boys with and without attention deficit/hyperactivity disorder. Neuropsychopharmacology 2015, 40, 2298–2306. [Google Scholar] [CrossRef] [Green Version]
- Pei-Chen Chang, J.; Su, K.P.; Mondelli, V.; Satyanarayanan, S.K.; Yang, H.-T.; Chiang, Y.-J.; Chen, H.-T.; Pariante, C.M. High-dose eicosapentaenoic acid (EPA) improves attention and vigilance in children and adolescents with attention deficit hyperactivity disorder (ADHD) and low endogenous EPA levels. Transl. Psychiatry 2019, 9, 303–312. [Google Scholar] [CrossRef]
- Cornu, C.; Mercier, C.; Ginhoux, T.; Masson, S.; Mouchet, J.; Nony, P.; Kassai, B.; Laudy, V.; Berquin, P.; Franc, N.; et al. A double-blind placebo-controlled randomised trial of omega-3 supplementation in children with moderate ADHD symptoms. Eur. Child Adolesc. Psychiatry 2018, 27, 377–384. [Google Scholar] [CrossRef]
- Crippa, A.; Tesei, A.; Sangiorgio, F.; Salandi, A.; Trabattoni, S.; Grazioli, S.; Agostoni, C.; Molteni, M.; Nobile, M. Behavioral and cognitive effects of docosahexaenoic acid in drug-naïve children with attention-deficit/hyperactivity disorder: A randomized, placebo-controlled clinical trial. Eur. Child Adolesc.Psychiatry 2019, 28, 571–583. [Google Scholar] [CrossRef]
- Dean, A.J.; Bor, W.; Adam, K.; Bowling, F.G.; Bellgrove, M.A. A Randomized, Controlled, Crossover Trial of Fish Oil Treatment for Impulsive Aggression in Children and Adolescents with Disruptive Behavior Disorders. J. Child Adolesc. Psychopharmacol. 2014, 24, 140–148. [Google Scholar] [CrossRef]
- Dubnov-Raz, G.; Khoury, Z.; Wright, I.; Raz, R.; Berger, I. The effect of alpha-linolenic acid supplementation on ADHD symptoms in children: A randomized controlled double-blind study. Front. Hum. Neurosci. 2014, 8, 780–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafsson, P.A.; Birberg-Thornberg, U.; Duchén, K.; Landgren, M.; Malmberg, K.; Pelling, H.; Strandvik, B.; Karlsson, T. EPA supplementation improves teacher-rated behaviour and oppositional symptoms in children with ADHD. Acta Paediatr. 2010, 99, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Hariri, M.; Djazayery, A.; Djalali, M.; Saedisomeolia, A.; Rahimi, A.; Abdolahian, E. Effect of n-3 supplementation on hyperactivity, oxidative stress and inflammatory mediators in children with attention-deficit-hyperactivity disorder. Malays J. Nutr. 2012, 18, 329–335. [Google Scholar] [PubMed]
- Manor, I.; Magen, A.; Keidar, D.; Rosen, S.; Tasker, H.; Cohen, T.; Richter, Y.; Zaaroor-Regev, D.; Manor, Y.; Weizman, A. The effect of phosphatidylserine containing Omega3 fatty-acids on attention-deficit hyperactivity disorder symptoms in children: A double-blind placebo-controlled trial, followed by an open-label extension. Eur. Psychiatry 2012, 27, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Matsudaira, T.; Gow, R.V.; Kelly, J.; Murphy, C.; Potts, L.; Sumich, A.; Ghebremeskel, K.; Crawford, M.A.; Taylor, E. Biochemical and Psychological Effects of Omega-3/6 Supplements in Male Adolescents with Attention-Deficit/Hyperactivity Disorder: A Randomized, Placebo-Controlled, Clinical Trial. J. Child Adolesc. Psychopharmacol. 2015, 25, 775–782. [Google Scholar] [CrossRef] [Green Version]
- Milte, C.M.; Parletta, N.; Buckley, J.D.; Coates, A.M.; Young, R.M.; Howe, P.R.C. Eicosapentaenoic and docosahexaenoic acids, cognition, and behavior in children with attention-deficit/hyperactivity disorder: A randomized controlled trial. Nutrition 2012, 28, 670–677. [Google Scholar] [CrossRef]
- Milte, C.M.; Parletta, N.; Buckley, J.D.; Coates, A.M.; Young, R.M.; Howe, P.R.C. Increased Erythrocyte Eicosapentaenoic Acid and Docosahexaenoic Acid Are Associated With Improved Attention and Behavior in Children With ADHD in a Randomized Controlled Three-Way Crossover Trial. J. Atten. Disord. 2015, 19, 954–964. [Google Scholar] [CrossRef]
- Mohammadzadeh, S.; Baghi, N.; Yousefi, F.; Yousefzamani, B. Effect of omega-3 plus methylphenidate as an alternative therapy to reduce attention deficit-hyperactivity disorder in children. Korean J. Pediatr. 2019, 62, 360–366. [Google Scholar] [CrossRef]
- Perera, H.; Jeewandara, K.C.; Seneviratne, S.; Guruge, C. Combined ω3 and ω6 supplementation in children with attention-deficit hyperactivity disorder (ADHD) refractory to methylphenidate treatment: A double-blind, placebo-controlled study. J. Child Neurol. 2012, 27, 747–753. [Google Scholar] [CrossRef]
- Rodríguez, C.; García, T.; Areces, D.; Fernández, E.; García-Noriega, M.; Domingo, J.C. Supplementation with high-content docosahexaenoic acid triglyceride in attention-deficit hyperactivity disorder: A randomized double-blind placebo-controlled trial. Neuropsychiatr. Dis. Treat. 2019, 15, 1193–1209. [Google Scholar] [CrossRef] [Green Version]
- Widenhorn-Müller, K.; Schwanda, S.; Scholz, E.; Spitzer, M.; Bode, H. Effect of supplementation with long-chain ω-3 polyunsaturated fatty acids on behavior and cognition in children with attention deficit/hyperactivity disorder (ADHD): A randomized placebo-controlled intervention trial. Prostaglandins Leukot Essent Fatty Acids 2014, 91, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.E.; DiSilvestro, R.A.; Bozzolo, D.; Bozzolo, H.; Crowl, L.; Fernandez, S.; Ramadan, Y.; Thompson, S.; Mo, X.; Abdel-Rasoul, M.; et al. Zinc for attention-deficit/hyperactivity disorder: Placebo-controlled double-blind pilot trial alone and combined with amphetamine. J. Child Adolesc. Psychopharmacol. 2011, 21, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noorazar, S.G.; Malek, A.; Aghaei, S.M.; Yasamineh, N.; Kalejahi, P. The efficacy of zinc augmentation in children with attention deficit hyperactivity disorder under treatment with methylphenidate: A randomized controlled trial. Asian J. Psychiatry 2020, 48, 101868. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mohammadbeigi, A.; Sheykholeslam, H.; Moshiri, E.; Dorreh, F. Omega-3 and Zinc supplementation as complementary therapies in children with attention-deficit/hyperactivity disorder. J. Res. Pharm. Pract. 2016, 5, 22–26. [Google Scholar]
- Dehbokri, N.; Noorazar, G.; Ghaffari, A.; Mehdizadeh, G.; Sarbakhsh, P.; Ghaffary, S. Effect of vitamin D treatment in children with attention-deficit hyperactivity disorder. World J. Pediatr. 2019, 15, 78–84. [Google Scholar] [CrossRef]
- Elshorbagy, H.H.; Barseem, N.F.; Abdelghani, W.E.; Suliman, H.A.I.; Al-shokary, A.H.; Abdulsamea, S.E.; Elsadek, A.E.; Abdel Maksoud, Y.H.; Nour El Din, D.M.A.E.H. Impact of Vitamin D Supplementation on Attention-Deficit Hyperactivity Disorder in Children. Ann. Pharmacother. 2018, 52, 623–631. [Google Scholar] [CrossRef]
- Borlase, N.; Melzer, T.; Darling, K.; Eggleston, M.J.F.; Rucklidge, J. Resting-state networks and neurometabolites in children with ADHD after 10weeks of treatment with micronutrients: Results of a randomised placebo-controlled trial. Nutr. Neurosci. 2019, 1, 1–11. [Google Scholar] [CrossRef]
- Hemamy, M.; Heidari-Beni, M.; Askari, G.; Karahmadi, M.; Maracy, M. Effect of Vitamin D and Magnesium Supplementation on Behavior Problems in Children with Attention-Deficit Hyperactivity Disorder. Int. J. Prev. Med. 2020, 24, 11–14. [Google Scholar]
- Rucklidge, J.J.; Eggleston, M.J.F.; Johnstone, J.M.; Darling, K.; Frampton, C.M. Vitamin-mineral treatment improves aggression and emotional regulation in children with ADHD: A fully blinded, randomized, placebo-controlled trial. J. Child Psychol. Psychiatry 2018, 59, 232–246. [Google Scholar] [CrossRef]
- Darling, K.A.; Eggleston, M.J.F.; Retallick-Brown, H.; Rucklidge, J.J. Mineral-Vitamin Treatment Associated with Remission in Attention-Deficit/Hyperactivity Disorder Symptoms and Related Problems: 1-Year Naturalistic Outcomes of a 10-Week Randomized Placebo-Controlled Trial. J. Child Adolesc. Psychopharmacol. 2019, 29, 688–704. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Eggleston, M.J.F.; Darling, K.A.; Stevens, A.J.; Kennedy, M.A.; Frampton, C.M. Can we predict treatment response in children with ADHD to a vitamin-mineral supplement? An investigation into pre-treatment nutrient serum levels, MTHFR status, clinical correlates and demographic variables. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Adar Levine, A.; Kol-Degani, H.; Kav-Venaki, L. A compound herbal preparation (CHP) in the treatment of children with ADHD: A randomized controlled trial. J. Attent. Disord. 2010, 14, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.J.; Kim, I.; Kim, J.B.; Moon, Y.; Whang, M.C.; Lee, K.M.; Jung, S.P. Effects of Korean red ginseng extract on behavior in children with symptoms of inattention and hyperactivity/impulsivity: A double-blind randomized placebo-controlled trial. J. Child Adolesc. Psychopharmacol. 2014, 24, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Li, Z.W.; Wang, S.Z.; Qi, F.H.; Zhao, L.; Lv, H.; Li, A.Y. Ningdong granule: A complementary and alternative therapy in the treatment of attention deficit/hyperactivity disorder. Psychopharmacology 2011, 216, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Motaharifard, M.S.; Effatpanah, M.; Akhondzadeh, S.; Rahimi, H.; Yasrebi, S.A.; Nejatbakhsh, F. Effect of sweet almond syrup versus methylphenidate in children with ADHD: A randomized triple-blind clinical trial. Complement. Ther. Clin. Pract. 2019, 36, 170–175. [Google Scholar] [CrossRef]
- Salehi, B.; Imani, R.; Mohammadi, M.R.; Fallah, J.; Mohammadi, M.; Ghanizadeh, A.; Akhondzadeh, S. Ginkgo biloba for attention-deficit/hyperactivity disorder in children and adolescents: A double blind, randomized controlled trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 76–80. [Google Scholar] [CrossRef]
- Shakibaei, F.; Radmanesh, M.; Salari, E.; Mahaki, B. Ginkgo biloba in the treatment of attention-deficit/hyperactivity disorder in children and adolescents. A randomized, placebo-controlled, trial. Complement. Ther. Clin. Pract. 2015, 21, 61–67. [Google Scholar] [CrossRef]
- Tan, M.L.; Foong, S.C.; Foong, W.C.; Yusuff, Y.; Chettiar, S.M. Tocotrienol-rich fractions (TRF) supplementation in school-going children with Attention Deficit/Hyperactive Disorder (ADHD): A randomized controlled trial. BMC Nutr. 2016, 2, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Pärtty, A.; Kalliomäki, M.; Wacklin, P.; Salminen, S.; Isolauri, E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: A randomized trial. Pediatr. Res. 2015, 77, 823–828. [Google Scholar] [CrossRef]
- Howard, A.L.; Robinson, M.; Smith, G.J.; Ambrosini, G.L.; Piek, J.P.; Oddy, W.H. ADHD is associated with a “Western” dietary pattern in adolescents. J. Atten. Disord. 2011, 15, 403–411. [Google Scholar] [CrossRef]
- Woo, H.D.; Kim, D.W.; Hong, Y.S.; Kim, Y.M.; Seo, J.H.; Choe, B.M.; Park, J.H.; Kang, J.-W.; Yoo, J.-H.; Chueh, H.W.; et al. Dietary patterns in children with attention deficit/hyperactivity disorder (ADHD). Nutrients 2014, 6, 1539–1553. [Google Scholar] [CrossRef] [PubMed]

| (a) | |||||
| Authors, Year | Sample and Age | Methodology and Durations | Daily Doses | Outcome Measures | Main Results |
| Anand et al., 2016 [34] | 50 ADHD (35 males), no other neuropsychiatric comorbidities. Age range: 4–11 years (6 ± 2.1 years). | 4 months DBRCT 25 subjects taking ATX+ PUFA. 25 subjects taking ATX. | ATX: 0.5 mg/kg/day. PUFA: EPA 180 mg + DHA 120 mg/day. | CPRS-R: parent-rated behavioral indexes. | CPRS-R: Non significant trend: supplementation group improved in ADHD scores as compared to the control group. Improvement was more evident, although not significant, in males with combined type of ADHD. |
| Assareh et al., 2017 [35] | 40 ADHD (30 males), ODD was present in 21 subjects. Age range: 6–12 (PUFA group: 9 ± 2 years; Placebo group 9.2 ± 2 years). | 10 weeks DBRCT Unspecified subjects taking MPH +DHA + EPA+ Omega 6. Unspecified subjects taking MPH + Placebo. | MPH: 0.3 mg/kg/day (in 2 doses). Increased to 1 mg/kg/day for 2 weeks. EPA:33 mg/day. DHA: 241 mg/day. Omega 6: 180 mg/day. Placebo: similar to other capsules. | Parent rated ADHD-RS every two weeks. | ADHD-RS: Significant time effect: both groups showed an improvement in symptomatology over time. |
| Barragán et al., 2017 [36] | 90 ADHD (60 males), no other neuropsychiatric comorbidities. Age range: 6–12 years (8.27 ± 1.74 years). | 12 months RPT 20 subjects taking MPH. 22 subjects taking Omega-3/6. 27 subjects taking MPH + Omega-3/6. | MPH: 0.3 mg/kg/ day, increased to 0.5 mg/kg/day after the first 2 weeks. The dose was increased to 1 mg/kg/day depending on response and tolerability. Omega-3/6: EPA: 558 mg\day. DHA: 174 mg\day. GLA: 60 mg\day. | Parent rated ADHD-RS. CGI-S: assessment of severity as reported by clinician and parents. | ADHD-RS and CGI-S: - Significant time x treatment effect: MPH + supplementation group showed greater improvements compared to supplementation alone. CGI-S: - Slow decrease in Omega-3/6 groups, compared with a rapid decrease and subsequent slight increase in the MPH-containing groups. - Adverse events were numerically less frequent with Omega-3/6 or MPH + Omega-3/6 than MPH alone. |
| Behdani et al., 2013 [37] | 69 ADHD (55 males), no other neuropsychiatric comorbidities. Age range: 7–15 years (8.7 ± 1.7 years). | Eight weeks DBRCT 36 subjects taking MPH + Omega 3 33 subjects taking MPH + Placebo. | MPH: 2.5–5 mg/day. Increased 2.5–5 mg weekly, to attain a final dose of 1 mg/kg (in two or three doses). Omega-3:1000 mg\2 times a day (240 mg of DHA and 360 mg of EPA). Placebo: similar to Omega 3 capsules. | Parent and teacher rated ADHD-RS. | ADHD-RS: Significant time effect: reduction in both parent’s and teacher’s rating scores in both groups. |
| Bos et al., 2015 [38] | 38 ADHD; 38 TD (76 males), no other neuropsychiatric comorbidities. Age range: 8–14 years (ADHD:10.3± 2.0 years; TD: 10.9 ± 2.0 years). | 16 weeks DBRPCT 19 ADHD* taking Omega 3. 19 ADHD* taking Placebo. 20 TD taking Omega 3. 18 TD taking placebo. *subjects took MPH before trial. | Omega 3 fortified margarine: 10 g (650 mg DHA and 650 mg EPA).Placebo: 10 g of similar margarine. | CBCL (parent-rated) SWAN (parent-rated) fMRI with G/No-Go paradigm | CBCL: Significant main effect of treatment: supplementation group had less attention problems compared to placebo groups. |
| Pei-Chen Chang et al., 2019 [39] | 92 ADHD (79 males), ODD was present in 51 subjects. Age range: 6–18 years (9.49 ± 3.05 years). | 12 weeks DBRPCT 48 subjects taking PUFAs. 44 subjects taking Placebo. | PUFAs (EPA): 1.2 g/day. Placebo: 1.2 g/day soybean oil. | CPT: computerized continuous performance test to evaluate attention measures. Parent, teacher and self-rated SNAP IV: checklist of DSM-IV ADHD symptoms. | CPT: Significant time x treatment interaction effect: supplementation group showed greater improvement in focused attention compared to placebo group. |
| Cornu et al., 2018 [40] | 162 ADHD (127 males), no other neuropsychiatric comorbidities. Age range 6–15 years (6.9 ± 2.9 years). | Three months DBRPCT 77 subjects taking Omega 3. 80 subjects taking Placebo. | Omega 3 for subjects aged 6–8: EPA 336 mg + DHA 84 mg/day. Omega 3 for subjects aged 9–11: EPA 504 mg + DHA 126 mg/day. Omega 3 for subjects aged 12–15: EPA 672 mg + DHA 168 mg/day. Supplementation capsules also contained 100 mg vitamin A + 1.25 mg vitamin D + 3.5 mg vitamin E. Placebo: similar to Omega 3. | Parent-rated ADHD-RS-IV: ADHD symptomatology as outlined in the DSM-IV-TR. CPRS-R: parent-rated behavioral indexes. “L’Alouette” test: french reading test. Attentional Performance Tests for Children. Children’s Depression Inventory: self-rated depression symptomatology. | ADHD-RS: the severity score decreased in both groups. The decrease was significantly higher in the placebo group. |
| Crippa et al., 2019 [41] | 50 ADHD (46 males), no other neuropsychiatric comorbidities. Age range: 7–14 years (Omega 3 group: 11.06 ± 1.85 years; Placebo group: 10.91 ± 1.42 years). | Six months DBRPCT 25 subjects taking Omega 3. 25 subjects taking Placebo. | Omega 3: DHA 500 mg/day. Placebo: similar to Omega 3. | Parent-rated ADHD-RS version IV: ADHD symptomatology as outlined in the DSM-IV-TR. CPRS-R: parent-rated behavioral indexes. SDQ: parent-rated emotional and behavioral indexes. CHQ: parent-rated measure of quality of life. CGI and C-GAS: assessment, severity and improvement of symptoms as reported by clinician. | A main amelioration effect over time was found in the following measures: ADHD-RS, CPRS-R ADHD index, CPRS-R Global Index restless – impulsive, CPRS-R Global Index total, DSM-IV hyperactive– impulsive scale and DSM-IV total, SDQ Hyperactivity scale and on SDQ total difficulties score, CGI severity, C-GAS. Children in the DHA group showed significantly higher amelioration effect compared to the placebo group in the CHQ. Psychosocial summary and emotional problems on SDQ. |
| Dean et al., 2014 [42] | 16 ADHD, 11 CD (17 males), no other neuropsychiatric comorbidities. Age range: 7–14 years (10.3 ± 2.2 years). | Six weeks RPCCT+ Follow-up 12 subjects taking fish oil capsules for six weeks (Phase 1) followed by placebo capsules for six weeks (Phase II). Nine subjects taking placebo capsules for six weeks (Phase I) followed by fish oil for six weeks (Phase II). | Fish oil: 4 g/day, (400 mg EPA + 2000 mg DHA). Placebo: 4 g\day (low polyphenol, olive oil and 10 mg standard fish oil to assist in maintaining blinding). | Children’s Aggression Scale – Parent Version. MOAS (parent-rated) SDQ (parent-rated) Family Assessment Device General Functioning Scale (FAD) ADHD-RS (parent-rated) Cognitive functioning: -Executive control trail-making task. -Response inhibition–stop signal task Cognitive control: -Eriksen flanker task. | Aggressive behaviour: No effect of fish oil treatment was observed on changes in total scores despite it increased serum concentrations of EPA and total omega-3 s. SDQ: Fish oil group worsened in Conduct Subscale but improved in Hyperactivity Subscale. No effect of fish oil supplementation was observed for other SDQ subscales or SDQ total score, the ADHD rating scale, or family functioning (FAD). Cognitive measures: Fish oil supplementation did not lead to any changes on the stop signal task, trail-making task, or flanker task. |
| Dubnov-Raz et al., 2014 [43] | 17 drug naïve ADHD (10 males), no other neuropsychiatric comorbidities. Age range: 6–16 years (ALA group: 11.1 ± 3.00 years; Placebo group: 10.9 ± 2.30 years). | Two months DBRPCT Nine subjects taking Alpha-Linolenic Acid (ALA). Eight subjects taking Placebo. | ALA: 1 g/day. Placebo: similar to ALA. | MOXO-CPT: standardized computerized continuous performance test designed to evaluate ADHD-related symptoms. Four performance indices: attention, timing, impulsivity, and hyperactivity. CPRS-R: parent-rated behavioral indexes. CTRS-R: teacher-rated behavioral indexes. | No significant between-group difference was found in the changes of the various measures of ADHD symptoms throughout the study period. |
| Gustafsson et al., 2010 [44] | 82 ADHD (number of males unspecified), no other neuropsychiatric comorbidities. Age range: 7–12 years (no mean age declared). | 15 weeks DBRCT 40 subjects taking Omega 3. 42 subjects taking Placebo. | Omega 3: EPA 500 mg+ DHA 2.7 mg/day. Active capsules also contained 10 mg Vitamin E. Placebo: similar to Omega 3. | CPRS-R: parent-rated behavioral indexes. CTRS-R: teacher-rated behavioral indexes. | CTRS-inattention / cognitive subscale: children in supplementation group showed significant amelioration effect. CTRS total score: -48% of the children receiving supplementation vs. 9% of placebo improved ≥25%. -Among the less hyperactive ⁄ impulsive children, 36% of the ones receiving supplementation vs. 18% receiving placebo improved ≥25%. -Among the more hyperactive ⁄ impulsive children, 8 ⁄ 13 receiving supplementation vs. 1 ⁄ 9 receiving placebo improved ≥25%. |
| Hariri et al., 2012 [45] | 103 ADHD (74 males), no other neuropsychiatric comorbidities. Age-range: 6–11. (Omega 3 group: 7.90 ± 1.53 years; Placebo group: 7.90 ± 1.45 years). | 15 week DBRPCT 53 subjects taking Omega 3. 50 subjects taking Placebo. | Omega 3: EPA 635 mg+ DHA 195 mg/day day. Placebo: similar to Omega 3. | ASQ-P: parent-rated behavioral indexes. | ASQ-P: children in EPA + DHA group showed significant improvement. |
| Manor et al., 2012 [46] | 147 ADHD (104 males), no other neuropsychiatric comorbidities. Age-range: 6–13 years (Supplementation group: 9.2 ± 2.0 years; Placebo group: 9.2 ± 1.8 years). | 15 weeks DBRPCT + 15 weeks OL 100 subjects taking Phosphatidylserine (PS) + -Omega 3. 47 subjects taking Placebo. | PS: 300 mg/day. Omega 3: 120 mg/day (EPA/DHA ratio of 2:1). Placebo: similar to supplementation. | CPRS-R: parent-rated behavioral indexes. CTRS-R: teacher-rated behavioral indexes. SDQ: parent-rated emotional and behavioral indexes. CHQ: parent-rated measure of quality of life. | CPRS-R: -Significant reduction in the Global restless/impulsive subscale in supplementation group -Children with more severe symptomatology revealed a significant reduction in the ADHD-Index and hyperactive components. CPRS-R and CTRS-R: Children that switched to supplementation group from placebo showed a significant reduction in subscales severity scores compared to baseline. CHQ: significant improvement in Parent impact-emotional (PE) subscale in supplementation group. |
| Matsudaira et al., 2015 [47] | 76 ADHD (76 males), no other neuropsychiatric comorbidities. Age-range: 12–16 years (LC-PUFA group: 13.7 ± 1.2 years; Placebo group: 13.7 ± 1.1 years). | Three months DBRPCT 38 subjects taking LC-PUFA 38 subjects taking Placebo. | LC-PUFA: EPA 558 mg + DHA 174 mg + CLA 60 mg + vitamin E 9.6 mg/day. Placebo: similar to LC-PUFA. | CTRS-R: teacher-rated behavioral indexes (specifically, the authors considered the ADHD index). | No between-group difference was found in the changes of the various measures of ADHD symptoms throughout the study period. |
| Milte et al., 2012 [48] | 87 ADHD or parent-rated symptoms higher than the 90th percentile on the CPRS-R (67 males), including parent-reported learning difficulties. Age range: 6–13 years (EPA-rich group: 8.77 ± 1.76 years; DHA-rich group: 8.89 ± 1.60 years; LA-rich group: 9.14 ± 2.03 years). | Four months RCT 30 subjects taking EPA-rich oil. 28 subjects taking DHA-rich oil. 29 subjects taking LA oil. | EPA-rich oil: EPA 1109 mg+ DHA 108 mg/day. DHA-rich oil: DHA 1032 mg + EPA 264 mg/day. LA oil: 1467 mg/day. Each capsule also contained low concentration of vitamin E. | WIAT-III word reading and spelling subtests: literacy assessment. WISC-III vocabulary subtest: literacy assessment. CPRS-R: parent-rated behavioral indexes. Abbreviated test of everyday attention for children: attention assessment. Computerized go/no-go task: inhibition assessment. | No between-group or within-group difference was found in the changes of the various measures of symptoms throughout the study period. In a subgroup of 17 children with learning difficulties an increased erythrocyte DHA was more strongly associated with improved word reading (r = 0.683), improved spelling (r = 0.556), an improved ability to divide attention (r = 0.676), and lower parent ratings of oppositional behavior (r = 0.777), hyperactivity (r = 0.702), restlessness (r = 0.705), and overall ADHD symptoms (r = 0.665). |
| Milte et al., 2015 [49] | 87 ADHD or parent-rated symptoms higher than the 90th percentile on the CPRS-R (67 males), including parent-reported learning difficulties. Age range: 6–13 years (8.91 ± 1.73 years). | 12 months Three-way, crossover clinical trial Group 1: EPA→DHA→LA Group 2: DHA→LA→EPA Group 3: LA→EPA→DHA | EPA-rich oil: EPA 1109 mg+ DHA 108 mg/day. DHA-rich oil: DHA 1032 mg + EPA 264 mg/day. LA oil: 1467 mg/day. Each capsule also contained low concentration of vitamin E. | WIAT-III word reading and spelling subtests: literacy assessment. WISC-III vocabulary subtest: literacy assessment. CPRS-R: parent-rated behavioral indexes. Abbreviated Test of Everyday Attention for Children: attention assessment. Computerized go/no-go task: inhibition assessment. | No between-group or within-group difference was found in the changes of the various measures of symptoms throughout the study period. An increased proportion of erythrocyte EPA + DHA was associated with improved spelling (r = 0.365) and attention (r = −0.540) and reduced oppositional behavior (r = −0.301), hyperactivity (r = −0.310), cognitive problems (r = −0.326), DSM-IV hyperactivity (r = −0.270) and DSM-IV inattention (r = −0.343). |
| Mohammadzadeh et al., 2019 [50] | 60 ADHD (49 males), no other neuropsychiatric comorbidities. Age range: 6–12 years (PUFA group: 8.20 ± 1.72 years; Placebo Group:7.7 ± 1.65 years). | Eight weeks DBRCT 31 subjects taking MPH + PUFA. 29 subjects taking MPH + Placebo. | Omega 3: EPA 180 mg + DHA 120 mg/day (from second week 2 times a day). Placebo: similar to Omega 3. MPH: 10 mg/day (in 2 doses); 20–30 mg/kg/day (in 2 doses) from second week. | ADHD-RS-IV parent rated | No significant effect was found. |
| Perera et al., 2012 [51] | 94 ADHD (69 males), including ODD, CD, SLD and tics comorbidities. Age range: 6–12 years (Omega 3 group: 9.4 ± 1.5 years; Placebo group: 9.2 ± 1.5 years). | Six months DBRPCT 48 subjects * taking Omega 3/6. 46 subjects* taking Placebo. *subjects took MPH before trial. | Omega capsules: Omega 3 296.37 mg+ Omega 6 180.75 mg (ratio 1.6:1). Placebo: similar to Omega 3/6. | Parent-rated checklist assessing the following domains: aggressiveness, restlessness, inattention, distractibility, easy anger, impulsiveness, fighting, cooperation, completing work, wait for turn, academic performance. Total score range: 11–33. | Significant reduction in severity score in treatment group compared to placebo in the following measures: aggressiveness, restlessness, completing work, and academic performance, inattention, impulsiveness, and cooperation with parents and teachers. |
| Rodríguez et al., 2020 [52] | 66 ADHD (47 males), including SLD, Anxiety, Tourette’s syndrome, psychomotor/behavioral problems comorbidities. Age range: 6–18 years (11.7 ± 3.1 years). | Six months DBRPCT 32 subjects * taking PUFA 34 subjects * taking Placebo * psychostimulant medication was allowed | PUFA sachet: DHA 1000 mg+ EPA 90 mg+ DPA 150 mg + vitamin E 4.5 mg+ carbohydrates 0.94 g (1 sachet/day in subjects ≤32 kg; 2 sachets/day in subjects >32 kg). Placebo: similar to PUFA sachet. | CPRS: parent-rated behavioral indexes. EDAH scale: parent-rated behavioral indexes. AULA Nesplora virtual test: test of attentional processes, impulsivity and motor activity. D2-test: paper and pencil test for selective and sustained attention. | CPRS: Significant within-group time effect: supplementation group showed improvement in behavioral variables; placebo group showed a behavioral symptomatology worsening. EDAH scale: Significant time x treatment interaction effect: supplementation group in post – treatment condition showed better behavioral indexes compared to placebo group. AULA Nesplora virtual test: Non-significant trend of higher amelioration in supplementation group. d-2 test: significant within-group time effect: both study groups showed improvements in cognitive variables. |
| Widenhorn-Müller et al., 2014 [53] | 95 ADHD (74 males), no other neuropsychiatric comorbidities. Age range: 6–12 years (Omega 3 group: 8.90 ± 1.48 years; Placebo group: 8.92 ± 1.24 years). | Four months DBRPCT 46 subjects taking Omega 3. 49 subjects taking Placebo. | Omega 3: EPA 600 mg + DHA 120 mg/day. Active capsules also contained 15 mg Vitamin E. Placebo: similar to Omega 3. | HAWIK-IV: General cognitive ability, working memory, speed of information processing. DIS-YPS-II: parent-and teacher-rated questionnaires corresponding to the ICD-10 and DSM-IV diagnostic criteria for ADHD. CBCL: parent-rated questionnaire addressing behavioral and emotional problems. TRF: teacher-rated questionnaire addressing behavioral and emotional problems and academic performance. KITAP and TAP: computerized test batteries as measures for attentional performance. | HAWIK-IV: significant time x treatment interaction: supplementation group showed an improvement in working memory function compared to placebo – taking group. Improved working memory correlated with increased erythrocyte EPA and DHA and decreased AA. |
| (b) | |||||
| Authors, Year | Sample and Age | Methodology and Durations | Daily Doses | Outcome Measures | Main Results |
| Abbasi et al., 2011 [15] | 40 ADHD (28 males), no other neuropsychiatric comorbidities. Age range: 7–13 years (ALC group:8.84 ± 2.03 years; Placebo group: 8.36 ± 1.53 years). | Six weeks DBRPCT 19 subjects taking MPH + Acetyl-L-carnitine (ALC). 19 subjects taking MPH + Placebo. | MPH week 1: 10 mg/day. MPH week 2: 20 mg/day. MPH week 3: 30 mg/day. ALC: 500–1500 mg/kg/day. Placebo + MPH: 20–30 mg/day/Kg. | Teacher and Parent rated ADHD-RS-IV. | Teacher and Parent rated ADHD-RS-IV: no significant between-groups outcome results. However, those in the ALC group experienced fewer adverse events than the placebo group regarding headaches and irritability. |
| Ghajar et al., 2018 [17] | 50 ADHD (40 males), not excluding ODD. Age range: 6–17 years (l-carnosine group: 9.12 ± 2.18 years; Placebo group: 8.28 ± 1.59 years). | Eight weeks DBRPCT 25 subjects taking MPH + l-carnosine. 25 subjects taking MPH + Placebo. | MPH: 0.5–1.5 mg/kg/day. Week 1:10 mg/day. Week 2-end: 20 mg/day. Subjects >30 kg: 30 mg/day. l-carnosine: 800 mg/day. Placebo: 800 mg/day. | Primary outcome: Parent rated ADHD-RS-IV. Secondary outcome: Teacher rated ADHD-RS-IV. Both rated at baseline and at weeks four and eight. | Primary outcome: Parent rated ADHD-RS-IV. Significant time x treatment interaction effect both at weeks 4 and 8: MPH + supplementation group showed greater improvements compared to MPH + placebo group. Secondary outcome: Teacher rated ADHD-RS-IV. No significant time x treatment interaction effect. |
| Hirayama et al., 2013 [21] | 36 ADHD (34 males), no other neuropsychiatric comorbidities. Age range: 4–14 years (Phosphatidylserine group: 9.1 ± 1.7 years; Placebo: 8.7 ± 3.0 years). | Eight weeks DBRPCT 19 subjects taking Phosphatidylserine (PS). 17 subjects taking Placebo. | PS: 100 mg/day. Placebo: 100 mg/day. | ADHD diagnostic criteria of DSM-IV-TR. WISC-III (Digit Span Test). go/no-go experiment. | Significant treatment effect: supplementation group showed post-treatment improvements compared to pre-treatment condition in ADHD, AD and HD symptoms (DSM-IV-TR), short-term auditory memory (WISC-III) and total number of errors in the reverse differentiation test (inattention – impulsivity), as well as total inattention errors and total errors over time (Go/No-Go). No significant differences were observed in other measurements and in the placebo group. |
| (c) | |||||
| Authors, Year | Sample and Age | Methodology and Durations | Daily Doses | Outcome Measures | Main Results |
| Arnold et al., 2011 [54] | 52 ADHD (43 males), ODD, CD comorbidities. Age range: 6–14 years (Zinc group 1: 9.61 ± 3.36 years; Zinc group 2: 8.89 ± 2.31 years; Placebo group: 10.24 ± 2.69 years). | 13 weeks (8 + 5) DBRPCT + 8 weeks OL Phase 1 28 subjects taking Zinc glycinate (group 1 and 2). 24 subjects taking Placebo. Phase 2/3 28 subjects taking Zinc glycinate + d-amphetamine. 24 subjects taking Placebo + d-amphetamine. | Zinc glycinate group 1: 15 mg/day (20 subjects). Zinc glycinate group 2: 15 mg/2 times day (8 subjects). AMPH: 25 kg: 5 mg/day; 25–45 kg: 10 mg/day; >45 kg: 15 mg/day. Placebo: similar to Zinc glycinate. | Parent and Teacher rated SNAP IV: checklist of DSM-IV ADHD symptoms. CPRS-R: parent-rated behavioral indexes. Short-term recognition memory task. Continuous performance task 11. Seat activity using a ‘‘wiggle’’ seat. CGI-S and CGI-I: assessment of severity and improvement as reported by clinician. Parent and child children’s depression inventory. | Phase 1: Parent and teacher rating showed no consistent tendency of superiority of zinc over placebo. Neuropsychological cognitive motor results are inconsistent, although a bit more favorable to zinc. Phase 2/3: Optimal mg/kg AMPH dose with in zinc group 2 was 37% lower than with placebo. |
| Noorazar et al., 2020 [55] | 60 ADHD (48 males), no other neuropsychiatric comorbidities. Age range: 7–12 years (Control group: 9.30 ± 1.38 years; Zinc group: 8.87 ± 1.97 years). | Six weeks DBRPCT 30 subjects taking MPH+ Zinc sulfate syrup. 30 subjects taking MPH + Placebo. | MPH: 0.5–1 mg/kg/day. Zinc sulfate syrup: 10 mg/day Placebo: 10 mg/day. | CPRS-R: parent-rated behavioral indexes. | CPRS-R. Significant time x treatment interaction effect: MPH + supplementation group showed greater improvements compared to MPH + placebo group in inattention score. |
| Salehi et al., 2016 [56] | 150 ADHD (111 males), no other neuropsychiatric comorbidities. Age range: 6–15 years (Control group: 9.12 ± 2.2 years; Omega 3 group: 8.6 ± 1.7 years; Zinc group: 9.5 ± 2.5 years). | Eight weeks DBRCT 50 subjects taking MPH+ Placebo. 50 subjects taking MPH+ Omega 3. 50 subjects taking MPH+ Zinc sulfate. | MPH: 0 mg/day for subjects <20 kg; 10 mg/2 times a day > 20 kg. Omega 3: 100 mg EPA for subjects <25 kg; 200 mg for subjects 26–35 kg;400 mg for subjects >35 kg/day. Zinc sulfate: 22 mg/day. | CPRS-R (parent-rated behavioral indexes) and CTRS-R (teacher-rated behavioral indexes) every 2 weeks. | CPRS-R and CTRS-R: Significant time x treatment interaction effects in children with attention deficit disorder subtype: - zinc group showed greater improvements compared to placebo group; - Omega 3 group showed greater improvements compared to the zinc group. |
| Dehbokri et al., 2019 [57] | 96 ADHD (80 males), no other neuropsychiatric comorbidities. Age range: 2–18 years (Vitamin D group: 9.76 ± 2.38 years; Placebo group: 8.58 ± 2.02 years). | Six weeks DBRCT 51 subjects taking MPH + Vitamin D. 45 subjects taking MPH + Placebo. | MPH: unspecified. Vitamin D3: 50,000 IU/week. Placebo: similar to Vitamin D3. | CPRS-R: parent-rated behavioral indexes. | CPRS-R: Significant time x treatment interaction effect: supplementation group showed a significant decrease in hyperactivity, impulsivity and attention problems compared to placebo group. These results improved considerably in patients with insufficient levels of Vitamin D at baseline. |
| Elshorbagy et al., 2018 [58] | 35 ADHD with vitamin D deficiency, including ODD, SLD comorbidities (number of males unspecified). Age range: 7–14 years (9.3 ± 2.6 years). | 12 weeks Case-Control Prospective Interventional Study 16 subjects taking MPH + Vitamin D. 19 subjects taking MPH + Placebo. | MPH: 0.3–1 mg/kg/3 times a day. Vitamin D3: 3000 IU/day. Placebo: similar to Vitamin D3. | CPRS-R: parent-rated behavioral indexes. Weekly Parent Ratings Behaviour. | CPRS-R: Significant time x treatment interaction effect: ADHD who received vitamin D showed a significant improvement in conceptual level, inattention, opposition, hyperactivity and impulsivity compared with placebo group. |
| Mohammadpour et al., 2018 [25] | 62 ADHD (46 males), no other neuropsychiatric comorbidities. Age range: 5–12 years (Vitamin D group: 7.70 ± 1.77 years; Placebo group: 8.03 ± 1.44 years). | Eight weeks DBRPCT 25 subjects taking MPH + Vitamin D. 29 subjects taking MPH + Placebo. | MPH: 0.3–1 mg/kg/3 times a day. Vitamin D tablets: 2000 IU/day. Placebo: similar to Vitamin D. | WPREMB: parent-rated morning and evening behavioural indexes. Parent rated ADHD-RS-IV. CPRS-R: parent-rated behavioral indexes. | WPREMB: Significant time x treatment interaction effect: ADHD who received vitamin D showed a significant improvement in total score and evening symptoms compared to placebo group. ADHD-RS: Significant within-group time effect: ADHD who received vitamin D showed an improvement in total score. |
| (d) | |||||
| Authors, year | Sample and age | Methodology and durations | Daily doses | Outcome measures | Main results |
| Borlase et al., 2019 [59] | 27 ADHD drug naïve (27 males), excluding only ASD and epilepsy comorbidities. Age range: 7–12 (Micronutrients group: 10.75 ± 1.50 years; Placebo group: 10.17 ± 1.36 years). | 10 weeks DBRPCT 13 subjects taking Micronutrients (DEN Formula). 14 subjects taking Placebo. | Micronutrients (comprising 13 vitamins, 17 minerals, 4 amino acids): titration over a week up to 12 capsules/day (in 3 doses). If there was no clinical response after four weeks, subjects could choose to take 15 pills/day. Placebo: similar to micronutrients. | CGI-I: assessment of improvement as reported by clinician. Clinician-rated ADHD-RS-IV. CPRS-R: parent-rated behavioral indexes. CTRS-R: teacher-rated behavioral indexes. Magnetic Resonance Imaging (MRI) | Questionnaires: Significant advantage of supplementation group over placebo for general functioning, emotional dysregulation, aggression and inattention. MRI: No significant between-groups differences. In the treatment group there was a non-significant trend for: - decreased choline in the striatum; - decreased glutamate in the prefrontal cortex; - increased grey matter in the anterior thalamus; - increased white matter in the fornix; - improved network integrity of the default mode network, dorsal attention network and frontal executive network. |
| Hemamy et al., 2020 [60] | 66 ADHD (46 males), no other neuropsychiatric comorbidities. Age range: 6–12 years (Vitamin D group: 9.06 ± 1.76 years; Placebos group: 9.15 ± 1.46 years). | Eight weeks DBRCT 33 subjects taking MPH + Vitamin D + Magnesium. 33 subjects taking MPH + Placebos. | MPH: unspecified. Vitamin D: 50,000 IU/week. Mg: 6 mg/kg/day. Placebo: similar to Vitamin D or Mg. | CPRS-R: parent-rated behavioral indexes. | CPRS-R: Significant time x treatment interaction effect: ADHD who received supplementation showed a significant improvement in conduct problem score, social problem and anxiety score compared to placebo group. |
| Rucklidge et al., 2018 [61] | 93 ADHD (69 males), excluding only ASD and epilepsy comorbidities. Age range: 7–12 years (Micronutrients group: 10.06 ± 1.56 years; Placebo group: 9.43 ± 1.53 years). | 10 weeks FBRPCT 47 subjects taking Micronutrients. 46 subjects taking Placebo. | Micronutrients: 3–12/15 capsule/day divided into 3 doses (it contains 13 vitamins, 17 minerals, 4 amino acids). Placebo: similar to micronutrients. | CGI-I and C-GAS: assessment of severity and improvement as reported by clinician. Clinician-rated ADHD-RS-IV. CPRS-R: parent-rated behavioral indexes. CTRS-R: teacher-rated behavioral indexes. SDQ: parent- and teacher-rated emotional and behavioral indexes. BRIEF: teacher-rated behavioural measures of executive skills in everyday environment. | CGI-I: the number of responders in supplementation group was 20 (51%) versus 11 (27%) on placebo. Clinician-rated ADHD-RS-IV. Improvement in inattention and hyperactivity symptoms, aggression, emotional dysregulation, conduct problem and problem behaviour in ADHD who received supplementation compared with placebo group. |
| Darling et al., 2019 [62] | 84 ADHD no drug naïve (62 males). See Rucklidge 2018. 43 subjects from Micronutrients group; 41 subjects from Placebo group. | Naturalistic Follow-up Study after 12 month post-baseline 19 subjects stayed on trial micronutrients. 21 subjects switched to medications. 35 subjects stopped all treatments. Nine subjects mixed micronutrients and medications. 27/84 subjects added psychological/ other intervention. | Micronutrients: 8–15 capsule/day (it contains 13 vitamins, 17 minerals, 4 amino acids). | CPRS-R: parent-rated behavioral indexes. SDQ: parent-rated emotional and behavioral indexes. Parent-rated CMRS for a measure of emotion dysregulation. Parent-rated SCARED-R for a measure of anxiety symptoms. Eating Behaviour Questionnaire. Acceptability of Treatment questionnaire. | More of those who stayed on supplementation (84%) were identified as ‘‘Much’’ or ‘‘Very Much’’ improved overall relative to baseline functioning, compared to 50% of those who switched to psychiatric medications and 21% of those who discontinued treatment. 79% of those still taking micronutrients, 42% of those using medications, and 23% of those who discontinued treatment were considered remitters based on parent-reported ADHD. |
| Rucklidge et al., 2019 [63] | 71 ADHD (55 males). See Rucklidge 2018. Age range: 7–12 (9.7 ± 1.5 years). | Data from Rucklidge (2018) +10 weeks OL 40 subjects from RCT phase (taking micronutrients). 31 subjects from OL phase (taking micronutrients). | Micronutrients: 3–12/15 capsule/day divided into 3 doses (it contains 13 vitamins, 17 minerals, 4 amino acids). No Placebo. | Clinician-rated ADHD-RS-IV. CGI-I and C-GAS: assessment of severity and improvement as reported by clinician. CPRS-R: parent-rated behavioral indexes. Parent-rated CMRS for a measure of emotion dysregulation. SDQ: parent-rated emotional and behavioral indexes. | Varying predictors were found across outcomes: lower pre-treatment folate and B12 levels, being female, greater severity of symptoms and co-occurring disorders in pre-treatment condition, more pregnancy complications and fewer birth problems were identified as possible predictors of greater improvement for outcome measures. |
| (e) | |||||
| Authors, year | Sample and age | Methodology and durations | Daily doses | Outcome measures | Main results |
| Katz et al., 2010 [64] | 92 ADHD (92 males), no other neuropsychiatric comorbidities. Age range: 6–12 years (CHP group:9.82± 1.56 years; Placebo group: 9.36 ± 1.97 years). | Four months DBRPCT 73 subjects taking Compound Herbal Preparation (CHP). 19 subjects taking Placebo. | CHP: 3 mL three times daily, before meals, diluted in 50 to 60 mL of water. Placebo: similar to CHP. | TOVA task to measure attention Daily side effect questionnaire | TOVA task: Significant within-group time effect: supplementation group showed significant improvement in the 4 subscales and overall scores, compared with no improvement in the control group. No serious side effects were reported. |
| Ko et al., 2014 [65] | 70 ADHD/ADHD NOS (44 males), no other neuropsychiatric comorbidities. Age range: 6–15 (KRG group = 10.94 ± 2.26 years; Placebo group = 10.86 ± 2.41 years). | Eight weeks DBRPCT 33 subjects taking Korean Red Ginseng (KRG). 37 subjects taking Placebo. | KRG: 1g (extract/pouch) twice a day. Placebo dose: one pouch twice a day. | Primary outcome: DSM-IV criteria for inattention and hyperactivity scale scores. Secondary outcomes: QEEG TBR: EEG theta/beta ratio. Salivary cortisol. DHEA levels. | Primary outcome: Active treatment significantly improved the inattention scores and hyperactivity scores. Secondary outcomes: - Supplementation group showed a significantly decrease in QEEG TBR. - No significant effect of supplementation on cortisol and DHEA levels. - No serious adverse reactions to KRG. |
| Li et al., 2011 [66] | 72 ADHD (47 males), no other neuropsychiatric comorbidities. Age range: 6–13 years (NDG group: 9.3 ± 1.8 years; MPH group: 9.2 ± 2.2 years). | Eight weeks DBRCT 36 subjects taking MPH. 36 subjects taking Ningdong granule (NDG). | MPH: 1 mg/kg/day. NDG: 5 mg/kg/day. | Teacher and Parent ADHD-RS to measure behavior. Blood levels of dopamine (DA) and homovanillic acid (HVA) Side effect questionnaire | Teacher and parent ADHD-RS: Scores were reduced from baseline to week 8 in both groups, but there were no significant differences between NDG and MPH groups. MPH group had more side effects than NDG group (significant effect only in hypersomnia). DA levels showed no significant change during the study. HVA in supplementation group was higher at the end of the research, but there was no significant difference between two groups. HVA increasing was associated with improved scores of Teacher and Parent ADHD-RS. |
| Motaharifard et al., 2019 [67] | 50 ADHD (33 males), no other neuropsychiatric comorbidities. Age range: 6–14 years (MPH group: 7.5 ± 1.5 years; Sweet almond group: 6.6 ± 1.0 years). | Eight weeks TBRCT 25 subjects taking MPH+ Placebo syrup. 25 subjects taking Placebo tablet + Sweet almond syrup. | MPH: week 1–5 mg tablet twice daily; from week 2–10 mg tablet twice daily; subjects >30 kg received a 10 mg tablet thrice daily from the third week. Placebo syrup: 5 cc/3 times a day. Sweet almond syrup: 5 cc/3 times a day. Placebo tablet: 5 mg twice daily. | Teacher and Parent ADHD-RS to measure behavior, every 2 weeks. Side effects checklist. | Teacher and Parent ADHD-RS: No significant differences were observed between the two groups. Both groups exhibited a similar declining linear trend in ADHD symptoms over time. Sweet almond syrup had less side effects (only increased appetite). |
| Salehi et al., 2010 [68] | 50 ADHD (39 males), no other neuropsychiatric comorbidities. Age range: 6–14 years (G. biloba group: 9.12 ± 1.61 years; MPH group: 9.61 ± 2.26 years). | Six weeks DBRCT 25 subjects taking Ginkgo biloba. 25 subjects taking MPH. | G. biloba: 80–120 mg/day/kg (80 mg/day for <30 kg and 120 mg/day for >30 kg). MPH: 20–30 mg/day/kg (20 mg/day for <30 kg and 30 mg/day for >30 kg). | Primary outcomes: Parent and Teacher ADHD-RS to measure behavior. Secondary outcomes: Side effect checklist. Physiological parameters. | Parent and Teacher ADHD-RS: Supplementation was less effective than MPH. The difference between supplementation and MPH groups in the frequency of side effects was not significant, except for more frequent decreased appetite, headache and insomnia in the MPH group. |
| Shakibaei et al., 2015 [69] | 60 ADHD (39 males), no other neuropsychiatric comorbidities. Age range: 6–12 years (G. biloba group: 7.83 ± 1.21; Placebo group: 8.41 ± 1.40 years). | Six weeks DBRPCT 31 subjects taking MPH* + Ginkgo biloba. 29 subjects taking MPH* + Placebo. *subjects took MPH before the trial. | MPH: 20 mg/day (10 mg/b.i.d) for subjects <30 kg; 30 mg/day (10 mg/t d s) for subjects >30 kg. G. biloba: 80 mg/day (40 mg/b.i.d) for subjects <30 kg; 120 mg/day (40 mg/t d s) for subjects >30 kg. Placebo: similar to G. biloba tablet. | Primary outcomes: Parent and Teacher ADHD-RS to measure behavior. Secondary outcomes: C-GAS: assessment of symptoms severity as reported by clinician. Physiological parameters. Side effect checklist. | Parent and Teacher ADHD-RS: -A significant improvement was found in inattention score and parent total rating score in supplementation group. - Response rate was higher in supplementation group compared to placebo based only on parent rating. C-GAS: No significant between-group difference after treatment. No between-group significant difference in side effects. |
| Tan et al., 2016 [70] | 146 ADHD (124 males), excluding syndromes, inborn errors of metabolism, brain lesions, chronic liver disease, anticoagulant/antiplatelet drugs. Age range: 6–12 years (TRFa group: 9.4 ± 1.9; Placebo group: 9.4 ± 1.7). | One month run in with placebo + 6 months RPCT 73 subjects taking TRFa (43 subjects taking medication). 73 subjects taking Placebo (35 subjects taking medication). | TRFa capsules: 200 mg/day. Placebo: similar to TRF a. | VAPRS: parent-rated ADHD symptoms. VATRS: teacher-rated ADHD symptoms. Side effects questionnaire. Tocotrienol levels (blood exams). | VAPRS: significant improvement in both groups. VATRS: improvement in TRFa group but not statistically significant. Side effects: non-significant differences between groups. Tocotrienol levels: higher levels in TRFa group and significant correlation with the change in VAPRS. |
| (f) | |||||
| Authors, year | Sample and age | Methodology and durations | Daily doses | Outcome measures | Main results |
| Pärtty et al., 2015 [71] | 75 TD children (40 males). Age range at RCT: 0–6 months after birth. Age range at follow-up: 13 years. Diagnoses at 13 years: 3 ADHD; 1 AS; 2 ADHD + AS. | Six months after birth DBPCRT+ Follow-up at 13 years 40 subjects taking Lactobacillus rhamnosus GG. 35 subjects taking Placebo. | Lactobacillus rhamnosus GG: 1 × 1010 colony-forming units\day for 4 weeks before delivery + for 6 months after delivery. | ICD-10 diagnostic criteria for diagnoses of ADHD or Asperger syndrome (AS) filled in at follow-up. Gut microbiota: in situ hybridization (FISH) and qPCR and blood group secretor type. | ADHD or AS was diagnosed in 6/35 (17.1%) children in the placebo and none in the probiotic group (p = 0.008). The mean numbers of Bifidobacterium species bacteria in feces during the first 6 months of life was lower in children with ADHD or AS log cells/g than in healthy children. |
| Self Rating Scales | Parent Rating Scales | Teacher Rating Scales | Clinician Rated Scales | Psychometric Tests | Computerized Tasks | Neurophysiological Measures | |
|---|---|---|---|---|---|---|---|
| PUFAs | [39,40] | [34,35,36,37,38,39,40,41,42,43,44,45,46,48,49,50,51,52,53] | [37,39,43,44,46,47,53] | [36,41] | [40,42,48,49,52,53] | [38,39,42,43,48,49,52] [53] | [38] |
| Peptides and amino acids derivatives | [15,17] | [15,17] | [21] | [21] | [21] | ||
| Single micronutrient | [54,55,56,57,58,25] | [54,56] | [54] | [54] | [54] | ||
| Micronutrients mix | [59,60,61,62,63] | [59,61] | [59,61,63] | [59] | |||
| Plant / herbal extracts | [64,66,67,68,69,70] | [64,66,67,68,69,70] | [66,67,68,69,70] | [64,65] | [64] | [65] | |
| Probiotics | [71] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosi, E.; Grazioli, S.; Villa, F.M.; Mauri, M.; Gazzola, E.; Pozzi, M.; Molteni, M.; Nobile, M. Use of Non-Pharmacological Supplementations in Children and Adolescents with Attention Deficit/Hyperactivity Disorder: A Critical Review. Nutrients 2020, 12, 1573. https://doi.org/10.3390/nu12061573
Rosi E, Grazioli S, Villa FM, Mauri M, Gazzola E, Pozzi M, Molteni M, Nobile M. Use of Non-Pharmacological Supplementations in Children and Adolescents with Attention Deficit/Hyperactivity Disorder: A Critical Review. Nutrients. 2020; 12(6):1573. https://doi.org/10.3390/nu12061573
Chicago/Turabian StyleRosi, Eleonora, Silvia Grazioli, Filippo Maria Villa, Maddalena Mauri, Erica Gazzola, Marco Pozzi, Massimo Molteni, and Maria Nobile. 2020. "Use of Non-Pharmacological Supplementations in Children and Adolescents with Attention Deficit/Hyperactivity Disorder: A Critical Review" Nutrients 12, no. 6: 1573. https://doi.org/10.3390/nu12061573





