Effects of Age on Acute Appetite-Related Responses to Whey-Protein Drinks, Including Energy Intake, Gastric Emptying, Blood Glucose, and Plasma Gut Hormone Concentrations—A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Silver, A.J. Anorexia in the elderly. Neurobiol. Aging 1988, 9, 9–16. [Google Scholar] [CrossRef]
- Soenen, S.; Chapman, I.M. Body weight, anorexia, and undernutrition in older people. J. Am. Med. Dir. Assoc. 2013, 14, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Groen, B.B.; Res, P.T.; Pennings, B.; Hertle, E.; Senden, J.M.; Saris, W.H.; van Loon, L.J. Intragastric protein administration stimulates overnight muscle protein synthesis in elderly men. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E52–E60. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Walrand, S.; Beelen, M.; Gijsen, A.P.; Kies, A.K.; Boirie, Y.; Saris, W.H.; van Loon, L.J. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J. Nutr. 2009, 139, 1707–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Giezenaar, C.; Hutchison, A.T.; Luscombe-Marsh, N.D.; Chapman, I.; Horowitz, M.; Soenen, S. Effect of age on blood glucose and plasma insulin, glucagon, ghrelin, CCK, GIP, and GLP-1 responses to whey protein ingestion. Nutrients 2017, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Giezenaar, C.; Trahair, L.G.; Rigda, R.; Hutchison, A.T.; Feinle-Bisset, C.; Luscombe-Marsh, N.D.; Hausken, T.; Jones, K.L.; Horowitz, M.; Chapman, I.; et al. Lesser suppression of energy intake by orally ingested whey protein in healthy older men compared with young controls. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R845–R854. [Google Scholar] [CrossRef]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3–36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef] [Green Version]
- Soenen, S.; Giezenaar, C.; Hutchison, A.T.; Horowitz, M.; Chapman, I.; Luscombe-Marsh, N.D. Effects of intraduodenal protein on appetite, energy intake, and antropyloroduodenal motility in healthy older compared with young men in a randomized trial. Am. J. Clin. Nutr. 2014, 100, 1108–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolls, B.J.; Dimeo, K.A.; Shide, D.J. Age-related impairments in the regulation of food intake. Am. J. Clin. Nutr. 1995, 62, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.B.; Fuss, P.; Heyman, M.B.; Evans, W.J.; Tsay, R.; Rasmussen, H.; Fiatarone, M.; Cortiella, J.; Dallal, G.E.; Young, V.R. Control of food intake in older men. JAMA 1994, 272, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Blom, W.A.; Lluch, A.; Stafleu, A.; Vinoy, S.; Holst, J.J.; Schaafsma, G.; Hendriks, H.F. Effect of a high-protein breakfast on the postprandial ghrelin response. Am. J. Clin. Nutr. 2006, 83, 211–220. [Google Scholar] [CrossRef]
- Goetze, O.; Steingoetter, A.; Menne, D.; van der Voort, I.R.; Kwiatek, M.A.; Boesiger, P.; Weishaupt, D.; Thumshirn, M.; Fried, M.; Schwizer, W. The effect of macronutrients on gastric volume responses and gastric emptying in humans: a magnetic resonance imaging study. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G11–G17. [Google Scholar] [CrossRef] [Green Version]
- Giezenaar, C.; Lange, K.; Hausken, T.; Jones, K.L.; Horowitz, M.; Chapman, I.; Soenen, S. Acute Effects of Substitution, and Addition, of Carbohydrates and Fat to Protein on Gastric Emptying, Blood Glucose, Gut Hormones, Appetite, and Energy Intake. Nutrients 2018, 10, 1451. [Google Scholar] [CrossRef] [Green Version]
- Giezenaar, C.; van der Burgh, Y.; Lange, K.; Hatzinikolas, S.; Hausken, T.; Jones, K.L.; Horowitz, M.; Chapman, I.; Soenen, S. Effects of substitution, and adding of carbohydrate and fat to whey-protein on energy intake, appetite, gastric emptying, glucose, insulin, ghrelin, CCK and GLP-1 in healthy older men-a randomized controlled trial. Nutrients 2018, 10, 113. [Google Scholar] [CrossRef] [Green Version]
- Gentilcore, D.; Hausken, T.; Horowitz, M.; Jones, K.L. Measurements of gastric emptying of low- and high-nutrient liquids using 3D ultrasonography and scintigraphy in healthy subjects. Neurogastroenterol. Motil. 2006, 18, 1062–1068. [Google Scholar] [CrossRef]
- Parker, B.A.; Ludher, A.K.; Loon, T.K.; Horowitz, M.; Chapman, I.M. Relationships of ratings of appetite to food intake in healthy older men and women. Appetite 2004, 43, 227–233. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Bland, J.M.; Altman, D.G. Calculating correlation coefficients with repeated observations: part 1-correlation within subjects. Br. Med. J. 1995, 310, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giezenaar, C.; Chapman, I.; Luscombe-Marsh, N.; Feinle-Bisset, C.; Horowitz, M.; Soenen, S. Ageing is associated with decreases in appetite and energy intake--a meta-analysis in healthy adults. Nutrients 2016, 8, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayner, C.K.; MacIntosh, C.G.; Chapman, I.M.; Morley, J.E.; Horowitz, M. Effects of age on proximal gastric motor and sensory function. Scand. J. Gastroenterol. 2000, 35, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.; Hochstenbach-Waelen, A.; Westerterp-Plantenga, M.S. Efficacy of alpha-lactalbumin and milk protein on weight loss and body composition during energy restriction. Obesity (Silver Spring) 2011, 19, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Weigle, D.S.; Breen, P.A.; Matthys, C.C.; Callahan, H.S.; Meeuws, K.E.; Burden, V.R.; Purnell, J.Q. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am. J. Clin. Nutr. 2005, 82, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.; Westerterp-Plantenga, M.S. Proteins and satiety: implications for weight management. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 747–751. [Google Scholar] [CrossRef] [Green Version]
- Pennings, B.; Groen, B.; de Lange, A.; Gijsen, A.P.; Zorenc, A.H.; Senden, J.M.; van Loon, L.J. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E992–E999. [Google Scholar] [CrossRef] [Green Version]
- Sturm, K.; MacIntosh, C.G.; Parker, B.A.; Wishart, J.; Horowitz, M.; Chapman, I.M. Appetite, food intake, and plasma concentrations of cholecystokinin, ghrelin, and other gastrointestinal hormones in undernourished older women and well-nourished young and older women. J. Clin. Endocrinol. Metab. 2003, 88, 3747–3755. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, M. Integrated upper gastrointestinal response to food intake. Gastroenterology 2006, 131, 640–658. [Google Scholar] [CrossRef]
- Horowitz, M.; Maddern, G.J.; Chatterton, B.E.; Collins, P.J.; Harding, P.E.; Shearman, D.J. Changes in gastric emptying rates with age. Clin. Sci. (Lond) 1984, 67, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Soenen, S.; Rayner, C.K.; Horowitz, M.; Jones, K.L. Gastric emptying in the elderly. Clin. Geriatr. Med. 2015, 31, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.; Phillips, L.; Trahair, L.; Hatzinikolas, S.; Horowitz, M.; Jones, K.L. Longitudinal changes in the blood pressure responses to, and gastric emptying of, an oral glucose load in healthy older subjects. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Niedereichholz, U.; Ettler, R.; Holst, J.J.; Orskov, C.; Ritzel, R.; Schmiegel, W.H. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am. J. Physiol. 1997, 273, E981–E988. [Google Scholar] [CrossRef] [PubMed]
- MacIntosh, C.G.; Horowitz, M.; Verhagen, M.A.; Smout, A.J.; Wishart, J.; Morris, H.; Goble, E.; Morley, J.E.; Chapman, I.M. Effect of small intestinal nutrient infusion on appetite, gastrointestinal hormone release, and gastric myoelectrical activity in young and older men. Am. J. Gastroenterol. 2001, 96, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Trahair, L.G.; Horowitz, M.; Rayner, C.K.; Gentilcore, D.; Lange, K.; Wishart, J.M.; Jones, K.L. Comparative effects of variations in duodenal glucose load on glycemic, insulinemic, and incretin responses in healthy young and older subjects. J. Clin. Endocrinol. Metab. 2012, 97, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Cowie, C.C.; Rust, K.F.; Byrd-Holt, D.D.; Gregg, E.W.; Ford, E.S.; Geiss, L.S.; Bainbridge, K.E.; Fradkin, J.E. Prevalence of Diabetes and High Risk for Diabetes Using A1C Criteria in the U.S. Population in 1988–2006. Diabetes Care 2010, 33, 562–568. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.G.; Boyko, E.J.; Strotmeyer, E.S.; Lewis, C.E.; Cawthon, P.M.; Hoffman, A.R.; Everson-Rose, S.A.; Barrett-Connor, E.; Orwoll, E.S.; Osteoporotic Fractures in Men Study Research Group. Association Between Insulin Resistance and Lean Mass Loss and Fat Mass Gain in Older Men without Diabetes Mellitus. J. Am. Geriatr. Soc. 2011, 59, 1217–1224. [Google Scholar] [CrossRef] [Green Version]
- MacIntosh, C.G.; Andrews, J.M.; Jones, K.L.; Wishart, J.M.; Morris, H.A.; Jansen, J.B.; Morley, J.E.; Horowitz, M.; Chapman, I.M. Effects of age on concentrations of plasma cholecystokinin, glucagon-like peptide 1, and peptide YY and their relation to appetite and pyloric motility. Am. J. Clin. Nutr. 1999, 69, 999–1006. [Google Scholar] [CrossRef]
- Murphy, K.G.; Bloom, S.R. Gut hormones in the control of appetite. Exp. Physiol. 2004, 89, 507–516. [Google Scholar] [CrossRef] [Green Version]
- MacIntosh, C.G.; Morley, J.E.; Wishart, J.; Morris, H.; Jansen, J.B.; Horowitz, M.; Chapman, I.M. Effect of exogenous cholecystokinin (CCK)-8 on food intake and plasma CCK, leptin, and insulin concentrations in older and young adults: evidence for increased CCK activity as a cause of the anorexia of aging. J. Clin. Endocrinol. Metab. 2001, 86, 5830–5837. [Google Scholar] [CrossRef]
- Ranganath, L.; Sedgwick, I.; Morgan, L.; Wright, J.; Marks, V. The ageing entero-insular axis. Diabetologia 1998, 41, 1309–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Francesco, V.; Barazzoni, R.; Bissoli, L.; Fantin, F.; Rizzotti, P.; Residori, L.; Antonioli, A.; Graziani, M.S.; Zanetti, M.; Bosello, O.; et al. The quantity of meal fat influences the profile of postprandial hormones as well as hunger sensation in healthy elderly people. J. Am. Med. Dir. Assoc. 2010, 11, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.; Marathe, C.S.; Phillips, L.K.; Trahair, L.G.; Hatzinikolas, S.; Huynh, L.; Wu, T.; Nauck, M.A.; Rayner, C.K.; Horowitz, M.; et al. Longitudinal Changes in Fasting and Glucose-Stimulated GLP-1 and GIP in Healthy Older Subjects. J. Clin. Endocrinol. Metab. 2019, 104, 6201–6206. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef]
- Giezenaar, C.; Luscombe-Marsh, N.D.; Hutchison, A.T.; Lange, K.; Hausken, T.; Jones, K.L.; Horowitz, M.; Chapman, I.; Soenen, S. Effect of gender on the acute effects of whey protein ingestion on energy intake, appetite, gastric emptying and gut hormone responses in healthy young adults. Nutr. Diabetes 2018, 8, 40. [Google Scholar] [CrossRef]
- Giezenaar, C.; Trahair, L.G.; Luscombe-Marsh, N.D.; Hausken, T.; Standfield, S.; Jones, K.L.; Lange, K.; Horowitz, M.; Chapman, I.; Soenen, S. Effects of randomized whey-protein loads on energy intake, appetite, gastric emptying, and plasma gut-hormone concentrations in older men and women. Am. J. Clin. Nutr. 2017, 106, 865–877. [Google Scholar] [CrossRef] [Green Version]
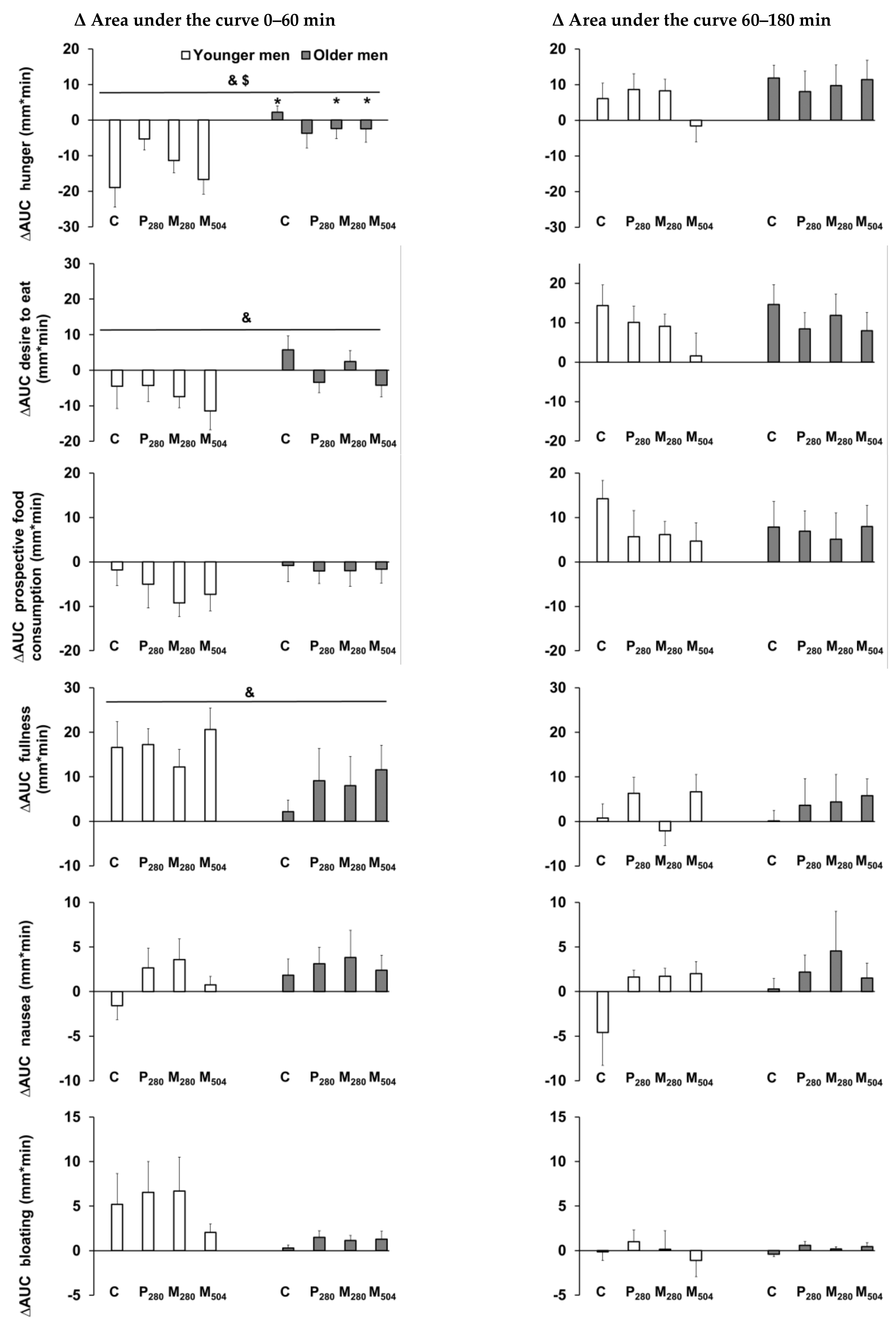

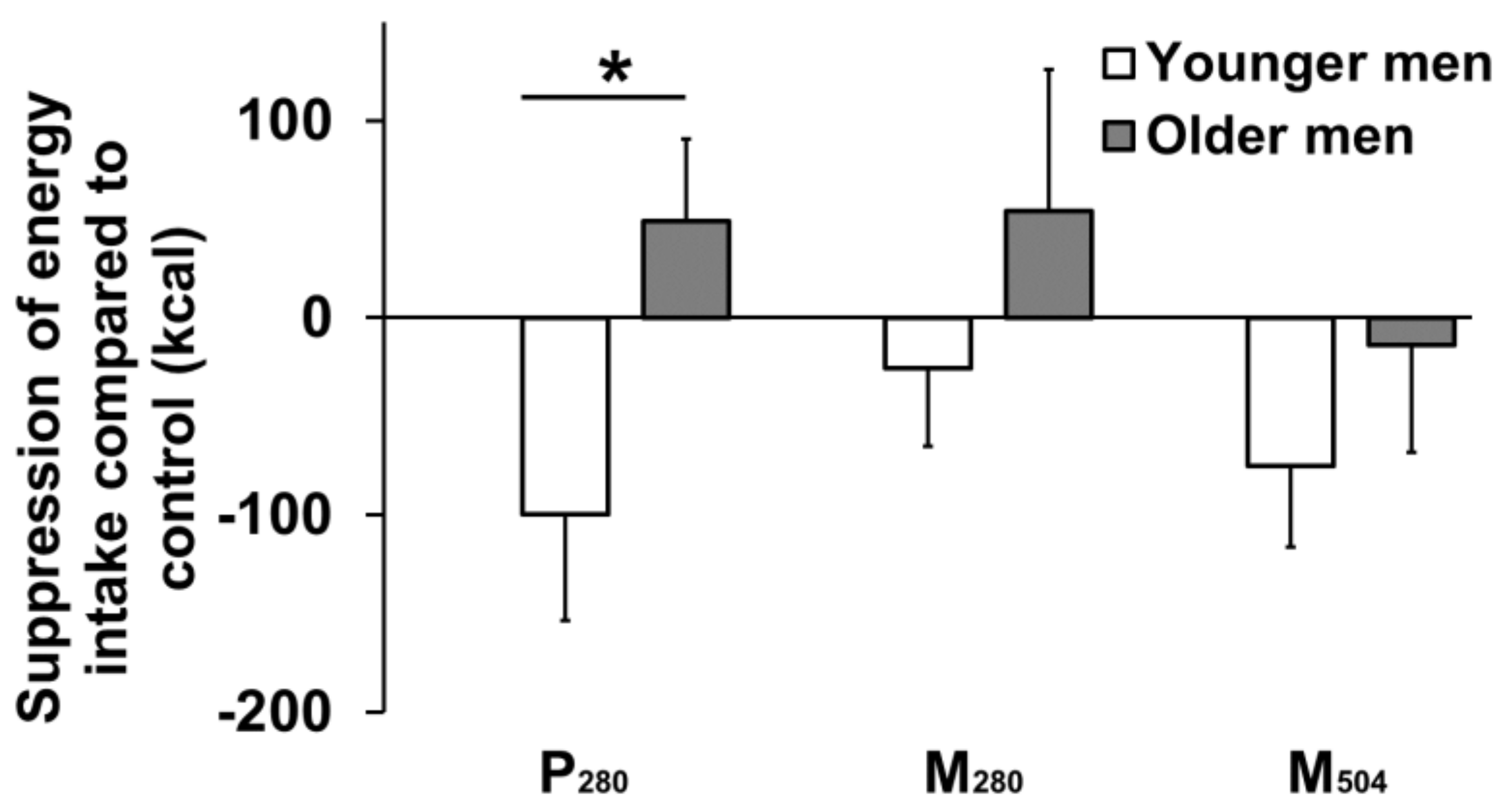
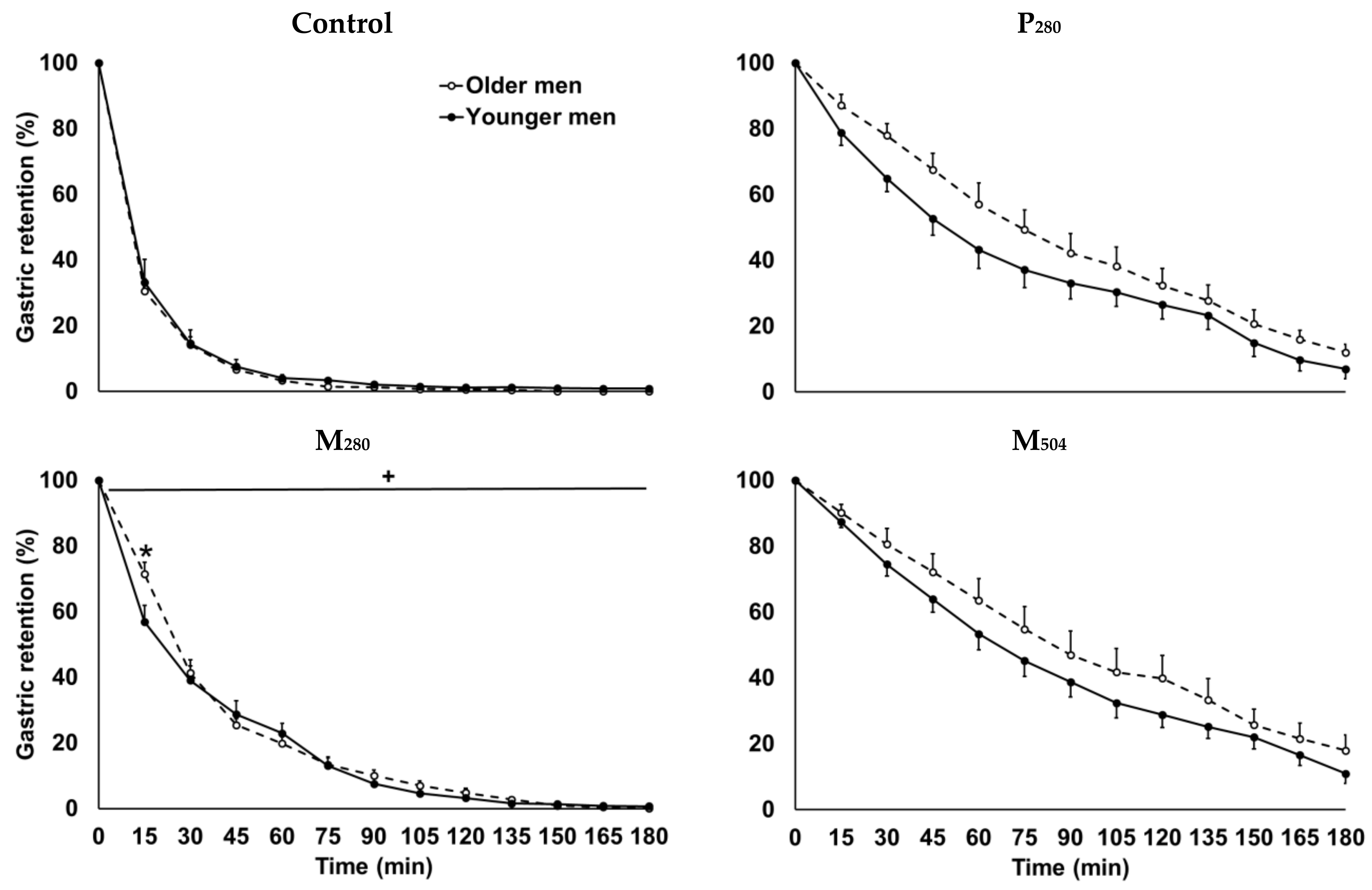
| Baseline Values | Younger Men (n = 13) | Older Men (n = 13) | Age Effect (p value) |
|---|---|---|---|
| Blood glucose (mmol/L) | 5.3 ± 0.1 | 5.7 ± 0.1 | 0.003 |
| Plasma insulin (mU/L) | 4.9 ± 1 | 5.2 ± 2 | 0.93 |
| Plasma ghrelin (pg/mL) | 1779 ± 236 | 1659 ± 165 | 0.67 |
| Plasma CCK (pmol/L) | 1.3 ± 0.1 | 2.0 ± 0.2 | 0.008 |
| Plasma GLP-1 (pmol/L) | 20 ± 2 | 15 ± 1 | 0.017 |
| Hunger (mm) | 46 ± 10 | 31 ± 13 | 0.34 |
| Desire to eat (mm) | 50 ± 6 | 30 ± 12 | 0.26 |
| Prospective food consumption (mm) | 56 ± 6 | 46 ± 14 | 0.73 |
| Fullness (mm) | 4 ± 1 | 2 ± 1 | 0.76 |
| Nausea (mm) | 6 ± 2 | 3 ± 1 | 0.08 |
| Bloating (mm) | 6 ± 2 | 3 ± 1 | 0.09 |
| Gastric volume (mL) | 37 ± 3 | 33 ± 4 | 0.31 |
| Younger Men (n = 13) | Older Men (n = 13) | Age Effect (p value) | |
|---|---|---|---|
| Control day | |||
| Energy intake (kcal) | 1218 ± 109 | 972 ± 87 | 0.09 |
| Protein (g) | 21 ± 1 | 20 ± 1 | 0.28 |
| Fat (g) | 32 ± 2 | 30 ± 1 | 0.36 |
| Carbohydrates (g) | 47 ± 3 | 52 ± 2 | 0.13 |
| P280 | |||
| Energy intake (kcal) | 1118 ± 105 | 1020 ± 95 | 0.50 |
| Protein (g) | 21 ± 1 | 20 ± 1 | 0.24 |
| Fat (g) | 32 ± 2 | 29 ± 1 | 0.10 |
| Carbohydrates (g) | 47 ± 3 | 52 ± 2 | 0.34 |
| M280 | |||
| Energy intake (kcal) | 1193 ± 113 | 1026 ± 76 | 0.23 |
| Protein (g) | 21 ± 1 | 20 ± 1 | 0.42 |
| Fat (g) | 32 ± 2 | 31 ± 1 | 0.48 |
| Carbohydrates (g) | 46 ± 3 | 51 ± 2 | 0.18 |
| M504 | |||
| Energy intake (kcal) | 1143 ± 112 | 957 ± 76 | 0.18 |
| Protein (g) | 21 ± 1 | 21 ± 1 | 0.88 |
| Fat (g) | 32 ± 2 | 29 ± 1 | 0.22 |
| Carbohydrates (g) | 47 ± 3 | 52 ± 2 | 0.20 |
| Combined | Younger men | Older men | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| AUC0–60 min | ||||||
| Glucose | 0.06 | 0.63 | −0.01 | 0.96 | 0.14 | 0.46 |
| Insulin | 0.38 | 0.003 | 0.52 | 0.002 | ||
| Ghrelin | −0.31 | 0.014 | −0.42 | 0.015 | ||
| CCK | 0.61 | <0.001 | 0.65 | <0.001 | 0.65 | <0.001 |
| GLP−1 | 0.68 | <0.001 | 0.57 | <0.001 | 0.79 | <0.001 |
| Hunger | −0.27 | 0.039 | −0.39 | <0.001 | 0.02 | 0.91 |
| Desire to eat | −0.26 | 0.039 | −0.41 | 0.015 | 0.11 | 0.60 |
| Prospective food consumption | −0.24 | 0.056 | −0.40 | 0.019 | 0.15 | 0.45 |
| Fullness | 0.26 | 0.043 | 0.28 | 0.11 | 0.24 | 0.22 |
| Nausea | −0.10 | 0.44 | −0.14 | 0.42 | −0.03 | 0.86 |
| Bloating | −0.25 | 0.053 | −0.31 | 0.07 | −0.28 | 0.16 |
| AUC60–180 min | ||||||
| Glucose | −0.13 | 0.33 | −0.31 | 0.079 | −0.02 | 0.93 |
| Insulin | 0.46 | <0.001 | 0.76 | <0.001 | 0.44 | 0.019 |
| Ghrelin | −0.70 | <0.001 | −0.74 | <0.001 | −0.72 | <0.001 |
| CCK | 0.71 | <0.001 | 0.75 | <0.001 | 0.72 | <0.001 |
| GLP−1 | 0.71 | <0.001 | 0.73 | <0.001 | 0.70 | <0.001 |
| Hunger | −0.04 | 0.76 | −0.23 | 0.19 | 0.07 | 0.73 |
| Desire to eat | 0.09 | 0.48 | −0.24 | 0.18 | 0.08 | 0.69 |
| Prospective food consumption | 0.02 | 0.90 | −0.15 | 0.40 | 0.16 | 0.41 |
| Fullness | −0.05 | 0.72 | −0.04 | 0.82 | 0.15 | 0.45 |
| Nausea | −0.14 | 0.26 | −0.27 | 0.13 | −0.12 | 0.56 |
| Bloating | −0.27 | 0.039 | 0.45 | 0.007 | −0.14 | 0.49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giezenaar, C.; Lange, K.; Hausken, T.; Jones, K.L.; Horowitz, M.; Chapman, I.; Soenen, S. Effects of Age on Acute Appetite-Related Responses to Whey-Protein Drinks, Including Energy Intake, Gastric Emptying, Blood Glucose, and Plasma Gut Hormone Concentrations—A Randomized Controlled Trial. Nutrients 2020, 12, 1008. https://doi.org/10.3390/nu12041008
Giezenaar C, Lange K, Hausken T, Jones KL, Horowitz M, Chapman I, Soenen S. Effects of Age on Acute Appetite-Related Responses to Whey-Protein Drinks, Including Energy Intake, Gastric Emptying, Blood Glucose, and Plasma Gut Hormone Concentrations—A Randomized Controlled Trial. Nutrients. 2020; 12(4):1008. https://doi.org/10.3390/nu12041008
Chicago/Turabian StyleGiezenaar, Caroline, Kylie Lange, Trygve Hausken, Karen L. Jones, Michael Horowitz, Ian Chapman, and Stijn Soenen. 2020. "Effects of Age on Acute Appetite-Related Responses to Whey-Protein Drinks, Including Energy Intake, Gastric Emptying, Blood Glucose, and Plasma Gut Hormone Concentrations—A Randomized Controlled Trial" Nutrients 12, no. 4: 1008. https://doi.org/10.3390/nu12041008





