Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis
Abstract
1. Introduction
2. The Immune System, COVID-19, Inflammation, and Oxidative Stress
3. Dietary Constituents as Key Factors of a Strong Immune System and Low Infection Risk
3.1. Macronutrients
3.1.1. Proteins
3.1.2. Lipids
3.1.3. Carbohydrates and Dietary Fiber
3.2. Micronutrients
3.2.1. Vitamins
Vitamin A
Vitamin D
Vitamin E
Vitamin C
B Vitamins
3.2.2. Minerals
Zinc
Iron
Copper
Selenium
3.3. Phytochemicals
3.3.1. Polyphenols
3.3.2. Carotenoids
4. Summary and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weston, S.; Frieman, M.B. COVID-19: Knowns, Unknowns, and Questions. mSphere 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Lake, M.A. What we know so far: COVID-19 current clinical knowledge and research. Clin. Med. (Lond.) 2020, 20, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M. Cardiovascular disease and COVID-19. Diabetes Metab. Syndr. 2020, 14, 247–250. [Google Scholar] [CrossRef]
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Kochi, A.N.; Tagliari, A.P.; Forleo, G.B.; Fassini, G.M.; Tondo, C. Cardiac and arrhythmic complications in patients with COVID-19. J. Cardiovasc. Electrophysiol. 2020, 31, 1003–1008. [Google Scholar] [CrossRef]
- Xie, P.; Ma, W.; Tang, H.; Liu, D. Severe COVID-19: A Review of Recent Progress With a Look Toward the Future. Front. Public Health 2020, 8, 189. [Google Scholar] [CrossRef]
- Chacko, S.A.; Song, Y.; Nathan, L.; Tinker, L.; de Boer, I.H.; Tylavsky, F.; Wallace, R.; Liu, S. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care 2010, 33, 304–310. [Google Scholar] [CrossRef]
- George, S.M.; Neuhouser, M.L.; Mayne, S.T.; Irwin, M.L.; Albanes, D.; Gail, M.H.; Alfano, C.M.; Bernstein, L.; McTiernan, A.; Reedy, J.; et al. Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2220–2228. [Google Scholar] [CrossRef]
- Ma, Y.; Hebert, J.R.; Li, W.; Bertone-Johnson, E.R.; Olendzki, B.; Pagoto, S.L.; Tinker, L.; Rosal, M.C.; Ockene, I.S.; Ockene, J.K.; et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition 2008, 24, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Pischon, T.; Hankinson, S.E.; Rifai, N.; Joshipura, K.; Willett, W.C.; Rimm, E.B. Dietary intake of trans fatty acids and systemic inflammation in women. Am. J. Clin. Nutr. 2004, 79, 606–612. [Google Scholar] [CrossRef] [PubMed]
- North, C.J.; Venter, C.S.; Jerling, J.C. The effects of dietary fibre on C-reactive protein, an inflammation marker predicting cardiovascular disease. Eur. J. Clin. Nutr. 2009, 63, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Jahns, L.; Conrad, Z.; Johnson, L.K.; Whigham, L.D.; Wu, D.; Claycombe-Larson, K.J. A diet high in carotenoid-rich vegetables and fruits favorably impacts inflammation status by increasing plasma concentrations of IFN-α2 and decreasing MIP-1β and TNF-α in healthy individuals during a controlled feeding trial. Nutr. Res. 2018, 52, 98–104. [Google Scholar] [CrossRef]
- Rodríguez, L.; Cervantes, E.; Ortiz, R. Malnutrition and gastrointestinal and respiratory infections in children: A public health problem. Int. J. Environ. Res. Public Health 2011, 8, 1174–1205. [Google Scholar] [CrossRef]
- Gabriele, M.; Pucci, L. Diet Bioactive Compounds: Implications for Oxidative Stress and Inflammation in the Vascular System. Endocr. Metab. Immune Disord. Drug Targets 2017, 17, 264–275. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Rubin, L.P.; Ross, A.C.; Stephensen, C.B.; Bohn, T.; Tanumihardjo, S.A. Metabolic effects of inflammation on vitamin A and carotenoids in humans and animal models. Adv. Nutr. 2017, 8, 197–212. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Lowe, G.D.; Rumley, A.; Bruckdorfer, K.R.; Whincup, P.H. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am. J. Clin. Nutr. 2006, 83, 567–574. [Google Scholar] [CrossRef]
- Khan, N.; Khymenets, O.; Urpi-Sarda, M.; Tulipani, S.; Garcia-Aloy, M.; Monagas, M.; Mora-Cubillos, X.; Llorach, R.; Andres-Lacueva, C. Cocoa polyphenols and inflammatory markers of cardiovascular disease. Nutrients 2014, 6, 844–880. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.; Gao, Y.; Liu, Y.; Ge, F. Carotenoids from the peel of Shatian pummelo (Citrus grandis Osbeck) and its antimicrobial activity. Am. Eur. J. Agric. Environ. Sci. 2010, 7, 110–115. [Google Scholar]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tu, L. Implications of gastrointestinal manifestations of COVID-19. Lancet Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Glaab, E.; Ostaszewski, M. The Role of Spike-ACE2 Interaction in Pulmonary Blood Pressure Regulation. FAIRDOM Hub. 2020. Available online: https://fairdomhub.org/models/709 (accessed on 27 May 2020).
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, S.A. Chapter 17-Innate Immunity. In Kelley and Firestein’s Textbook of Rheumatology, 10th ed.; Firestein, G.S., Budd, R.C., Gabriel, S.E., McInnes, I.B., O’Dell, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 247–287. [Google Scholar] [CrossRef]
- Spiering, M.J. Primer on the Immune System. Alcohol Res. 2015, 37, 171–175. [Google Scholar]
- Lee, J.H.; Jung, J.Y.; Jeong, Y.J.; Park, J.H.; Yang, K.H.; Choi, N.K.; Kim, S.H.; Kim, W.J. Involvement of both mitochondrial- and death receptor-dependent apoptotic pathways regulated by Bcl-2 family in sodium fluoride-induced apoptosis of the human gingival fibroblasts. Toxicology 2008, 243, 340–347. [Google Scholar] [CrossRef]
- Park, J.; Min, J.S.; Kim, B.; Chae, U.B.; Yun, J.W.; Choi, M.S.; Kong, I.K.; Chang, K.T.; Lee, D.S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-kappaB pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef]
- Perez, G.I.; Acton, B.M.; Jurisicova, A.; Perkins, G.A.; White, A.; Brown, J.; Trbovich, A.M.; Kim, M.R.; Fissore, R.; Xu, J.; et al. Genetic variance modifies apoptosis susceptibility in mature oocytes via alterations in DNA repair capacity and mitochondrial ultrastructure. Cell Death Differ. 2007, 14, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Role of oxidative stress in infectious diseases. A review. Folia Microbiol. 2013, 58, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress in Infection and Consequent Disease. Oxid. Med. Cell Longev. 2017, 2017, 3496043. [Google Scholar] [CrossRef]
- Beck, M.A.; Handy, J.; Levander, O.A. The role of oxidative stress in viral infections. Ann. N. Y. Acad. Sci. 2000, 917, 906–912. [Google Scholar] [CrossRef]
- Rahman, I.; Adcock, I.M. Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 2006, 28, 219–242. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- De la Fuente, M.; Hernanz, A.; Vallejo, M.C. The immune system in the oxidative stress conditions of aging and hypertension: Favorable effects of antioxidants and physical exercise. Antioxid. Redox Signal. 2005, 7, 1356–1366. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Maker, G.L.; Hood, S.D.; Drummond, P.D. A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Brusselle, G.; Bracke, K. Targeting immune pathways for therapy in asthma and chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2014, 11 (Suppl. 5), S322–S328. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, A.V.; Komarova, E.A. p53 and the Carcinogenicity of Chronic Inflammation. Cold Spring Harb. Perspect Med. 2016, 6, a026161. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Girard, S.; Kadhim, H.; Roy, M.; Lavoie, K.; Brochu, M.E.; Larouche, A.; Sebire, G. Role of perinatal inflammation in cerebral palsy. Pediatr. Neurol. 2009, 40, 168–174. [Google Scholar] [CrossRef]
- Hendrayani, S.F.; Al-Harbi, B.; Al-Ansari, M.M.; Silva, G.; Aboussekhra, A. The inflammatory/cancer-related IL-6/STAT3/NF-kappaB positive feedback loop includes AUF1 and maintains the active state of breast myofibroblasts. Oncotarget 2016, 7, 41974–41985. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar] [CrossRef]
- Dudley, D.J. 1 The immune system in health and disease. Baillière’s Clin. Obstet. Gynaecol. 1992, 6, 393–416. [Google Scholar] [CrossRef]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef]
- Goldberg, E.L.; Shaw, A.C.; Montgomery, R.R. How Inflammation Blunts Innate Immunity in Aging. Interdiscip Top Gerontol. Geriatr. 2020, 43, 1–17. [Google Scholar] [CrossRef]
- Fu, Y.; Cheng, Y.; Wu, Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol. Sin. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Lam, C.W.; Wu, A.K.; Ip, W.K.; Lee, N.L.; Chan, I.H.; Lit, L.C.; Hui, D.S.; Chan, M.H.; Chung, S.S.; et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004, 136, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Hill, T.; Li, K.; Peters, C.J.; Tseng, C.T. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J. Virol. 2009, 83, 3039–3048. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, Q.; Xu, J. Mechanism of thrombocytopenia in COVID-19 patients. Ann. Hematol. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Z.; Min, W. Mitochondria, Oxidative Stress and Innate Immunity. Front. Physiol. 2018, 9, 1487. [Google Scholar] [CrossRef]
- Crapo, J.D. Oxidative stress as an initiator of cytokine release and cell damage. Eur. Respir. J. 2003, 22, 4s–6s. [Google Scholar] [CrossRef]
- Veiga-Fernandes, H.; Mucida, D. Neuro-Immune Interactions at Barrier Surfaces. Cell 2016, 165, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Wick, G.; Hu, Y.; Schwarz, S.; Kroemer, G. Immunoendocrine communication via the hypothalamo-pituitary-adrenal axis in autoimmune diseases. Endocr. Rev. 1993, 14, 539–563. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, K.; Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014, 32, 609–634. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; La Cava, A. The intricate interface between immune system and metabolism. Trends Immunol. 2004, 25, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Osborn, O.; Olefsky, J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nature Med. 2012, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflugers Arch. 2012, 463, 121–137. [Google Scholar] [CrossRef]
- Majde, J.A.; Krueger, J.M. Links between the innate immune system and sleep. J. Allergy Clin. Immunol. 2005, 116, 1188–1198. [Google Scholar] [CrossRef]
- Marcos, A.; Nova, E.; Montero, A. Changes in the immune system are conditioned by nutrition. Eur. J. Clin. Nutr. 2003, 57, S66–S69. [Google Scholar] [CrossRef]
- Langley-Evans, S.C.; Carrington, L.J. Diet and the developing immune system. Lupus 2006, 15, 746–752. [Google Scholar] [CrossRef]
- Park, J.E.; Barbul, A. Understanding the role of immune regulation in wound healing. Am. J. Surg. 2004, 187, 11s–16s. [Google Scholar] [CrossRef]
- Stanton, J. Listening to the Ga: Cicely Williams’ discovery of kwashiorkor on the Gold Coast. Clio Med. 2001, 61, 149–171. [Google Scholar] [CrossRef] [PubMed]
- Barbeito-Andres, J.; Pezzuto, P.; Higa, L.M.; Dias, A.A.; Vasconcelos, J.M.; Santos, T.M.P.; Ferreira, J.; Ferreira, R.O.; Dutra, F.F.; Rossi, A.D.; et al. Congenital Zika syndrome is associated with maternal protein malnutrition. Sci. Adv. 2020, 6, eaaw6284. [Google Scholar] [CrossRef] [PubMed]
- França, T.; Ishikawa, L.; Zorzella-Pezavento, S.; Chiuso-Minicucci, F.; da Cunha, M.; Sartori, A. Impact of malnutrition on immunity and infection. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009, 15, 374–390. [Google Scholar] [CrossRef]
- Kohatsu, W.; Karpowicz, S. Chapter 88-Antiinflammatory Diet. In Integrative Medicine, 4th ed.; Rakel, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hebert, J.R. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 2009, 139, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Ceriello, A.; Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef]
- Fulop, T., Jr.; Wagner, J.R.; Khalil, A.; Weber, J.; Trottier, L.; Payette, H. Relationship between the response to influenza vaccination and the nutritional status in institutionalized elderly subjects. J. Gerontol. A Biol. Sci. Med. Sci. 1999, 54, M59–M64. [Google Scholar] [CrossRef]
- Taylor, A.K.; Cao, W.; Vora, K.P.; De La Cruz, J.; Shieh, W.J.; Zaki, S.R.; Katz, J.M.; Sambhara, S.; Gangappa, S. Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J. Infect Dis. 2013, 207, 501–510. [Google Scholar] [CrossRef]
- Chan, J.; Tian, Y.; Tanaka, K.E.; Tsang, M.S.; Yu, K.; Salgame, P.; Carroll, D.; Kress, Y.; Teitelbaum, R.; Bloom, B.R. Effects of protein calorie malnutrition on tuberculosis in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 14857–14861. [Google Scholar] [CrossRef]
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The Lipid Mediator Protectin D1 Inhibits Influenza Virus Replication and Improves Severe Influenza. Cell 2013, 153, 112–125. [Google Scholar] [CrossRef]
- Siegers, J.Y.; Novakovic, B.; Hulme, K.D.; Marshall, R.; Bloxham, C.J.; Thomas, W.G.; Reichelt, M.E.; Leijten, L.; van Run, P.; Knox, K.; et al. A high fat diet increases influenza A virus-associated cardiovascular damage. J. Infect. Dis. 2020, 1537–6613. [Google Scholar] [CrossRef]
- Cho, W.J.; Lee, D.K.; Lee, S.Y.; Sohn, S.H.; Park, H.L.; Park, Y.W.; Kim, H.; Nam, J.H. Diet-induced obesity reduces the production of influenza vaccine-induced antibodies via impaired macrophage function. Acta Virol. 2016, 60, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.L.; Molony, R.D.; Kudo, E.; Sidorov, S.; Kong, Y.; Dixit, V.D.; Iwasaki, A. Ketogenic diet activates protective gammadelta T cell responses against influenza virus infection. Sci. Immunol. 2019, 4, eaav2026. [Google Scholar] [CrossRef]
- Park, Y.; Subar, A.F.; Hollenbeck, A.; Schatzkin, A. Dietary fiber intake and mortality in the NIH-AARP diet and health study. Arch. Intern. Med. 2011, 171, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Pattaroni, C.; Lopez-Mejia, I.C.; Riva, E.; Pernot, J.; Ubags, N.; Fajas, L.; Nicod, L.P.; Marsland, B.J. Dietary Fiber Confers Protection against Flu by Shaping Ly6c(-) Patrolling Monocyte Hematopoiesis and CD8(+) T Cell Metabolism. Immunity 2018, 48, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef]
- Chen, H.; Zhuo, Q.; Yuan, W.; Wang, J.; Wu, T. Vitamin A for preventing acute lower respiratory tract infections in children up to seven years of age. Cochrane Database Syst. Rev. 2008, Cd006090. [Google Scholar] [CrossRef]
- Brown, N.; Roberts, C. Vitamin A for acute respiratory infection in developing countries: A meta-analysis. Acta Paediatr 2004, 93, 1437–1442. [Google Scholar] [CrossRef]
- Raharusun, P.; Priambada, S.; Budiarti, C.; Agung, E.; Budi, C. Patterns of COVID-19 Mortality and Vitamin D: An Indonesian Study. SSRN 2020. [Google Scholar] [CrossRef]
- Charan, J.; Goyal, J.P.; Saxena, D.; Yadav, P. Vitamin D for prevention of respiratory tract infections: A systematic review and meta-analysis. J. Pharmacol. Pharm. 2012, 3, 300–303. [Google Scholar] [CrossRef]
- Bergman, P.; Lindh, A.U.; Bjorkhem-Bergman, L.; Lindh, J.D. Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2013, 8, e65835. [Google Scholar] [CrossRef] [PubMed]
- Hemila, H. Vitamin E administration may decrease the incidence of pneumonia in elderly males. Clin. Interv. Aging 2016, 11, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Zhao, W.; Wang, J.; Wang, H.; Zhao, Y.; Tseng, Y.; Bu, H. Extra Dose of Vitamin C Based on a Daily Supplementation Shortens the Common Cold: A Meta-Analysis of 9 Randomized Controlled Trials. Biomed. Res. Int. 2018, 2018, 1837634. [Google Scholar] [CrossRef] [PubMed]
- Poudel-Tandukar, K.; Chandyo, R.K. Dietary B Vitamins and Serum C-Reactive Protein in Persons With Human Immunodeficiency Virus Infection: The Positive Living With HIV (POLH) Study. Food Nutr. Bull. 2016, 37, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Mossad, S.B.; Macknin, M.L.; Medendorp, S.V.; Mason, P. Zinc gluconate lozenges for treating the common cold. A randomized, double-blind, placebo-controlled study. Ann. Intern. Med. 1996, 125, 81–88. [Google Scholar] [CrossRef]
- Dhur, A.; Galan, P.; Hannoun, C.; Huot, K.; Hercberg, S. Effects of iron deficiency upon the antibody response to influenza virus in rats. J. Nutr. Biochem. 1990, 1, 629–634. [Google Scholar] [CrossRef]
- Goldson, A.J.; Fairweather-Tait, S.J.; Armah, C.N.; Bao, Y.; Broadley, M.R.; Dainty, J.R.; Furniss, C.; Hart, D.J.; Teucher, B.; Hurst, R. Effects of selenium supplementation on selenoprotein gene expression and response to influenza vaccine challenge: A randomised controlled trial. PLoS ONE 2011, 6, e14771. [Google Scholar] [CrossRef]
- Droebner, K.; Ehrhardt, C.; Poetter, A.; Ludwig, S.; Planz, O. CYSTUS052, a polyphenol-rich plant extract, exerts anti-influenza virus activity in mice. Antivir. Res. 2007, 76, 1–10. [Google Scholar] [CrossRef]
- Melikian, G.; Mmiro, F.; Ndugwa, C.; Perry, R.; Jackson, J.B.; Garrett, E.; Tielsch, J.; Semba, R.D. Relation of vitamin A and carotenoid status to growth failure and mortality among Ugandan infants with human immunodeficiency virus. Nutrition 2001, 17, 567–572. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Amaral, J.F.; Foschetti, D.A.; Assis, F.A.; Menezes, J.S.; Vaz, N.M.; Faria, A.M. Immunoglobulin production is impaired in protein-deprived mice and can be restored by dietary protein supplemntation. Braz J. Med. Biol. Res. 2006, 39, 1581–1586. [Google Scholar] [CrossRef]
- Jakulj, F.; Zernicke, K.; Bacon, S.L.; van Wielingen, L.E.; Key, B.L.; West, S.G.; Campbell, T.S. A high-fat meal increases cardiovascular reactivity to psychological stress in healthy young adults. J. Nutr. 2007, 137, 935–939. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Bell, D.S. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am. J. Cardiol. 2007, 100, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Jacques, P.F. Dietary Protein and Changes in Biomarkers of Inflammation and Oxidative Stress in the Framingham Heart Study Offspring Cohort. Curr. Dev. Nutr. 2019, 3, nzz019. [Google Scholar] [CrossRef]
- Kostovcikova, K.; Coufal, S.; Galanova, N.; Fajstova, A.; Hudcovic, T.; Kostovcik, M.; Prochazkova, P.; Jiraskova Zakostelska, Z.; Cermakova, M.; Sediva, B.; et al. Diet Rich in Animal Protein Promotes Pro-inflammatory Macrophage Response and Exacerbates Colitis in Mice. Front. Immunol. 2019, 10, 919. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.K.; McFarlane, S.I. The case for low carbohydrate diets in diabetes management. Nutr. Metab. (Lond.) 2005, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Chungchunlam, S.M.S.; Henare, S.J.; Ganesh, S.; Moughan, P.J. Effects of whey protein and its two major protein components on satiety and food intake in normal-weight women. Physiol. Behav. 2017, 175, 113–118. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Lemmens, S.G.; Westerterp, K.R. Dietary protein-its role in satiety, energetics, weight loss and health. Br. J. Nutr. 2012, 108 (Suppl. 2), S105–S112. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Gheewala, N.M.; O’Keefe, J.O. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J. Am. Coll. Cardiol. 2008, 51, 249–255. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, S.H.; Zeng, X.F.; Liu, H.; Qiao, S.Y. Branched-chain Amino Acids are Beneficial to Maintain Growth Performance and Intestinal Immune-related Function in Weaned Piglets Fed Protein Restricted Diet. Asian-Australas J. Anim. Sci. 2015, 28, 1742–1750. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Kim, S.-H.; Roszik, J.; Grimm, E.A.; Ekmekcioglu, S. Impact of l-Arginine Metabolism on Immune Response and Anticancer Immunotherapy. Front. Oncol. 2018, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Newsholme, P.; Marzuca-Nassr, G.N.; Takahashi, H.K.; Hirabara, S.M.; Cruzat, V.; Krause, M.; de Bittencourt, P.I., Jr. Regulatory principles in metabolism-then and now. Biochem. J. 2016, 473, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Lagranha, C.J.; Doi, S.Q.; Sellitti, D.F.; Procopio, J.; Pithon-Curi, T.C. Glutamine-dependent changes in gene expression and protein activity. Cell Biochem. Funct. 2005, 23, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Lagranha, C.J.; Doi, S.Q.; Sellitti, D.F.; Procopio, J.; Pithon-Curi, T.C.; Corless, M.; Newsholme, P. Molecular mechanisms of glutamine action. J. Cell Physiol. 2005, 204, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Kelly, B.; O’Neill, L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017, 18, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Coeffier, M.; Claeyssens, S.; Hecketsweiler, B.; Lavoinne, A.; Ducrotte, P.; Dechelotte, P. Enteral glutamine stimulates protein synthesis and decreases ubiquitin mRNA level in human gut mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G266–G273. [Google Scholar] [CrossRef]
- Jobin, C.; Hellerbrand, C.; Licato, L.L.; Brenner, D.A.; Sartor, R.B. Mediation by NF-kappa B of cytokine induced expression of intercellular adhesion molecule 1 (ICAM-1) in an intestinal epithelial cell line, a process blocked by proteasome inhibitors. Gut 1998, 42, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.; Oehler, R.; Manhart, N.; Exner, R.; Wessner, B.; Strasser, E.; Spittler, A. Regulative potential of glutamine--relation to glutathione metabolism. Nutrition 2002, 18, 217–221. [Google Scholar] [CrossRef]
- Hiscock, N.; Petersen, E.W.; Krzywkowski, K.; Boza, J.; Halkjaer-Kristensen, J.; Pedersen, B.K. Glutamine supplementation further enhances exercise-induced plasma IL-6. J. Appl. Physiol. (1985) 2003, 95, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.K. Nutrition, immunity and infection: From basic knowledge of dietary manipulation of immune responses to practical application of ameliorating suffering and improving survival. Proc. Natl. Acad. Sci. USA 1996, 93, 14304–14307. [Google Scholar] [CrossRef]
- Ah, O. Protein Energy Malnutrition and Susceptibility to Viral Infections as Zika and Influenza Viruses. J. Nutr. Food Sci. 2016, 6, 2. [Google Scholar] [CrossRef]
- Harbige, L.S. Fatty acids, the immune response, and autoimmunity: A question of n-6 essentiality and the balance between n-6 and n-3. Lipids 2003, 38, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, U.; Rinaldi, A.O.; Çelebi Sözener, Z.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Shipley, M.; Armitage, J.; Collins, R.; Harris, W. Plasma phospholipid fatty acids and CHD in older men: Whitehall study of London civil servants. Br. J. Nutr. 2009, 102, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Lennie, T.A.; Chung, M.L.; Habash, D.L.; Moser, D.K. Dietary fat intake and proinflammatory cytokine levels in patients with heart failure. J. Card Fail 2005, 11, 613–618. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern Med. 2014, 160, 398–406. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Bates, E.J. Eicosanoids, fatty acids and neutrophils: Their relevance to the pathophysiology of disease. Prostaglandins Leukot Essent Fatty Acids 1995, 53, 75–86. [Google Scholar] [CrossRef]
- Magnusson, J.; Kull, I.; Westman, M.; Hakansson, N.; Wolk, A.; Melen, E.; Wickman, M.; Bergstrom, A. Fish and polyunsaturated fat intake and development of allergic and nonallergic rhinitis. J. Allergy Clin. Immunol. 2015, 136, 1247–1253.e2. [Google Scholar] [CrossRef] [PubMed]
- Scaioli, E.; Liverani, E.; Belluzzi, A. The Imbalance between n-6/n-3 Polyunsaturated Fatty Acids and Inflammatory Bowel Disease: A Comprehensive Review and Future Therapeutic Perspectives. Int. J. Mol. Sci. 2017, 18, 2619. [Google Scholar] [CrossRef] [PubMed]
- van Elten, T.M.; van Rossem, L.; Wijga, A.H.; Brunekreef, B.; de Jongste, J.C.; Koppelman, G.H.; Smit, H.A. Breast milk fatty acid composition has a long-term effect on the risk of asthma, eczema, and sensitization. Allergy 2015, 70, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Hankinson, S.E.; Hotamisligil, G.S.; Rifai, N.; Willett, W.C.; Rimm, E.B. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation 2003, 108, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 560S–569S. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef]
- Rett, B.S.; Whelan, J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: A systematic review. Nutr. Metab. 2011, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Akbar, U.; Yang, M.; Kurian, D.; Mohan, C. Omega-3 Fatty Acids in Rheumatic Diseases: A Critical Review. J. Clin. Rheumatol. 2017, 23, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Andridge, R.; Malarkey, W.B.; Hwang, B.S.; Glaser, R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: A randomized controlled trial. Brain Behav. Immun. 2012, 26, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Andridge, R.; Malarkey, W.B.; Glaser, R. Omega-3 supplementation lowers inflammation and anxiety in medical students: A randomized controlled trial. Brain Behav. Immun. 2011, 25, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Schwerbrock, N.M.; Karlsson, E.A.; Shi, Q.; Sheridan, P.A.; Beck, M.A. Fish oil-fed mice have impaired resistance to influenza infection. J. Nutr. 2009, 139, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.J.B.; Roper, R.L. The effects of diets enriched in omega-3 polyunsaturated fatty acids on systemic vaccinia virus infection. Sci. Rep. 2017, 7, 15999. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.J.; Sheridan, P.A.; Karlsson, E.A.; Schultz-Cherry, S.; Shi, Q.; Beck, M.A. Diet-induced obese mice exhibit altered heterologous immunity during a secondary 2009 pandemic H1N1 infection. J. Immunol. 2013, 191, 2474–2485. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Manson, J.E.; Buring, J.E.; Stampfer, M.J.; Willett, W.C.; Ridker, P.M. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am. J. Clin. Nutr. 2002, 75, 492–498. [Google Scholar] [CrossRef]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef]
- Bullo, M.; Casas, R.; Portillo, M.P.; Basora, J.; Estruch, R.; Garcia-Arellano, A.; Lasa, A.; Juanola-Falgarona, M.; Aros, F.; Salas-Salvado, J. Dietary glycemic index/load and peripheral adipokines and inflammatory markers in elderly subjects at high cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 443–450. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Schwarz, Y.; Wang, C.; Breymeyer, K.; Coronado, G.; Wang, C.Y.; Noar, K.; Song, X.; Lampe, J.W. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J. Nutr. 2012, 142, 369–374. [Google Scholar] [CrossRef]
- Egger, G.; Dixon, J. Should obesity be the main game? Or do we need an environmental makeover to combat the inflammatory and chronic disease epidemics? Obes. Rev. 2009, 10, 237–249. [Google Scholar] [CrossRef]
- Bo, S.; Ciccone, G.; Guidi, S.; Gambino, R.; Durazzo, M.; Gentile, L.; Cassader, M.; Cavallo-Perin, P.; Pagano, G. Diet or exercise: What is more effective in preventing or reducing metabolic alterations? Eur. J. Endocrinol. 2008, 159, 685–691. [Google Scholar] [CrossRef]
- Galland, L. Diet and inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.M.; Champ, M.M.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef] [PubMed]
- Costabile, A.; Klinder, A.; Fava, F.; Napolitano, A.; Fogliano, V.; Leonard, C.; Gibson, G.R.; Tuohy, K.M. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: A double-blind, placebo-controlled, crossover study. Br. J. Nutr. 2008, 99, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Costabile, A.; Martin-Pelaez, S.; Vitaglione, P.; Klinder, A.; Gibson, G.R.; Fogliano, V. Potential prebiotic activity of oligosaccharides obtained by enzymatic conversion of durum wheat insoluble dietary fibre into soluble dietary fibre. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 283–290. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Mumford, S.L.; Rovner, A.J.; Zhang, C.; Chen, L.; Wactawski-Wende, J.; Perkins, N.J.; Schisterman, E.F.; BioCycle Study, G. Whole grains are associated with serum concentrations of high sensitivity C-reactive protein among premenopausal women. J. Nutr. 2010, 140, 1669–1676. [Google Scholar] [CrossRef]
- Gogebakan, O.; Kohl, A.; Osterhoff, M.A.; van Baak, M.A.; Jebb, S.A.; Papadaki, A.; Martinez, J.A.; Handjieva-Darlenska, T.; Hlavaty, P.; Weickert, M.O.; et al. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: The diet, obesity, and genes (DiOGenes) study: A randomized, controlled trial. Circulation 2011, 124, 2829–2838. [Google Scholar] [CrossRef]
- Goletzke, J.; Buyken, A.E.; Joslowski, G.; Bolzenius, K.; Remer, T.; Carstensen, M.; Egert, S.; Nothlings, U.; Rathmann, W.; Roden, M.; et al. Increased intake of carbohydrates from sources with a higher glycemic index and lower consumption of whole grains during puberty are prospectively associated with higher IL-6 concentrations in younger adulthood among healthy individuals. J. Nutr. 2014, 144, 1586–1593. [Google Scholar] [CrossRef]
- Herder, C.; Peltonen, M.; Koenig, W.; Sutfels, K.; Lindstrom, J.; Martin, S.; Ilanne-Parikka, P.; Eriksson, J.G.; Aunola, S.; Keinanen-Kiukaanniemi, S.; et al. Anti-inflammatory effect of lifestyle changes in the Finnish Diabetes Prevention Study. Diabetologia 2009, 52, 433–442. [Google Scholar] [CrossRef]
- Pol, K.; Christensen, R.; Bartels, E.M.; Raben, A.; Tetens, I.; Kristensen, M. Whole grain and body weight changes in apparently healthy adults: A systematic review and meta-analysis of randomized controlled studies. Am. J. Clin. Nutr. 2013, 98, 872–884. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Tungland, B. Chapter 2-Short-Chain Fatty Acid Production and Functional Aspects on Host Metabolism. In Human Microbiota in Health and Disease; Tungland, B., Ed.; Academic Press: Cambridge, UK, 2018; pp. 37–106. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133, 2485s–2493s. [Google Scholar] [CrossRef]
- Candido, E.P.; Reeves, R.; Davie, J.R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell 1978, 14, 105–113. [Google Scholar] [CrossRef]
- Vidali, G.; Boffa, L.C.; Bradbury, E.M.; Allfrey, V.G. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc. Natl. Acad. Sci. USA 1978, 75, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Boffa, L.C.; Vidali, G.; Mann, R.S.; Allfrey, V.G. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J. Biol. Chem. 1978, 253, 3364–3366. [Google Scholar]
- Riggs, M.G.; Whittaker, R.G.; Neumann, J.R.; Ingram, V.M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature 1977, 268, 462–464. [Google Scholar] [CrossRef]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Miyauchi, S.; Gopal, E.; Fei, Y.J.; Ganapathy, V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na(+)-coupled transporter for short-chain fatty acids. J. Biol. Chem. 2004, 279, 13293–13296. [Google Scholar] [CrossRef]
- Ritzhaupt, A.; Wood, I.S.; Ellis, A.; Hosie, K.B.; Shirazi-Beechey, S.P. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: Its potential to transport L-lactate as well as butyrate. J. Physiol. 1998, 513 Pt 3, 719–732. [Google Scholar] [CrossRef]
- Rechkemmer, G.; von Engelhardt, W. Concentration- and pH-dependence of short-chain fatty acid absorption in the proximal and distal colon of guinea pig (Cavia porcellus). Comp. Biochem. Physiol. A Comp. Physiol. 1988, 91, 659–663. [Google Scholar] [CrossRef]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Saemann, M.D.; Bohmig, G.A.; Osterreicher, C.H.; Burtscher, H.; Parolini, O.; Diakos, C.; Stockl, J.; Horl, W.H.; Zlabinger, G.J. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000, 14, 2380–2382. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.F.; Wang, J.; Yan, X.L.; Tian, F.; Zhao, J.B.; Wang, Y.J.; Jiang, T. Histone deacetylase inhibitor, butyrate, attenuates lipopolysaccharide-induced acute lung injury in mice. Respir. Res. 2010, 11, 33. [Google Scholar] [CrossRef]
- Segain, J.P.; Raingeard de la Bletiere, D.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottiere, H.M.; Galmiche, J.P. Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health Effects and Sources of Prebiotic Dietary Fiber. Curr. Dev. Nutr. 2018, 2, nzy005. [Google Scholar] [CrossRef]
- Myhrstad, M.C.W.; Tunsjo, H.; Charnock, C.; Telle-Hansen, V.H. Dietary Fiber, Gut Microbiota, and Metabolic Regulation-Current Status in Human Randomized Trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef]
- van den Munckhof, I.C.L.; Kurilshikov, A.; Ter Horst, R.; Riksen, N.P.; Joosten, L.A.B.; Zhernakova, A.; Fu, J.; Keating, S.T.; Netea, M.G.; de Graaf, J.; et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: A systematic review of human studies. Obes. Rev. 2018, 19, 1719–1734. [Google Scholar] [CrossRef]
- Qu, H.; Zhang, Y.; Chai, H.; Gao, Z.Y.; Shi, D.Z. Effects of microbiota-driven therapy on inflammatory responses in elderly individuals: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0211233. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the Human Gut Microbiome: An International Review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef]
- Dubourg, G.; Edouard, S.; Raoult, D. Relationship between nasopharyngeal microbiota and patient’s susceptibility to viral infection. Expert Rev. Anti. Infect. Ther. 2019, 17, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Schenck, L.P.; Surette, M.G.; Bowdish, D.M.E. Composition and immunological significance of the upper respiratory tract microbiota. FEBS Lett. 2016, 590, 3705–3720. [Google Scholar] [CrossRef] [PubMed]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Marsland, B.J.; Trompette, A.; Gollwitzer, E.S. The Gut-Lung Axis in Respiratory Disease. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. 2), S150–S156. [Google Scholar] [CrossRef] [PubMed]
- Hanada, S.; Pirzadeh, M.; Carver, K.Y.; Deng, J.C. Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia. Front. Immunol. 2018, 9, 2640. [Google Scholar] [CrossRef]
- Chen, C.J.; Wu, G.H.; Kuo, R.L.; Shih, S.R. Role of the intestinal microbiota in the immunomodulation of influenza virus infection. Microbes Infect. 2017, 19, 570–579. [Google Scholar] [CrossRef]
- Li, N.; Ma, W.-T.; Pang, M.; Fan, Q.-L.; Hua, J.-L. The Commensal Microbiota and Viral Infection: A Comprehensive Review. Front. Immunol. 2019, 10, 1551. [Google Scholar] [CrossRef]
- Botic, T.; Klingberg, T.D.; Weingartl, H.; Cencic, A. A novel eukaryotic cell culture model to study antiviral activity of potential probiotic bacteria. Int. J. Food Microbiol. 2007, 115, 227–234. [Google Scholar] [CrossRef]
- Bandoro, C.; Runstadler, J.A. Bacterial Lipopolysaccharide Destabilizes Influenza Viruses. mSphere 2017, 2, e00267-17. [Google Scholar] [CrossRef]
- Chen, H.W.; Liu, P.F.; Liu, Y.T.; Kuo, S.; Zhang, X.Q.; Schooley, R.T.; Rohde, H.; Gallo, R.L.; Huang, C.M. Nasal commensal Staphylococcus epidermidis counteracts influenza virus. Sci. Rep. 2016, 6, 27870. [Google Scholar] [CrossRef] [PubMed]
- Tuyama, A.C.; Cheshenko, N.; Carlucci, M.J.; Li, J.H.; Goldberg, C.L.; Waller, D.P.; Anderson, R.A.; Profy, A.T.; Klotman, M.E.; Keller, M.J.; et al. ACIDFORM inactivates herpes simplex virus and prevents genital herpes in a mouse model: Optimal candidate for microbicide combinations. J. Infect Dis. 2006, 194, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Ferrey, A.J.; Choi, G.; Hanna, R.M.; Chang, Y.; Tantisattamo, E.; Ivaturi, K.; Park, E.; Nguyen, L.; Wang, B.; Tonthat, S.; et al. A Case of Novel Coronavirus Disease 19 in a Chronic Hemodialysis Patient Presenting with Gastroenteritis and Developing Severe Pulmonary Disease. Am. J. Nephrol. 2020, 51, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Stevens, J.; Heiss, G.; Rose, K.M.; London, S.J. Dietary fiber, lung function, and chronic obstructive pulmonary disease in the atherosclerosis risk in communities study. Am. J. Epidemiol. 2008, 167, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.T.; Elson, C.O.; Fouser, L.A.; Kolls, J.K. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu. Rev. Pathol. 2013, 8, 477–512. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sun, Y.; Cai, R.; Chen, Y.; Gu, B. The impact of dietary fiber and probiotics in infectious diseases. Microb. Pathog. 2020, 140, 103931. [Google Scholar] [CrossRef]
- Araujo, G.V.; Oliveira Junior, M.H.; Peixoto, D.M.; Sarinho, E.S. Probiotics for the treatment of upper and lower respiratory-tract infections in children: Systematic review based on randomized clinical trials. J. Pediatr. (Rio J.) 2015, 91, 413–427. [Google Scholar] [CrossRef]
- Harrington, D. Laboratory Assessment of Vitamin Status; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Semba, R.D. Vitamin A, immunity, and infection. Clin. Infect. Dis. 1994, 19, 489–499. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef]
- Müller, O.; Krawinkel, M. Malnutrition and health in developing countries. CMAJ 2005, 173, 279–286. [Google Scholar] [CrossRef]
- Ross, A.C. Diet in vitamin A research. Methods Mol. Biol. 2010, 652, 295–313. [Google Scholar] [CrossRef]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.-A.; Biesalski, H.K. Beta-carotene is an important vitamin A source for humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Vyas, K.S. A global clinical view on vitamin A and carotenoids. Am J Clin Nutr 2012, 96, 1204s–1206s. [Google Scholar] [CrossRef] [PubMed]
- McCullough, F.S.; Northrop-Clewes, C.A.; Thurnham, D.I. The effect of vitamin A on epithelial integrity. Proc. Nutr. Soc. 1999, 58, 289–293. [Google Scholar] [CrossRef]
- Wang, J.L.; Swartz-Basile, D.A.; Rubin, D.C.; Levin, M.S. Retinoic acid stimulates early cellular proliferation in the adapting remnant rat small intestine after partial resection. J. Nutr. 1997, 127, 1297–1303. [Google Scholar] [CrossRef]
- Rochette-Egly, C.; Germain, P. Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs). Nucl. Recept Signal. 2009, 7, e005. [Google Scholar] [CrossRef]
- Hiemstra, I.H.; Beijer, M.R.; Veninga, H.; Vrijland, K.; Borg, E.G.; Olivier, B.J.; Mebius, R.E.; Kraal, G.; den Haan, J.M. The identification and developmental requirements of colonic CD169(+) macrophages. Immunology 2014, 142, 269–278. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, S.Y.; Yun, Y.J.; Kim, J.K.; Lee, J.M.; Shin, M.; Song, D.K.; Hong, C.W. Retinoic acid induces hypersegmentation and enhances cytotoxicity of neutrophils against cancer cells. Immunol. Lett. 2017, 182, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.K.; Hou, W.S. Retinoic acid modulates interferon-gamma production by hepatic natural killer T cells via phosphatase 2A and the extracellular signal-regulated kinase pathway. J. Interferon Cytokine Res. 2015, 35, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Beijer, M.R.; Molenaar, R.; Goverse, G.; Mebius, R.E.; Kraal, G.; den Haan, J.M. A crucial role for retinoic acid in the development of Notch-dependent murine splenic CD8- CD4- and CD4+ dendritic cells. Eur. J. Immunol. 2013, 43, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Duriancik, D.M.; Hoag, K.A. Vitamin A deficiency alters splenic dendritic cell subsets and increases CD8(+)Gr-1(+) memory T lymphocytes in C57BL/6J mice. Cell Immunol. 2010, 265, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, C.A.; Spencer, S.P.; Torabi-Parizi, P.; Grainger, J.R.; Roychoudhuri, R.; Ji, Y.; Sukumar, M.; Muranski, P.; Scott, C.D.; Hall, J.A.; et al. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J. Exp. Med. 2013, 210, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Worbs, T.; Hammerschmidt, S.I.; Forster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017, 17, 30–48. [Google Scholar] [CrossRef]
- Stephensen, C.B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001, 21, 167–192. [Google Scholar] [CrossRef]
- Timoneda, J.; Rodríguez-Fernández, L.; Zaragozá, R.; Marín, M.P.; Cabezuelo, M.T.; Torres, L.; Viña, J.R.; Barber, T. Vitamin A Deficiency and the Lung. Nutrients 2018, 10, 1132. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 Illness in Native and Immunosuppressed States: A Clinical-Therapeutic Staging Proposal. J. Heart Lung Transplant. 2020. [Google Scholar] [CrossRef]
- Villamor, E.; Fawzi, W.W. Effects of Vitamin A Supplementation on Immune Responses and Correlation with Clinical Outcomes. Clin. Microbiol. Rev. 2005, 18, 446–464. [Google Scholar] [CrossRef]
- Gardner, E.M.; Bernstein, E.D.; Popoff, K.A.; Abrutyn, E.; Gross, P.; Murasko, D.M. Immune response to influenza vaccine in healthy elderly: Lack of association with plasma β-carotene, retinol, α-tocopherol, or zinc. Mech. Ageing Dev. 2000, 117, 29–45. [Google Scholar] [CrossRef]
- Penkert, R.R.; Rowe, H.M.; Surman, S.L.; Sealy, R.E.; Rosch, J.; Hurwitz, J.L. Influences of Vitamin A on Vaccine Immunogenicity and Efficacy. Front. Immunol. 2019, 10, 1576. [Google Scholar] [CrossRef]
- Mosekilde, L. Vitamin D and the elderly. Clin. Endocrinol. (Oxf.) 2005, 62, 265–281. [Google Scholar] [CrossRef]
- von Essen, M.R.; Kongsbak, M.; Schjerling, P.; Olgaard, K.; Odum, N.; Geisler, C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat. Immunol. 2010, 11, 344–349. [Google Scholar] [CrossRef]
- Sigmundsdottir, H.; Pan, J.; Debes, G.F.; Alt, C.; Habtezion, A.; Soler, D.; Butcher, E.C. DCs metabolize sunlight-induced vitamin D3 to ’program’ T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 2007, 8, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Gruber-Bzura, B.M. Vitamin D and Influenza-Prevention or Therapy? Int. J. Mol. Sci. 2018, 19, 2419. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Du, J.; Huang, L.; Wang, Y.; Shi, Y.; Lin, H. Preventive Effects of Vitamin D on Seasonal Influenza A in Infants: A Multicenter, Randomized, Open, Controlled Clinical Trial. Pediatr. Infect. Dis. J. 2018, 37, 749–754. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Yu, M.; Sun, J. The efficacy of vitamin D therapy for patients with COPD: A meta-analysis of randomized controlled trials. Ann. Palliat. Med. 2020, 9, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.M.; Subramanian, S.; Laird, E.; Kenny, R.A. Editorial: Low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment. Pharmacol. Ther. 2020. [Google Scholar] [CrossRef]
- Laird, E.; Rhodes, J.; Kenny, R.A. Vitamin D and Inflammation: Potential Implications for Severity of Covid-19. Ir. Med. J. 2020, 113, 81. [Google Scholar]
- Strain, J.J.; Mulholland, C.W. Vitamin C and vitamin E--synergistic interactions in vivo? Exs 1992, 62, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Buendia, P.; Ramirez, R.; Aljama, P.; Carracedo, J. Klotho Prevents Translocation of NFkappaB. Vitam Horm 2016, 101, 119–150. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Sagoo, P.; Chan, C.; Yates, J.B.; Campbell, J.; Beutelspacher, S.C.; Foxwell, B.M.; Lombardi, G.; George, A.J. Inhibition of NF-kappa B and oxidative pathways in human dendritic cells by antioxidative vitamins generates regulatory T cells. J. Immunol. 2005, 174, 7633–7644. [Google Scholar] [CrossRef] [PubMed]
- Xuan, N.T.; Trang, P.T.; Van Phong, N.; Toan, N.L.; Trung, D.M.; Bac, N.D.; Nguyen, V.L.; Hoang, N.H.; Van Hai, N. Klotho sensitive regulation of dendritic cell functions by vitamin E. Biol. Res. 2016, 49, 45. [Google Scholar] [CrossRef]
- Stiff, A.; Trikha, P.; Mundy-Bosse, B.; McMichael, E.; Mace, T.A.; Benner, B.; Kendra, K.; Campbell, A.; Gautam, S.; Abood, D.; et al. Nitric Oxide Production by Myeloid-Derived Suppressor Cells Plays a Role in Impairing Fc Receptor-Mediated Natural Killer Cell Function. Clin. Cancer Res. 2018, 24, 1891–1904. [Google Scholar] [CrossRef] [PubMed]
- Beharka, A.A.; Han, S.N.; Adolfsson, O.; Wu, D.; Smith, D.; Lipman, R.; Cao, G.; Meydani, M.; Meydani, S.N. Long-term dietary antioxidant supplementation reduces production of selected inflammatory mediators by murine macrophages. Nutr. Res. 2000, 20, 281–296. [Google Scholar] [CrossRef]
- Meydani, S.N.; Meydani, M.; Blumberg, J.B.; Leka, L.S.; Siber, G.; Loszewski, R.; Thompson, C.; Pedrosa, M.C.; Diamond, R.D.; Stollar, B.D. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA 1997, 277, 1380–1386. [Google Scholar] [CrossRef]
- Adolfsson, O.; Huber, B.T.; Meydani, S.N. Vitamin E-enhanced IL-2 production in old mice: Naive but not memory T cells show increased cell division cycling and IL-2-producing capacity. J. Immunol. 2001, 167, 3809–3817. [Google Scholar] [CrossRef]
- Marko, M.G.; Ahmed, T.; Bunnell, S.C.; Wu, D.; Chung, H.; Huber, B.T.; Meydani, S.N. Age-associated decline in effective immune synapse formation of CD4(+) T cells is reversed by vitamin E supplementation. J. Immunol. 2007, 178, 1443–1449. [Google Scholar] [CrossRef]
- Marko, M.G.; Pang, H.J.; Ren, Z.; Azzi, A.; Huber, B.T.; Bunnell, S.C.; Meydani, S.N. Vitamin E reverses impaired linker for activation of T cells activation in T cells from aged C57BL/6 mice. J. Nutr. 2009, 139, 1192–1197. [Google Scholar] [CrossRef]
- Meydani, S.N.; Han, S.N.; Wu, D. Vitamin E and immune response in the aged: Molecular mechanisms and clinical implications. Immunol. Rev. 2005, 205, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Tantcheva, L.P.; Stoeva, E.S.; Galabov, A.S.; Braykova, A.A.; Savov, V.M.; Mileva, M.M. Effect of vitamin E and vitamin C combination on experimental influenza virus infection. Methods Find Exp. Clin. Pharmacol. 2003, 25, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Han, S.N.; Meydani, S.N. Vitamin E and infectious diseases in the aged. Proc. Nutr. Soc. 1999, 58, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Han, S.N.; Wu, D.; Ha, W.K.; Beharka, A.; Smith, D.E.; Bender, B.S.; Meydani, S.N. Vitamin E supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunology 2000, 100, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Kowdley, K.V.; Mason, J.B.; Meydani, S.N.; Cornwall, S.; Grand, R.J. Vitamin E deficiency and impaired cellular immunity related to intestinal fat malabsorption. Gastroenterology 1992, 102, 2139–2142. [Google Scholar] [CrossRef]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef]
- Ghani, S.M.A.; Goon, J.A.; Azman, N.; Zakaria, S.N.A.; Hamid, Z.; Ngah, W.Z.W. Comparing the effects of vitamin E tocotrienol-rich fraction supplementation and alpha-tocopherol supplementation on gene expression in healthy older adults. Clinics (Sao Paulo) 2019, 74, e688. [Google Scholar] [CrossRef]
- Neupane, B.; Walter, S.D.; Krueger, P.; Marrie, T.; Loeb, M. Predictors of inhospital mortality and re-hospitalization in older adults with community-acquired pneumonia: A prospective cohort study. BMC Geriatr. 2010, 10, 22. [Google Scholar] [CrossRef]
- Carr, A.C.; Shaw, G.M.; Fowler, A.A.; Natarajan, R. Ascorbate-dependent vasopressor synthesis: A rationale for vitamin C administration in severe sepsis and septic shock? Crit. Care 2015, 19, 418. [Google Scholar] [CrossRef]
- Englard, S.; Seifter, S. The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 1986, 6, 365–406. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, C.; Vissers, M.C. Ascorbate as a co-factor for fe- and 2-oxoglutarate dependent dioxygenases: Physiological activity in tumor growth and progression. Front. Oncol. 2014, 4, 359. [Google Scholar] [CrossRef] [PubMed]
- Mandl, J.; Szarka, A.; Banhegyi, G. Vitamin C: Update on physiology and pharmacology. Br. J. Pharmacol. 2009, 157, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Young, J.I.; Zuchner, S.; Wang, G. Regulation of the Epigenome by Vitamin C. Annu. Rev. Nutr. 2015, 35, 545–564. [Google Scholar] [CrossRef]
- Kim, K.P.; Shin, K.O.; Park, K.; Yun, H.J.; Mann, S.; Lee, Y.M.; Cho, Y. Vitamin C Stimulates Epidermal Ceramide Production by Regulating Its Metabolic Enzymes. Biomol. Ther. (Seoul) 2015, 23, 525–530. [Google Scholar] [CrossRef]
- Pasonen-Seppanen, S.; Suhonen, T.M.; Kirjavainen, M.; Suihko, E.; Urtti, A.; Miettinen, M.; Hyttinen, M.; Tammi, M.; Tammi, R. Vitamin C enhances differentiation of a continuous keratinocyte cell line (REK) into epidermis with normal stratum corneum ultrastructure and functional permeability barrier. Histochem. Cell Biol. 2001, 116, 287–297. [Google Scholar] [CrossRef]
- Ponec, M.; Weerheim, A.; Kempenaar, J.; Mulder, A.; Gooris, G.S.; Bouwstra, J.; Mommaas, A.M. The formation of competent barrier lipids in reconstructed human epidermis requires the presence of vitamin C. J. Investig. Dermatol. 1997, 109, 348–355. [Google Scholar] [CrossRef]
- Savini, I.; Catani, M.V.; Rossi, A.; Duranti, G.; Melino, G.; Avigliano, L. Characterization of keratinocyte differentiation induced by ascorbic acid: Protein kinase C involvement and vitamin C homeostasis. J. Investig. Dermatol. 2002, 118, 372–379. [Google Scholar] [CrossRef]
- Uchida, Y.; Behne, M.; Quiec, D.; Elias, P.M.; Holleran, W.M. Vitamin C stimulates sphingolipid production and markers of barrier formation in submerged human keratinocyte cultures. J. Investig. Dermatol. 2001, 117, 1307–1313. [Google Scholar] [CrossRef]
- Duarte, T.L.; Cooke, M.S.; Jones, G.D. Gene expression profiling reveals new protective roles for vitamin C in human skin cells. Free Radic. Biol. Med. 2009, 46, 78–87. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Ward, S.; Wayne, J.S.; Brophy, D.F.; Fowler, A.A., 3rd; Yager, D.R.; Natarajan, R. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int. Wound J. 2016, 13, 572–584. [Google Scholar] [CrossRef]
- Bozonet, S.M.; Carr, A.C.; Pullar, J.M.; Vissers, M.C. Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold kiwifruit. Nutrients 2015, 7, 2574–2588. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.H.; Rhea, E.M.; Qu, Z.C.; Hecker, M.R.; May, J.M. Intracellular ascorbate tightens the endothelial permeability barrier through Epac1 and the tubulin cytoskeleton. Am. J. Physiol. Cell Physiol. 2016, 311, C652–c662. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Raghavan, S.A.; Saini, R.; Dikshit, M. Ascorbate-mediated enhancement of reactive oxygen species generation from polymorphonuclear leukocytes: Modulatory effect of nitric oxide. J. Leukoc Biol. 2004, 75, 1070–1078. [Google Scholar] [CrossRef]
- Huijskens, M.J.; Walczak, M.; Koller, N.; Briede, J.J.; Senden-Gijsbers, B.L.; Schnijderberg, M.C.; Bos, G.M.; Germeraad, W.T. Technical advance: Ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. J. Leukoc. Biol. 2014, 96, 1165–1175. [Google Scholar] [CrossRef]
- Manning, J.; Mitchell, B.; Appadurai, D.A.; Shakya, A.; Pierce, L.J.; Wang, H.; Nganga, V.; Swanson, P.C.; May, J.M.; Tantin, D.; et al. Vitamin C promotes maturation of T-cells. Antioxid. Redox Signal. 2013, 19, 2054–2067. [Google Scholar] [CrossRef]
- Huijskens, M.J.; Walczak, M.; Sarkar, S.; Atrafi, F.; Senden-Gijsbers, B.L.; Tilanus, M.G.; Bos, G.M.; Wieten, L.; Germeraad, W.T. Ascorbic acid promotes proliferation of natural killer cell populations in culture systems applicable for natural killer cell therapy. Cytotherapy 2015, 17, 613–620. [Google Scholar] [CrossRef]
- Hemila, H. Vitamin C and Infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef]
- Cai, Y.; Li, Y.F.; Tang, L.P.; Tsoi, B.; Chen, M.; Chen, H.; Chen, X.M.; Tan, R.R.; Kurihara, H.; He, R.R. A new mechanism of vitamin C effects on A/FM/1/47(H1N1) virus-induced pneumonia in restraint-stressed mice. Biomed. Res. Int. 2015, 2015, 675149. [Google Scholar] [CrossRef]
- Hemila, H.; Louhiala, P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.; Chakravorty, N.K.; Annan, G.; Habibzadeh, N.; Schorah, C.J. The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int. J. Vitam Nutr. Res. 1994, 64, 212–219. [Google Scholar]
- Vorilhon, P.; Arpajou, B.; Vaillant Roussel, H.; Merlin, E.; Pereira, B.; Cabaillot, A. Efficacy of vitamin C for the prevention and treatment of upper respiratory tract infection. A meta-analysis in children. Eur. J. Clin. Pharmacol. 2019, 75, 303–311. [Google Scholar] [CrossRef]
- Keil, S.D.; Bowen, R.; Marschner, S. Inactivation of Middle East respiratory syndrome coronavirus (MERS-CoV) in plasma products using a riboflavin-based and ultraviolet light-based photochemical treatment. Transfusion 2016, 56, 2948–2952. [Google Scholar] [CrossRef]
- Jones, H.D.; Yoo, J.; Crother, T.R.; Kyme, P.; Ben-Shlomo, A.; Khalafi, R.; Tseng, C.W.; Parks, W.C.; Arditi, M.; Liu, G.Y.; et al. Nicotinamide exacerbates hypoxemia in ventilator-induced lung injury independent of neutrophil infiltration. PLoS ONE 2015, 10, e0123460. [Google Scholar] [CrossRef] [PubMed]
- Willis-Carr, J.I.; St Pierre, R.L. Effects of vitamin B6 deficiency on thymic epithelial cells and T lymphocyte differentiation. J. Immunol. 1978, 120, 1153–1159. [Google Scholar]
- Ha, C.; Miller, L.T.; Kerkvliet, N.I. The effect of vitamin B6 deficiency on cytotoxic immune responses of T cells, antibodies, and natural killer cells, and phagocytosis by macrophages. Cell. Immunol. 1984, 85, 318–329. [Google Scholar] [CrossRef]
- Axelrod, A.E. Immune processes in vitamin deficiency states. Am. J. Clin. Nutr. 1971, 24, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, A.V.; Bykovskaja, S.N.; Luchanskaja, L.M.; Rauschenbach, M.O. Pyridoxine deficiency and cytotoxicity of T lymphocytes in vitro. Cell. Immunol. 1978, 38, 187–192. [Google Scholar] [CrossRef]
- Cheng, C.H.; Chang, S.J.; Lee, B.J.; Lin, K.L.; Huang, Y.C. Vitamin B6 supplementation increases immune responses in critically ill patients. Eur. J. Clin. Nutr. 2006, 60, 1207–1213. [Google Scholar] [CrossRef]
- Morris, M.S.; Sakakeeny, L.; Jacques, P.F.; Picciano, M.F.; Selhub, J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J. Nutr. 2010, 140, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Paniz, C.; Bertinato, J.F.; Lucena, M.R.; De Carli, E.; Amorim, P.; Gomes, G.W.; Palchetti, C.Z.; Figueiredo, M.S.; Pfeiffer, C.M.; Fazili, Z.; et al. A Daily Dose of 5 mg Folic Acid for 90 Days Is Associated with Increased Serum Unmetabolized Folic Acid and Reduced Natural Killer Cell Cytotoxicity in Healthy Brazilian Adults. J. Nutr. 2017, 147, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Tamura, J.; Kubota, K.; Murakami, H.; Sawamura, M.; Matsushima, T.; Tamura, T.; Saitoh, T.; Kurabayshi, H.; Naruse, T. Immunomodulation by vitamin B12: Augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin. Exp. Immunol. 1999, 116, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.C.; Downey, L.A.; Simpson, T.; McPhee, G.; Oliver, C.; Stough, C. The Effect of a High-Dose Vitamin B Multivitamin Supplement on the Relationship between Brain Metabolism and Blood Biomarkers of Oxidative Stress: A Randomized Control Trial. Nutrients 2018, 10, 1860. [Google Scholar] [CrossRef] [PubMed]
- Christen, W.G.; Cook, N.R.; Van Denburgh, M.; Zaharris, E.; Albert, C.M.; Manson, J.E. Effect of Combined Treatment With Folic Acid, Vitamin B6, and Vitamin B12 on Plasma Biomarkers of Inflammation and Endothelial Dysfunction in Women. J. Am. Heart Assoc. 2018, 7, e008517. [Google Scholar] [CrossRef] [PubMed]
- Alpert, P.T. The Role of Vitamins and Minerals on the Immune System. Home Health Care Manag. Pract. 2017, 29, 199–202. [Google Scholar] [CrossRef]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [PubMed]
- Abdelhaleim, A.F.; Abdo Soliman, J.S.; Amer, A.Y.; Abdo Soliman, J.S. Association of Zinc Deficiency with Iron Deficiency Anemia and its Symptoms: Results from a Case-control Study. Cureus 2019, 11, e3811. [Google Scholar] [CrossRef]
- Wessells, K.R.; Brown, K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef]
- Black, R.E. Zinc deficiency, infectious disease and mortality in the developing world. J. Nutr. 2003, 133, 1485s–1489s. [Google Scholar] [CrossRef]
- Bhutta, Z.A. Iron and zinc deficiency in children in developing countries. BMJ (Clin. Res. ed.) 2007, 334, 104–105. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Contribution of selected vitamins and trace elements to immune function. Ann. Nutr. Metab. 2007, 51, 301–323. [Google Scholar] [CrossRef] [PubMed]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006, 50, 85–94. [Google Scholar] [CrossRef]
- Bulyk, M.L.; Huang, X.; Choo, Y.; Church, G.M. Exploring the DNA-binding specificities of zinc fingers with DNA microarrays. Proc. Natl. Acad. Sci. USA 2001, 98, 7158–7163. [Google Scholar] [CrossRef]
- Andreini, C.; Bertini, I.; Cavallaro, G. Minimal functional sites allow a classification of zinc sites in proteins. PLoS ONE 2011, 6, e26325. [Google Scholar] [CrossRef]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef]
- Barnett, J.B.; Hamer, D.H.; Meydani, S.N. Low zinc status: A new risk factor for pneumonia in the elderly? Nutr. Rev. 2010, 68, 30–37. [Google Scholar] [CrossRef]
- Shaw, J.G.; Friedman, J.F. Iron deficiency anemia: Focus on infectious diseases in lesser developed countries. Anemia 2011, 2011, 260380. [Google Scholar] [CrossRef]
- Oppenheimer, S.J. Iron and its relation to immunity and infectious disease. J. Nutr. 2001, 131, 616S–633S. [Google Scholar] [CrossRef]
- Semba, R.D.; Bloem, M.W. The anemia of vitamin A deficiency: Epidemiology and pathogenesis. Eur. J. Clin. Nutr. 2002, 56, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bloem, M.W. Interdependence of vitamin A and iron: An important association for programmes of anaemia control. Proc. Nutr. Soc. 1995, 54, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Thurnham, D.I. Vitamin A, iron, and haemopoiesis. Lancet 1993, 342, 1312–1313. [Google Scholar] [CrossRef]
- Cherayil, B.J. The role of iron in the immune response to bacterial infection. Immunologic. Res. 2011, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Drakesmith, H.; Prentice, A. Viral infection and iron metabolism. Nat. Rev. Microbiol. 2008, 6, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Kim, R.Y.; Karim, R.; Mayall, J.R.; Martin, K.L.; Shahandeh, A.; Abbasian, F.; Starkey, M.R.; Loustaud-Ratti, V.; Johnstone, D.; et al. Role of iron in the pathogenesis of respiratory disease. Int. J. Biochem. Cell Biol. 2017, 88, 181–195. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Sun, J.; Krause, D.; Mastro, A.; Handte, G. Immune function is impaired in iron-deficient, homebound, older women. Am. J. Clin. Nutr. 2004, 79, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Jayaweera, J.; Reyes, M.; Joseph, A. Childhood iron deficiency anemia leads to recurrent respiratory tract infections and gastroenteritis. Sci. Rep. 2019, 9, 12637. [Google Scholar] [CrossRef]
- Besold, A.N.; Culbertson, E.M.; Culotta, V.C. The Yin and Yang of copper during infection. JBIC J. Biol. Inorganic Chem. 2016, 21, 137–144. [Google Scholar] [CrossRef]
- Saeed, F.; Nadeem, M.; Ahmed, R.S.; Tahir Nadeem, M.; Arshad, M.S.; Ullah, A. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds—A review. Food Agric. Immunol. 2016, 27, 205–229. [Google Scholar] [CrossRef]
- Weiss, G.; Carver, P.L. Role of divalent metals in infectious disease susceptibility and outcome. Clin. Microbiol. Infect 2018, 24, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Al-Quraishy, S.; Dkhil, M.A.; Wunderlich, F.; Sies, H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv. Nutr. 2015, 6, 73–82. [Google Scholar] [CrossRef]
- Harthill, M. Review: Micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol. Trace Elem. Res. 2011, 143, 1325–1336. [Google Scholar] [CrossRef]
- Stoffaneller, R.; Morse, N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants (Basel) 2018, 7, 66. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Stranges, S.; Marshall, J.R.; Natarajan, R.; Donahue, R.P.; Trevisan, M.; Combs, G.F.; Cappuccio, F.P.; Ceriello, A.; Reid, M.E. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: A randomized trial. Ann. Intern. Med. 2007, 147, 217–223. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Lee, S.J.; Lee, M.K.; Lee, W.-Y.; Yong, S.J.; Kim, S.-H. Serum selenium levels in patients with respiratory diseases: A prospective observational study. J. Thorac. Dis. 2016, 8, 2068–2078. [Google Scholar] [CrossRef]
- Mahmoodpoor, A.; Hamishehkar, H.; Shadvar, K.; Ostadi, Z.; Sanaie, S.; Saghaleini, S.H.; Nader, N.D. The Effect of Intravenous Selenium on Oxidative Stress in Critically Ill Patients with Acute Respiratory Distress Syndrome. Immunol. Investig. 2019, 48, 147–159. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am. J. Clin. Nutr. 2006, 84, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Bermudez, O.I.; Tucker, K.L. Plasma C-reactive protein and homocysteine concentrations are related to frequent fruit and vegetable intake in Hispanic and non-Hispanic white elders. J. Nutr. 2004, 134, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A.; Yoshida, D.; Yamaji, T.; Mizoue, T.; Takayanagi, R.; Kono, S. Dietary patterns and C-reactive protein in Japanese men and women. Am. J. Clin. Nutr. 2008, 87, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- di Giuseppe, R.; Di Castelnuovo, A.; Centritto, F.; Zito, F.; De Curtis, A.; Costanzo, S.; Vohnout, B.; Sieri, S.; Krogh, V.; Donati, M.B.; et al. Regular consumption of dark chocolate is associated with low serum concentrations of C-reactive protein in a healthy Italian population. J. Nutr. 2008, 138, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Stote, K.S.; Clevidence, B.A.; Novotny, J.A.; Henderson, T.; Radecki, S.V.; Baer, D.J. Effect of cocoa and green tea on biomarkers of glucose regulation, oxidative stress, inflammation and hemostasis in obese adults at risk for insulin resistance. Eur. J. Clin. Nutr. 2012, 66, 1153–1159. [Google Scholar] [CrossRef]
- Chun, O.K.; Chung, S.J.; Claycombe, K.J.; Song, W.O. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in U.S. adults. J. Nutr. 2008, 138, 753–760. [Google Scholar] [CrossRef]
- Macready, A.L.; George, T.W.; Chong, M.F.; Alimbetov, D.S.; Jin, Y.; Vidal, A.; Spencer, J.P.; Kennedy, O.B.; Tuohy, K.M.; Minihane, A.M.; et al. Flavonoid-rich fruit and vegetables improve microvascular reactivity and inflammatory status in men at risk of cardiovascular disease--FLAVURS: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 479–489. [Google Scholar] [CrossRef]
- Martinez-Lopez, S.; Sarria, B.; Sierra-Cinos, J.L.; Goya, L.; Mateos, R.; Bravo, L. Realistic intake of a flavanol-rich soluble cocoa product increases HDL-cholesterol without inducing anthropometric changes in healthy and moderately hypercholesterolemic subjects. Food Funct. 2014, 5, 364–374. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Venable, A.S.; Henning, A.L.; Prado, E.A.; Best Sampson, J.N.; Vingren, J.L.; Hill, D.W. Natural cocoa consumption: Potential to reduce atherogenic factors? J. Nutr. Biochem. 2015, 26, 626–632. [Google Scholar] [CrossRef]
- Tokede, O.A.; Gaziano, J.M.; Djousse, L. Effects of cocoa products/dark chocolate on serum lipids: A meta-analysis. Eur. J. Clin. Nutr. 2011, 65, 879–886. [Google Scholar] [CrossRef]
- Flammer, A.J.; Sudano, I.; Wolfrum, M.; Thomas, R.; Enseleit, F.; Periat, D.; Kaiser, P.; Hirt, A.; Hermann, M.; Serafini, M.; et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur. Heart J. 2012, 33, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Necozione, S.; di Giosia, P.; Barnabei, R.; Allegaert, L.; Bernaert, H.; Ferri, C. Cocoa consumption dose-dependently improves flow-mediated dilation and arterial stiffness decreasing blood pressure in healthy individuals. J. Hypertens. 2015, 33, 294–303. [Google Scholar] [CrossRef]
- West, S.G.; McIntyre, M.D.; Piotrowski, M.J.; Poupin, N.; Miller, D.L.; Preston, A.G.; Wagner, P.; Groves, L.F.; Skulas-Ray, A.C. Effects of dark chocolate and cocoa consumption on endothelial function and arterial stiffness in overweight adults. Br. J. Nutr. 2014, 111, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Kaul, T.N.; Middleton, E., Jr.; Ogra, P.L. Antiviral effect of flavonoids on human viruses. J. Med. Virol. 1985, 15, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.-C.; et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.N.; Galeano-Diaz, T.; Lopez, O.; Fernandez-Bolanos, J.G.; Sanchez, J.; De Miguel, C.; Gil, M.V.; Martin-Vertedor, D. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem. 2014, 163, 289–298. [Google Scholar] [CrossRef]
- Gimeno, E.; Fito, M.; Lamuela-Raventos, R.M.; Castellote, A.I.; Covas, M.; Farre, M.; de La Torre-Boronat, M.C.; Lopez-Sabater, M.C. Effect of ingestion of virgin olive oil on human low-density lipoprotein composition. Eur. J. Clin. Nutr. 2002, 56, 114–120. [Google Scholar] [CrossRef]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martinez, P.; Medina-Remon, A.; Lamuela-Raventos, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef]
- Rodrigo, R.; Miranda, A.; Vergara, L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin. Chim. Acta 2011, 412, 410–424. [Google Scholar] [CrossRef]
- Gonzalez-Gallego, J.; Sanchez-Campos, S.; Tunon, M.J. Anti-inflammatory properties of dietary flavonoids. Nutr. Hosp. 2007, 22, 287–293. [Google Scholar]
- Covas, M.I.; Nyyssonen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Baumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Hernaez, A.; Fernandez-Castillejo, S.; Farras, M.; Catalan, U.; Subirana, I.; Montes, R.; Sola, R.; Munoz-Aguayo, D.; Gelabert-Gorgues, A.; Diaz-Gil, O.; et al. Olive oil polyphenols enhance high-density lipoprotein function in humans: A randomized controlled trial. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases-Promises, Perspectives, and Pitfalls. Oxid. Med. Cell Longev. 2016, 2016, 9346470. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Sodagari, H.R.; Farzaei, M.H.; Abdolghaffari, A.H.; Gooshe, M.; Rezaei, N. The preventive and therapeutic potential of natural polyphenols on influenza. Expert Rev. Anti Infect. Ther. 2016, 14, 57–80. [Google Scholar] [CrossRef]
- Lin, L.-T.; Hsu, W.-C.; Lin, C.-C. Antiviral Natural Products and Herbal Medicines. J. Tradit. Complementary Med. 2014, 4, 24–35. [Google Scholar] [CrossRef]
- Mishra, S.; Pandey, A.; Manvati, S. Coumarin: An emerging antiviral agent. Heliyon 2020, 6, e03217. [Google Scholar] [CrossRef]
- Zu, M.; Yang, F.; Zhou, W.; Liu, A.; Du, G.; Zheng, L. In vitro anti-influenza virus and anti-inflammatory activities of theaflavin derivatives. Antiviral Res. 2012, 94, 217–224. [Google Scholar] [CrossRef]
- Fioravanti, R.; Celestino, I.; Costi, R.; Cuzzucoli Crucitti, G.; Pescatori, L.; Mattiello, L.; Novellino, E.; Checconi, P.; Palamara, A.T.; Nencioni, L.; et al. Effects of polyphenol compounds on influenza A virus replication and definition of their mechanism of action. Bioorg. Med. Chem. 2012, 20, 5046–5052. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Majeed, M.; Sahebkar, A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015, 34, 1101–1108. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Carotenoids and Markers of Oxidative Stress in Human Observational Studies and Intervention Trials: Implications for Chronic Diseases. Antioxidants (Basel) 2019, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; Xue, Q.; Semba, R.D.; Ferrucci, L.; Cappola, A.R.; Ricks, M.; Guralnik, J.; Fried, L.P. Serum antioxidants, inflammation, and total mortality in older women. Am. J. Epidemiol. 2006, 163, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Dalgard, C.; Nielsen, F.; Morrow, J.D.; Enghusen-Poulsen, H.; Jonung, T.; Horder, M.; de Maat, M.P. Supplementation with orange and blackcurrant juice, but not vitamin E, improves inflammatory markers in patients with peripheral arterial disease. Br. J. Nutr. 2009, 101, 263–269. [Google Scholar] [CrossRef]
- Naithani, R.; Huma, L.C.; Holland, L.E.; Shukla, D.; McCormick, D.L.; Mehta, R.G.; Moriarty, R.M. Antiviral activity of phytochemicals: A comprehensive review. Mini Rev. Med. Chem. 2008, 8, 1106–1133. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257s–261s. [Google Scholar] [CrossRef]
- Huang, J.; Weinstein, S.J.; Yu, K.; Männistö, S.; Albanes, D. Serum Beta Carotene and Overall and Cause-Specific Mortality. Circ. Res. 2018, 123, 1339–1349. [Google Scholar] [CrossRef]
- Bates, C.J.; Hamer, M.; Mishra, G.D. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: The National Diet and Nutrition Survey of people aged 65 years and over. Br. J. Nutr. 2011, 105, 123–132. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.; Thornquist, M.; Grizzle, J.; Rosenstock, L.; Barnhart, S.; Balmes, J.; Cherniack, M.G.; Cullen, M.R.; Glass, A.; et al. The beta-carotene and retinol efficacy trial (CARET) for chemoprevention of lung cancer in high risk populations: Smokers and asbestos-exposed workers. Cancer Res. 1994, 54, 2038s–2043s. [Google Scholar]
- Ulaszewska, M.M.; Weinert, C.H.; Trimigno, A.; Portmann, R.; Andres Lacueva, C.; Badertscher, R.; Brennan, L.; Brunius, C.; Bub, A.; Capozzi, F.; et al. Nutrimetabolomics: An Integrative Action for Metabolomic Analyses in Human Nutritional Studies. Mol. Nutr. Food Res. 2019, 63, e1800384. [Google Scholar] [CrossRef] [PubMed]
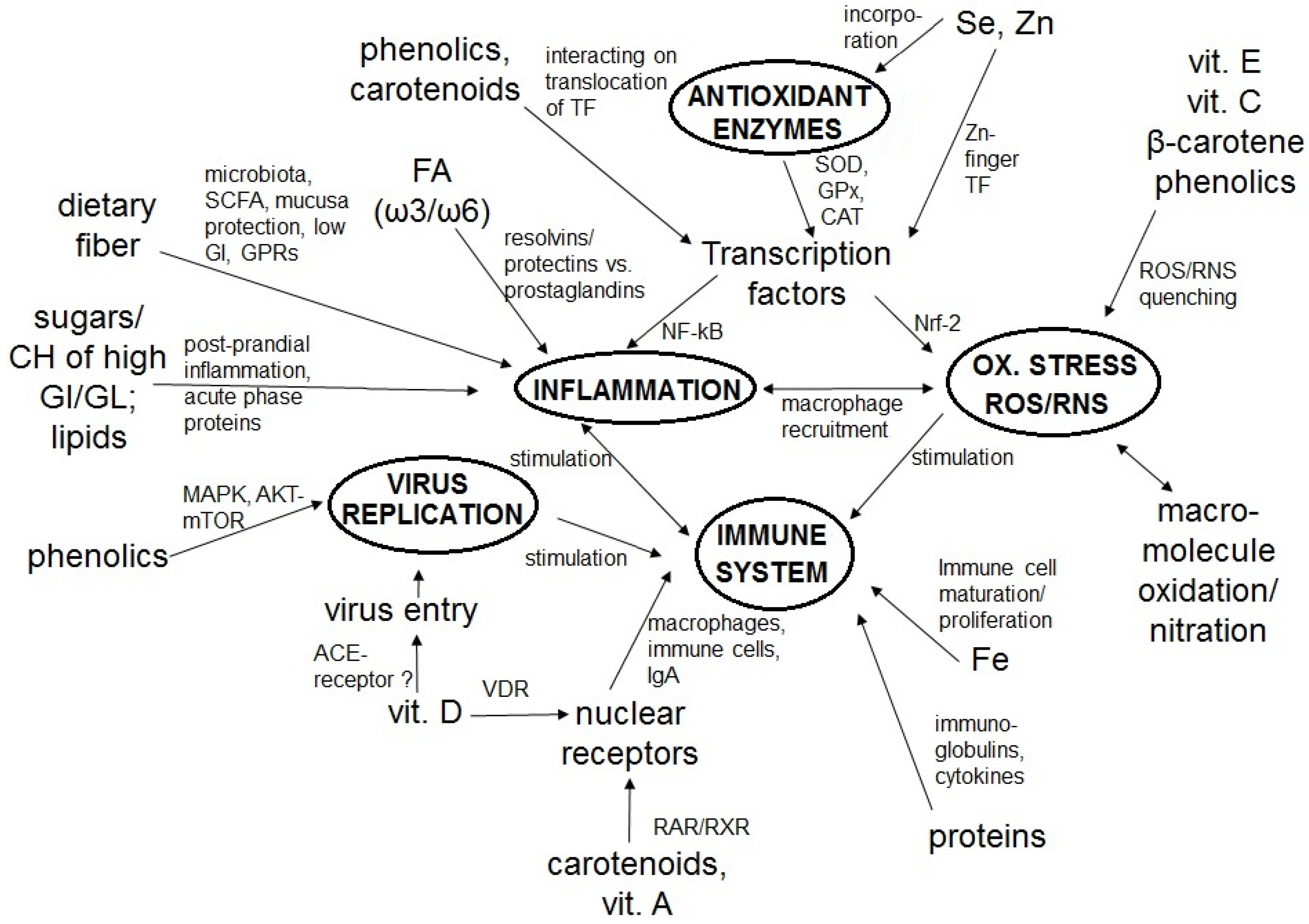
| Constituent | Study Design | Description | Main Findings | Ref |
|---|---|---|---|---|
| Proteins | Human cross-sectional study | 23 elderly patients subjected to influenza vaccination and measurement of their nutrient status. | Total protein status (determined by questionnaire) was slightly lower (p < 0.05) in influenza vaccine non-responders vs. responders (66 vs. 69 g/L). Similar results were found for iron, proposing that immune-response was compromised by poor nutrient status in this elderly population. | Fulop et al., 1999 [78] |
| Animal study (mice) | Group receiving a diet adequate in protein (AP; 18% of energy) vs. group receiving very low protein (VLP; 2%) for 3 weeks. Both groups were subjected to influenza infection. | Higher mortality in the VLP group (p < 0.001) vs. AP, 25 d post infection (p.i.). The AP vs. the VLP group showed a decreased virus titer by day 9 (p < 0.001) and an efficient clearance within 12 d (p < 0.001). %age of NK cells in lungs were reduced (p < 0.01) in VLP vs. AP group with higher (p < 0.001) neutrophil proportions in response to infection with influenza virus in each group, respectively. The VLP group had less influenza NP-specific CD8+ T cells at days 8 (p < 0.05), 15 (p < 0.05), and 30 (p = 0.001). Switching VLP to AP diet improved CD4+ and CD8+ T cell subset levels on days 8 (p < 0.01), 15 (p < 0.01), and 30 (p < 0.01) and increased IFN-γ (p < 0.001). | Taylor et al., 2013 [79] | |
| Animal study (mice) | Mycobacteria-infected mice fed 2% protein diet vs. control group receiving 20% protein diet for up to 30 days. | 100% of malnourished mice (fed 2% protein diet) succumbed to M. tuberculosis infection within 66 d p.i. Malnourished mice had a reduced expression of IFN-γ, TNF-α, and iNOS in the lungs. A mortal infection of M. tuberculosis in malnourished animals was reversed upon re-feeding with the 20% protein diet. | Chan et al., 1996 [80] | |
| Lipids | Animal study (mice) | Mice infected with H5N1 virus treated with omega-3 polyunsaturated fatty acid-derived lipid mediator protectin D1 (PD1), given 3 times i.v. | H5N1 virus pathogenicity decreased with higher levels of PD1. PD1 inhibited virus replication (p < 0.001) via influenza virus nucleoprotein mRNA expression at day 2 p.i. PD1 treatment within 12 h improved the survival (p < 0.05) and pathology of severe influenza (p < 0.001). | Morita et al., 2013 [81] |
| Animal study (mice) | Influenza A virus (IAV) infected mice fed with a high-fat (HF, 40% of energy) vs. low-fat (LF, 12% of energy) diet for 10 weeks. | HF mice were more susceptible to respiratory disease after IAV infection than were LF mice, with lower blood oxygen saturation (p < 0.05) and an increase in pulmonary viral load (p < 0.05). Decreased pro-inflammatory response to IAV in the serum of HF mice vs. LF for IL-6, IFN-γ, IFN-α, and IP-10 (p < 0.05). Antiviral response in the heart was reduced in HF mice after IAV infection, where higher viral loads were detected in the hearts of HF vs. LF mice (p < 0.05). Correlation between IAV-infected HF mice and viral infection in the heart, left ventricular mass, and thickening of the left ventricular wall, characterized by increased HIF-1α compared to LF group (p < 0.05). | Siegers et al., 2020 [82] | |
| Animal study (mice) | High-fat diet (HFD) 60% or regular-fat diet (RFD) 5% fat, administered to 4-week old mice for 10 weeks. Influenza vaccination was conducted after 10 weeks. | Functionality of macrophages was diminished after diet-induced obesity (p < 0.001) via lower CD86-expressing macrophages, lower release of IL-6 and TNF-α, increased Th1 cell subpopulation, and reduced proportion of Treg cells. Vaccination-induced antibody production was decreased in animals receiving HFD vs. RFD (p < 0.001) | Cho et al., 2016 [83] | |
| Lipids, carbo-hydrates | Animal study (mice) | Feeding mice with ketogenic, i.e., low carbohydrate diet (KD, 90% fat) vs. standard high-fat (60% fats, 20% lipids) diet (HFD) for 7 d before influenza A virus (IAV) infection. | KD protected mice from lethal IAV infection and disease (p < 0.05) compared to HFD-fed mice. KD resulted in an expansion of T-cells (p < 0.001), compared with the HFD group. KD-fed mice had better blood O2 saturation (p < 0.001). KD diet was significantly related to improved antiviral resistance (p < 0.001). | Goldberg et al., 2019 [84] |
| Fiber | Prospective human cohort study | Study evaluating dietary fiber intake versus health outcomes. n = 219,123 men and 168,999 women, aged 50–71 y. 9 y follow-up. | Consumption of dietary fiber correlated with lowered mortality from infectious and respiratory diseases. Per 10 g/d increase in dietary fiber, the multivariate RRs for infectious and respiratory diseases were 0.66 (CI: 0.52–0.84) and 0.82 (CI: 0.74–0.93) in men and 0.61 (CI: 0.44–0.85) and 0.66 (CI: 0.56–0.78) in women, respectively. | Park et al., 2011 [85] |
| Animal study (mice) | High-fiber diet (HFD)-fed mice vs. control group, subjected to viral influenza infection | Intake of dietary fiber improved influenza by prolonged survival (p < 0.05) and ameliorated clinical scores (p < 0.001). After 7 d, HFD-fed mice with high-dose infection had better lung function as shown by reduced pulmonary resistance (p > 0.01) and enhanced compliance in response to methacholine (p > 0.01). In HFD-fed mice, the excessive neutrophil influx into the airways was inhibited by blunted levels of CXCL1, produced by lung monocytes and macrophages (p < 0.001) vs. controls. Increased antiviral immunity by dietary fiber through CD8+ T cell activation (p < 0.01) vs. controls. (HFD)-fed mice showed enhanced adaptive immunity by changed CD8+ T cell metabolism (p < 0.05). | Trompette et al., 2018 [86] | |
| Animal study (mice) | Fiber-free diet group (LFD) vs. control group for up to 40 d, subjected to infection with mucosal pathogen Citrobacter rodentium | Low fiber intake resulting in increases in mucus-degrading microbiota and enhanced lethal colitis cases (p < 0.05). | Desai et al., 2017 [87] | |
| Vitamin A | Meta-analysis of RCTs. | Effects of vitamin A supplementation on acute lower respiratory tract infections (LRTI). 10 studies (n = 33,179 children). | Though some individual studies demonstrated a positive effect of vitamin A supplementation on LRTI, in pooled analyses, there was no effect of vitamin A supplementation on acute LRTI incidence or prevalence of symptoms. | Chen et al., 2008 [88] |
| Meta-analysis of RCTS. | Assessment of vitamin A supplementation on acute respiratory infection. 5 studies (n = 2177 children (1067 children under intervention, 1110 control). | Faster recovery from infection symptoms due to vitamin A, no differences in the placebo group: fever: OR: 0.03, CI: −0.10–0.17; oxygen requirement: OR: −0.08, CI: −0.31–0.16; increased respiratory rate: OR: −0.09, CI: −0.38 –0.19; hospital stay duration: OR: −0.06, CI: −0.52–0.40. | Brown and Roberts 2004 [89] | |
| Vitamin D | Retrospective human study | Study determining mortality patterns of COVID-19 and associated factors: Special focus on vitamin D status. 2 cohorts of 780 cases with confirmed infection of SARS-CoV-2 in Indonesia. | Vitamin D status is strongly associated with COVID-19 mortality (adjusted for age, sex, and comorbidity) (p < 0.001). Individuals with insufficient vitamin D status were ca. 12.6 as likely to die (OR 12.55). | Reharusun et al., 2020 [90] |
| Meta-analysis of RCTs | Assessment of vitamin D supplementation on respiratory tract infections. 5 clinical trials (n = 964 participants). | Significantly fewer respiratory tract infections were observed following a vitamin D supplementation. (OR: 0.58, CI: 0.417–0.812). In clinical trials there were beneficial effects on events of infections due to vitamin D supplementation in children (OR: 0.58, CI: 0.416–0.805) and adults (OR: 0.65, CI: 0.472–0.904). | Charan et al., 2012 [91] | |
| Meta-analysis of RCTs | Assessment of vitamin D supplementation on respiratory tract infection (RTI). 11 placebo-controlled studies (RTCs) (n = 5660 patients). | Vitamin D had protective effects against RTI (OR: 0.64; CI, 0.49- 0.84). This was more pronounced by individual daily dosing compared to bolus doses (OR = 0.51 vs. OR = 0.86, p = 0.01). | Bergman et al., 2013 [92] | |
| Vitamin E | Humans, RCT | Assessment of vitamin E supplementation and community acquired pneumonia. n = 7469 men 50–69 y. | Lower incidence of pneumonia in individuals receiving vitamin E supplements (RR: 0.28; CI: 0.11–0.69). | Hemila, 2016 [93] |
| Vitamin C | Meta-analysis of RCTs | Supplementation trials with vitamin C and observation of cold symptoms. 9 randomized controlled trials (n = 5500) in children (3 months–18 y of age). | Daily supplementation in vitamin C with extra doses reduced the time of having a common cold (mean difference = −0.56, 95% confidence interval (CI) (−1.03, −0.10)), fever (mean difference = −0.45, 95% CI (−0.78, −0.11)) and chest pain (mean difference = −0.40, 95% CI (−0.77, −0.03)). | Ran et al., 2018 [94] |
| B-vitamins | Human cross-sectional study | Observation of inflammation markers and nutrient status. HIV infected participants (n = 180 men, 134 women; 18–60 y). | Serum CRP concentrations were inversely associated with increased vitamin B intake including niacin, pyridoxine, and cobalamin (p for trend p < 0.01, p < 0.05 and p = 0.037, respectively) in men. Trends were observed in women. | Poudel-Tandukar et al., 2016 [95] |
| Zinc | Human double-blinded RCT | Patients in the zinc group (n = 50) received lozenges (13.3 mg of zinc gluconate) as long as they showed cold symptoms. Patients in the placebo group (n = 50) received 5% calcium lactate pentahydrate. | A faster decrease of the cold symptoms (median, 4.4 d vs. 7.6 d; p < 0.001), e.g., fewer days with coughing (median, 2 d compared with 4.5 d; p < 0.05), hoarseness (2 and 3 d; p < 0.05), headache (2 and 3 d; p < 0.05), nasal congestion (4 and 6 d; p < 0.01), and sore throat (1 and 3 d; p < 0.001) were found in the intervention group, supplemented with zinc, in comparison with the placebo group. | Mossad et al., 1996 [96] |
| Iron | Animal trial (Wistar rats) | Administration of low iron diet (4–5 mg powder), medium iron diet (15 mg), control group (35 mg) and normal iron intake diet group. At week 4, rats received injection of inactivated porcine influenza vaccine (HswIN1). | Following immunization, anemic rats exhibited decreased (p < 0.05) antibody titer vs. controls. Antibody synthesis was preserved in moderate iron deficiency, but was hampered by severe anemia. | Dhur et al., 1990 [97] |
| Selenium | Human randomized, double-blinded RCT | Evaluation of response to influenza vaccine. 12-weeks follow up. n = 119 (50–64y) 6 intervention groups: 50, 100, or 200 mgSe/day, meals with Se-enriched onions (50 mg se/day), unenriched onions and placebo. | SEPS1 mRNA (marker of inflammation) increased (p < 0.05) after one week of vaccine administration, being dependent on the dose of Se per each intervention arm. | Goldson 2011 [98] |
| Polyphenols | Animal study (mice) | Evaluation of effect of polyphenol extract from Cistus Incanus on avian influenza Aviurs (H7N7). Inbred female Balb/c and C57Bl/6 mice at the age of 6–8 weeks. | The polyphenol extract helped mice to not contract avian influenza, and to not alter bronchiole epithelial cells, as well as to keep constant the body temperature and the gross motor activity. | Droebner et al., 2007 [99] |
| Carotenoids | Longitudinal study with infants | Observation of β-carotene in plasma. 194 HIV-infected infants. | β-Carotene was related to increased risk of death during HIV infection (OR: 3.16, CI: 1.38 to 7.21; p < 0.01). | Melikian et al., 2001 [100] |
| Constituent | Major Food Sources | Quantity |
|---|---|---|
| Protein (g/100 g or mL) | Meat products: | |
| Beef | 25.3 | |
| Chicken | 19.3 | |
| Egg white | 11 | |
| Dairy products: | ||
| Yogurt | 3.5 | |
| Milk | 3.1 | |
| Cereals, roots, and tubers: | ||
| Potatoes | 2.4 | |
| Quinoa | 4.4 | |
| Legumes: | ||
| Soybeans (raw) | 25.9 | |
| Lipids (mg/100 g) | Fruits and vegetables: | |
| (High in omega-3) | Chia seeds | 1783 |
| Edamame | 361 | |
| Avocado | 111 | |
| Animal source: | ||
| Salmon | 2314 | |
| Tuna Fish | 1337 | |
| Whole grain food: | 18 | |
| Oatmeal | ||
| Carbohydrates (g/100 g) | Fruits and vegetables: | |
| Blueberries | 14.5 | |
| Figs | 19.2 | |
| Summer squash | 3.8 | |
| Whole grain food: | ||
| Oatmeal | 12 | |
| Whole-wheat bread | 42.7 | |
| Legumes: | ||
| Black beans | 23.7 | |
| Fiber (g/100 g) | Fruits and vegetables: | |
| Chia seeds | 34.4 | |
| Soybeans | 1.1 | |
| Orange | 2.4 | |
| Brussel Sprouts | 3.8 | |
| Legumes: | ||
| Lentils | 7.9 | |
| Chickpeas | 7.6 | |
| Vitamin A (µg/100 g) | Fruits and vegetables: | |
| Carrots (raw) | 835 | |
| Cantaloupe | 169 | |
| Mango | 54 | |
| Animal source: | ||
| Salmon | 13 | |
| Eggs | 160 | |
| Vitamin D (µg/100 g) | Vegetables: | |
| Portabella mushrooms | 0.33 | |
| Animal source: | ||
| Salmon | 14.4 | |
| Chicken | 0.14 | |
| Egg (whole, raw) | 2.1 | |
| Low fat yogurt | 0.03 | |
| Vitamin E (mg/100 g) | Fruits and vegetables: | |
| Sunflower seeds | 35.2 | |
| Nuts, almonds | 25.6 | |
| Blueberries | 0.6 | |
| Kiwi | 1.5 | |
| Broccoli | 0.8 | |
| Vitamin C (mg/100 g) | Fruits and vegetables: | |
| Oranges | 53.2 | |
| Broccoli | 89.2 | |
| Brussel sprouts | 85 | |
| Lemon | 53 | |
| Cauliflower | 48.2 | |
| Vitamins B6 (mg/100 g) | Plant source: | |
| Peanuts | 0.5 | |
| Lentils | 0.2 | |
| Animal source: | 1 | |
| Tuna fish | 0.4 | |
| Mollusks (raw) | ||
| Vitamin B12 (µg/100 g) | Animal source: | |
| Mollusks (raw) | 14.1 | |
| Plain yogurt | 0.4 | |
| Chicken breast | 0.2 | |
| Zinc (mg/100 g) | Plant source: | |
| Pumpkin and squash seeds | 7 | |
| Nuts | 3.1 | |
| Soybeans | 1.2 | |
| Animal source: | ||
| Beef | 7.4 | |
| Mollusks (raw) | 16.6 | |
| Lamb | 4.9 | |
| Iron (mg/100 g) | Fruits and vegetables: | |
| Apricots (dehydrated) | 2.7 | |
| Tomatoes (cherry) | 0.3 | |
| Peas | 1.5 | |
| Sunflower seeds | 5.3 | |
| Animal Source: | ||
| Mollusks | 5.1 | |
| Egg | 1.8 | |
| Veal (ground) | 1.4 | |
| Copper (mg/100 g) | Vegetables: | |
| Cashew nuts | 2.2 | |
| Tofu | 0.4 | |
| Mushrooms | 0.3 | |
| Animal Source: | ||
| Beef | 0.2 | |
| Oyster | 1.6 | |
| Cereals, roots, and tubers: | ||
| Sweet potato | 0.3 | |
| Quinoa | 0.2 | |
| Selenium (µg/100 g) | Plant source: | |
| Sunflower seeds | 53 | |
| Coconut meat | 17 | |
| Animal Source: | ||
| Mollusks | 77 | |
| Salmon | 47 | |
| Turkey, ham | 37 | |
| Polyphenols | Flavanone | |
| (mg/100 g) | Oranges (raw) | 42.6 |
| Grapefruit juice | 31.2 | |
| Anthocyanidin | ||
| Blueberries (raw) | 163.5 | |
| Strawberries (raw) | 33.6 | |
| Flavan-3-ol | ||
| Black tea | 115.3 | |
| Apple juice | 6 | |
| Carotenoids (mg/100 g) | α-Carotene | |
| Mixed frozen vegetables | 1.4 | |
| Tomatoes | 0.08 | |
| Tangerines | 0.08 | |
| β-Carotene | ||
| Spinach | 10.8 | |
| Kale | 9 | |
| Cantaloupe | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. https://doi.org/10.3390/nu12061562
Iddir M, Brito A, Dingeo G, Fernandez Del Campo SS, Samouda H, La Frano MR, Bohn T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients. 2020; 12(6):1562. https://doi.org/10.3390/nu12061562
Chicago/Turabian StyleIddir, Mohammed, Alex Brito, Giulia Dingeo, Sofia Sosa Fernandez Del Campo, Hanen Samouda, Michael R. La Frano, and Torsten Bohn. 2020. "Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis" Nutrients 12, no. 6: 1562. https://doi.org/10.3390/nu12061562
APA StyleIddir, M., Brito, A., Dingeo, G., Fernandez Del Campo, S. S., Samouda, H., La Frano, M. R., & Bohn, T. (2020). Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients, 12(6), 1562. https://doi.org/10.3390/nu12061562








