Distinguishing Low and High Water Consumers—A Paradigm of Disease Risk
Abstract
1. Introduction
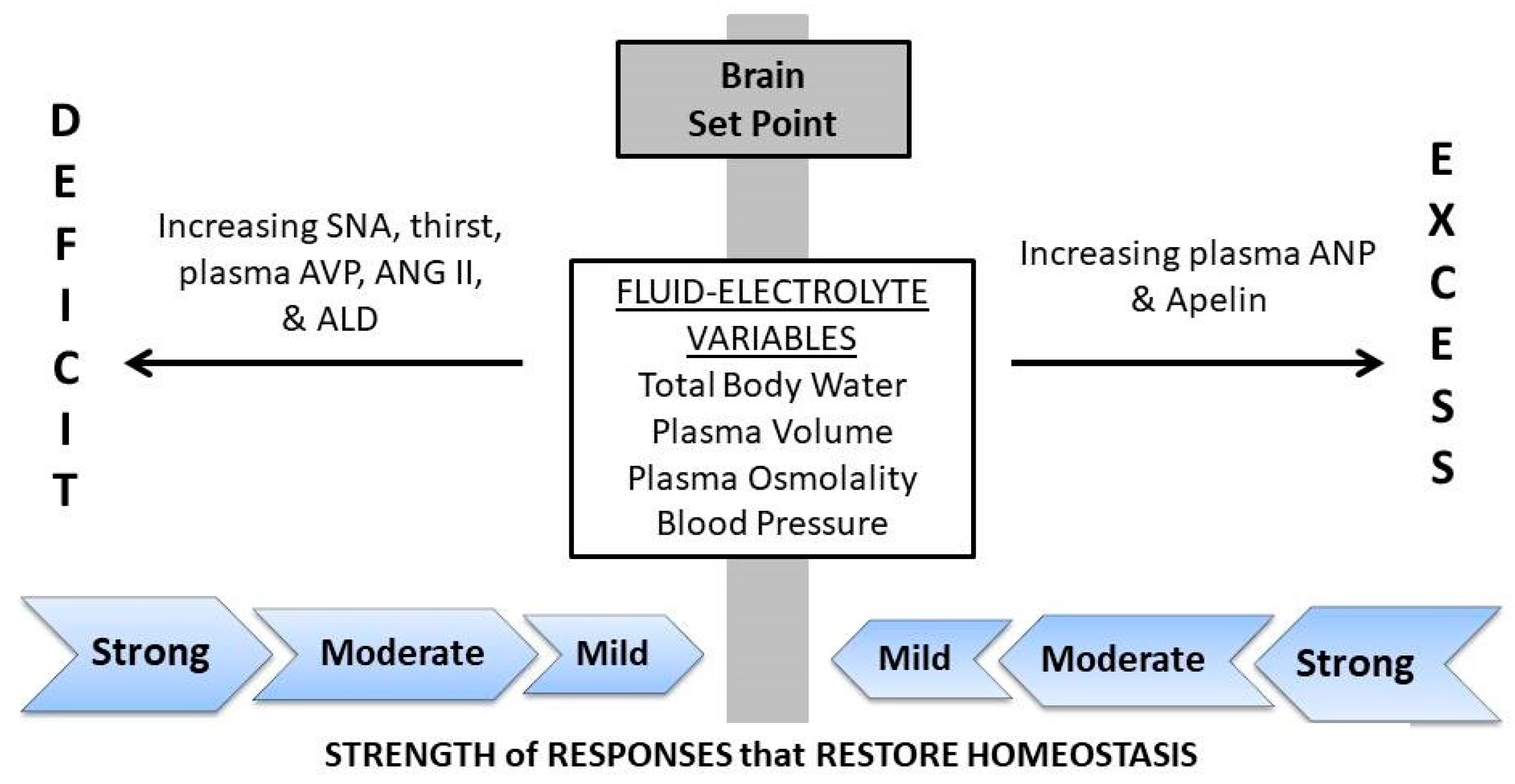
2. The Hydration Continuum
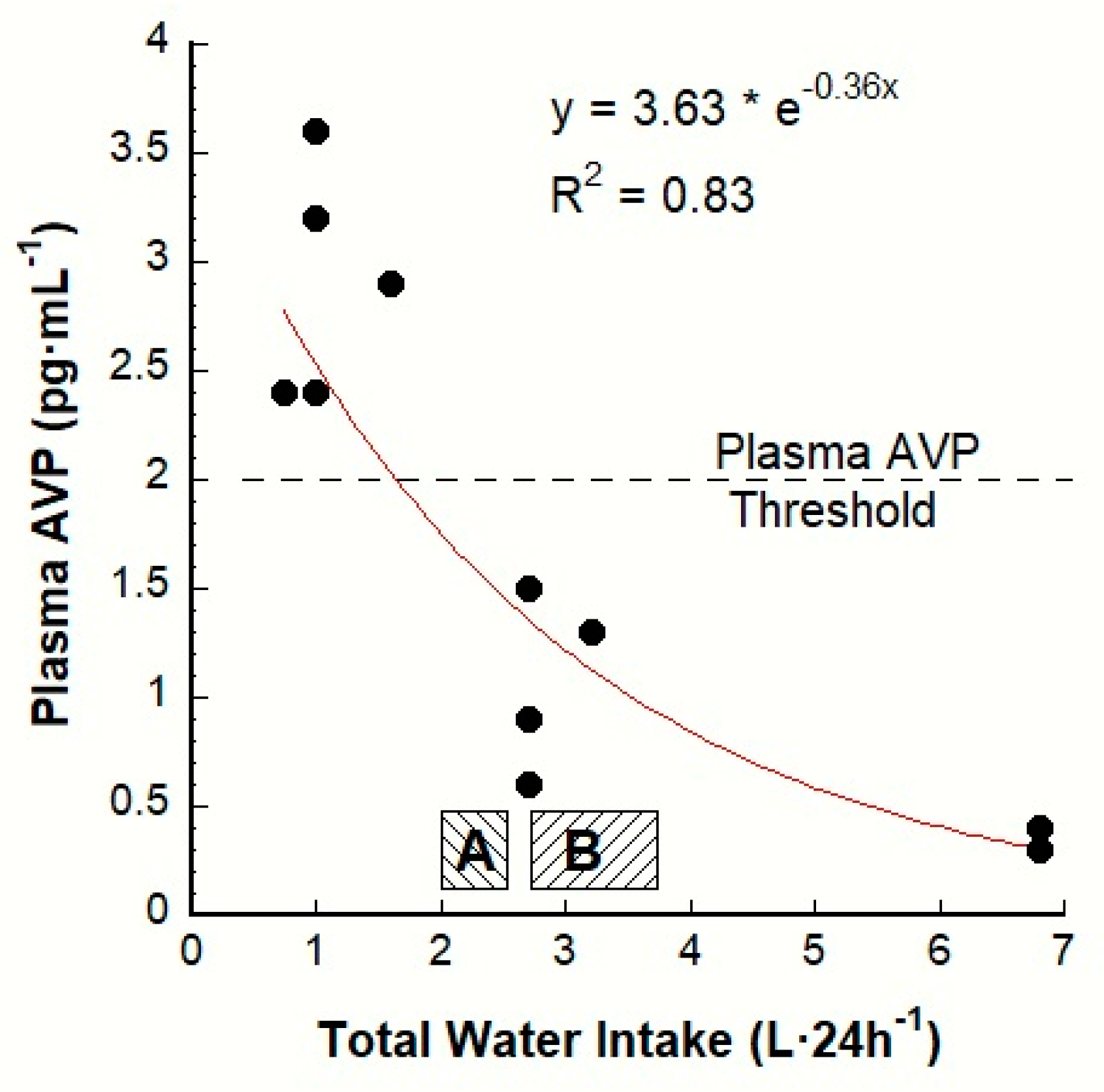
3. The AVP-TWI Relationship
4. Characteristics that Distinguish Low from High
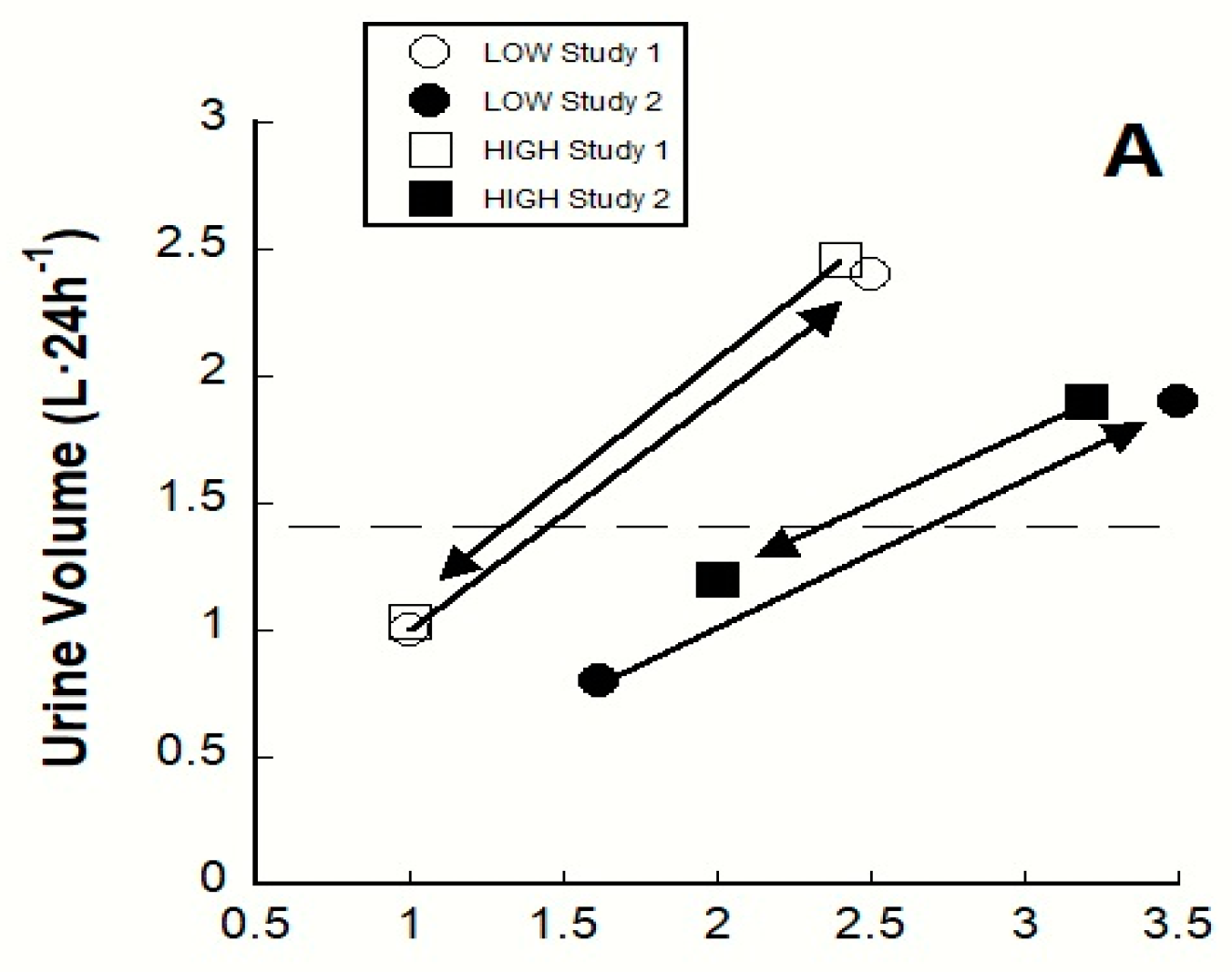
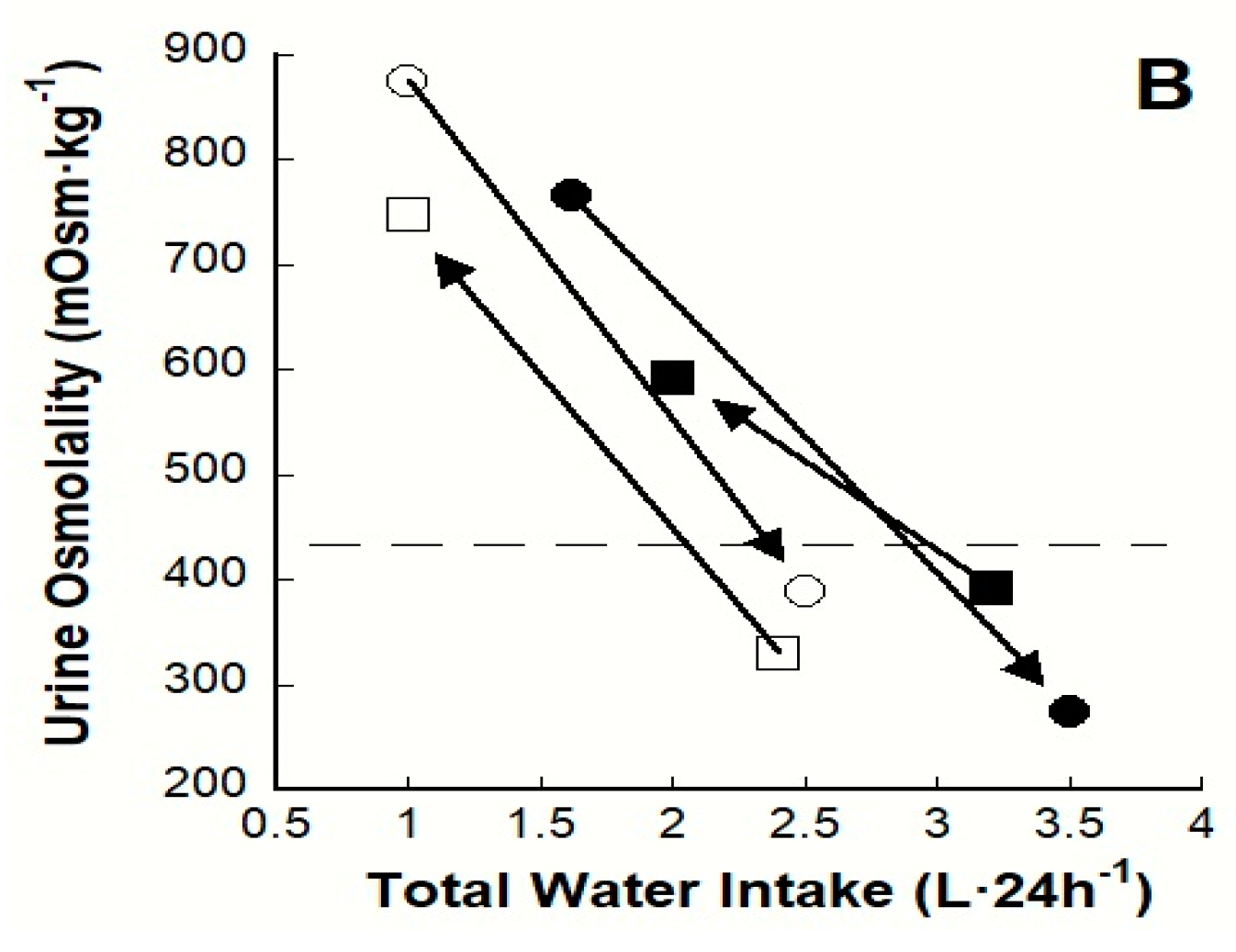
4.1. Longitudinal Clinical Trials
4.2. Observational Studies and Controlled Interventions
5. Possible Mechanisms of Morbidity and Mortality: AVP and Cortisol
6. A Theoretical Paradigm of Disease Risk
7. Future Research
7.1. Experimental Considerations
7.2. Variability of the POSM Threshold for Thirst
7.3. Renal Osmolar Excretion: Differences Due to Diet
7.4. Drinking Behavior & Fluid Composition
8. Nutritional Recommendations for LOW
8.1. Five Dietary and Hydration Goals
8.2. Select Solid Foods with a High Water Content
8.3. Act to Increase TWI
8.4. Reduce 24-h Osmolar Load
8.4.1. Dietary Protein
8.4.2. Dietary Salt
8.5. Self-assess Hydration Status
8.6. Avoid Overdrinking
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thornton, S.N. Thirst and hydration: Physiology and consequences of dysfunction. Physiol. Behav. 2010, 100, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Cheuvront, S.N.; Kenefick, R.W. Am I drinking enough? Yes, no, and maybe. J. Am. Coll. Nutr. 2016, 35, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Greenleaf, J.E. Problem: Thirst, drinking behavior, and involuntary dehydration. Med. Sci. Sports Exerc. 1992, 24, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimons, J.T. The physiological basis of thirst. Kidney Int. 1976, 10, 3–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galanth, C.; Hus-Citharel, A.; Li, B.; Llorens-Cortes, C. Apelin in the control of body fluid homeostasis and cardiovascular functions. Curr. Pharm. Des. 2012, 18, 789–798. [Google Scholar] [CrossRef]
- Azizi, M.; Iturrioz, X.; Blanchard, A.; Peyrard, S.; De Mota, N.; Chartrel, N.; Vaudry, H.; Corvol, P.; Llorens-Cortes, C. Reciprocal regulation of plasma apelin and vasopressin by osmotic stimuli. J. Am. Soc. Nephrol. 2008, 19, 1015–1024. [Google Scholar] [CrossRef]
- Melander, O. Vasopressin, from regulator to disease predictor for diabetes and cardiometabolic risk. Ann. Nutr. Metab. 2016, 68, 24–28. [Google Scholar] [CrossRef]
- Muñoz, C.X.; Johnson, E.C.; McKenzie, A.L.; Guelinckx, I.; Graverholt, G.; Casa, D.J.; Maresh, C.M.; Armstrong, L.E. Habitual total water intake and dimensions of mood in healthy young women. Appetite 2015, 92, 81–86. [Google Scholar] [CrossRef]
- Perrier, E.; Vergne, S.; Klein, A.; Poupin, M.; Rondeau, P.; Le Bellego, L.; Armstrong, L.E.; Lang, F.; Stookey, J.; Tack, I. Hydration biomarkers in free-living adults with different levels of habitual fluid consumption. Br. J. Nutr. 2013, 109, 1678–1687. [Google Scholar] [CrossRef]
- EFSA Panel on Dietic Products, Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for water. EFSA J. 2010, 8, 1459–1507. [Google Scholar]
- Institute of Medicine, Panel on Dietary Reference Intakes for Electrolytes and Water. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; National Academy Press: Washington, DC, USA, 2005. [Google Scholar]
- Türk, C.; Knoll, T.; Petrik, A. Guidelines on urolithiasis. Selection of procedure for active removal of kidney stones. In Proceedings of 26th European Association of Urology Congress, Vienna, Austria, 18–22 March 2011; pp. 18–22. [Google Scholar]
- Sontrop, J.M.; Dixon, S.N.; Garg, A.X.; Buendia-Jimenez, I.; Dohein, O.; Huang, S.H.; Clark, W.F. Association between water intake, chronic kidney disease, and cardiovascular disease: A cross-sectional analysis of NHANES data. Am. J. Nephrol. 2013, 37, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, G.F.; Craig, J.C.; Rochtchina, E.; Flood, V.M.; Wang, J.J.; Mitchell, P. Fluid and nutrient intake and risk of chronic kidney disease. Nephrology 2011, 16, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Finkielstein, V.A.; Goldfarb, D.S. Strategies for preventing calcium oxalate stones. CMAJ 2006, 174, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Hooton, T.M.; Vecchio, M.; Iroz, A.; Tack, I.; Dornic, Q.; Seksek, I.; Lotan, Y. Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections: A randomized clinical trial. JAMA Intern. Med. 2018, 178, 1509–1515. [Google Scholar] [CrossRef]
- Pross, N.; Demazières, A.; Girard, N.; Barnouin, R.; Metzger, D.; Klein, A.; Perrier, E.; Guelinckx, I. Effects of changes in water intake on mood of high and low drinkers. PLoS ONE 2014, 9, e94754. [Google Scholar] [CrossRef]
- Johnson, E.C.; Muñoz, C.X.; Jimenez, L.; Le Bellego, L.; Kupchak, B.R.; Kraemer, W.J.; Casa, D.J.; Maresh, C.M.; Armstrong, L.E. Hormonal and thirst modulated maintenance of fluid balance in young women with different levels of habitual fluid consumption. Nutrients 2016, 8, 302. [Google Scholar] [CrossRef]
- Manz, F. Hydration and disease. J. Am. Coll. Nutr. 2007, 26, 535S–541S. [Google Scholar] [CrossRef]
- Perrier, E.; Demazières, A.; Girard, N.; Pross, N.; Osbild, D.; Metzger, D.; Guelinckx, I.; Klein, A. Circadian variation and responsiveness of hydration biomarkers to changes in daily water intake. Eur. J. Appl. Physiol. 2013, 113, 2143–2151. [Google Scholar] [CrossRef]
- Johnson, E.C.; Muñoz, C.X.; Le Bellego, L.; Klein, A.; Casa, D.J.; Maresh, C.M.; Armstrong, L.E. Markers of the hydration process during fluid volume modification in women with habitual high or low daily fluid intakes. Eur. J. Appl. Physiol. 2015, 115, 1067–1074. [Google Scholar] [CrossRef]
- Adolph, E.F. Physiology of Man in the Desert; Interscience Publishers: Cummings Park, MA, USA, 1947. [Google Scholar]
- Ashcroft, F.M. Life at the Extremes; University of California Press: Oakland, CA, USA, 2002. [Google Scholar]
- Armstrong, L.E.; Johnson, E.C.; Ganio, M.S.; Judelson, D.A.; Vingren, J.L.; Kupchak, B.R.; Kunces, L.J.; Muñoz, C.X.; McKenzie, A.L.; Williamson, K.H. Effective body water and body mass changes during summer ultra-endurance road cycling. J. Sports Sci. 2015, 33, 125–135. [Google Scholar] [CrossRef]
- Ganio, M.S.; Armstrong, L.E.; Kavouras, S.A. Hydration. In Sport and Physical Activity in the Heat; Casa, D.J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 83–100. [Google Scholar]
- Negoianu, D.; Goldfarb, S. Just add water. J. Am. Soc. Nephrol. 2008, 19, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E. The challenges of linking chronic dehydration and fluid consumption to health outcomes. Nutr. Rev. 2012, 70, S121–S127. [Google Scholar] [CrossRef] [PubMed]
- Bankir, L.; Bouby, N.; Ritz, E. Vasopressin: A novel target for the prevention and retardation of kidney disease? Nat. Rev. Nephrol. 2013, 9, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Enhörning, S.; Wang, T.J.; Nilsson, P.M.; Almgren, P.; Hedblad, B.; Berglund, G.; Struck, J.; Morgenthaler, N.G.; Bergmann, A.; Lindholm, E.; et al. Plasma copeptin and the risk of diabetes mellitus. Circulation 2010, 121, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Corpeleijn, E.; Meijer, E.; Postmus, D.; Gansevoort, R.T.; Gans, R.O.; Struck, J.; Hillege, H.L.; Stolk, R.P.; Navis, G.; et al. Sex differences in the association between plasma copeptin and incident type 2 diabetes: The Prevention of Renal and Vascular Endstage Disease (PREVEND) study. Diabetologia 2012, 55, 1963–1970. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Welsh, P.; Papacosta, O.; Lennon, L.; Whincup, P.H.; Sattar, N. Copeptin, insulin resistance and risk of incident diabetes in older men. J. Clin. Endocrinol. Metab. 2015, 100, 3332–3339. [Google Scholar] [CrossRef]
- Roussel, R.; Fezeu, L.; Bouby, N.; Balkau, B.; Lantieri, O.; Alhenc-Gelas, F.; Marre, M.; Bankir, L. Low water intake and risk for new-onset hyperglycemia. Diabetes Care 2011, 34, 2551–2554. [Google Scholar] [CrossRef]
- Saleem, U.; Khaleghi, M.; Morgenthaler, N.G.; Bergmann, A.; Struck, J.; Mosley, T.H., Jr.; Kullo, I.J. Plasma carboxy-terminal provasopressin (copeptin): A novel marker of insulin resistance and metabolic syndrome. J. Clin. Endocrinol. Metab. 2009, 94, 2558–2564. [Google Scholar] [CrossRef]
- Enhörning, S.; Struck, J.; Wirfält, E.; Hedblad, B.; Morgenthaler, N.G.; Melander, O. Plasma copeptin, a unifying factor behind the metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E1065–E1072. [Google Scholar] [CrossRef]
- Enhörning, S.; Bankir, L.; Bouby, N.; Struck, J.; Hedblad, B.; Persson, M.; Morgenthaler, N.G.; Nilsson, P.M.; Melander, O. Copeptin, a marker of vasopressin, in abdominal obesity, diabetes and microalbuminuria: The prospective Malmö Diet and Cancer Study cardiovascular cohort. Int. J. Obes. 2013, 37, 598–603. [Google Scholar] [CrossRef]
- Fenske, W.K.; Schnyder, I.; Koch, G.; Walti, C.; Pfister, M.; Kopp, P.; Fassnacht, M.; Strauss, K.; Christ-Crain, M. Release and decay kinetics of copeptin vs AVP in response to osmotic alterations in healthy volunteers. J. Clin. Endocrinol. Metab. 2018, 103, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Riphagen, I.J.; Boertien, W.E.; Alkhalaf, A.; Kleefstra, N.; Gansevoort, R.T.; Groenier, K.H.; Van Hateren, K.J.; Struck, J.; Navis, G.; Bilo, H.J.; et al. Copeptin, a surrogate marker for arginine vasopressin, is associated with cardiovascular and all-cause mortality in patients with type 2 diabetes (ZODIAC-31). Diabetes Care 2013, 36, 3201–3207. [Google Scholar] [CrossRef] [PubMed]
- Velho, G.; Ragot, S.; El Boustany, R.; Saulnier, P.J.; Fraty, M.; Mohammedi, K.; Fumeron, F.; Potier, L.; Marre, M.; Hadjadj, S.; et al. Plasma copeptin, kidney disease, and risk for cardiovascular morbidity and mortality in two cohorts of type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Tasevska, I.; Enhörning, S.; Christensson, A.; Persson, M.; Nilsson, P.M.; Melander, O. Increased levels of copeptin, a surrogate marker of arginine vasopressin, are associated with an increased risk of chronic kidney disease in a general population. Am. J. Nephrol. 2016, 44, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Boertien, W.E.; Riphagen, I.J.; Drion, I.; Alkhalaf, A.; Bakker, S.J.; Groenier, K.H.; Struck, J.; de Jong, P.E.; Bilo, H.J.; Kleefstra, N.; et al. Copeptin, a surrogate marker for arginine vasopressin, is associated with declining glomerular filtration in patients with diabetes mellitus (ZODIAC-33). Diabetologia 2013, 56, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Johnson, E.C. Water intake, water balance, and the elusive daily water requirement. Nutrients 2018, 10, 1928. [Google Scholar] [CrossRef]
- Allen, M.D.; Springer, D.A.; Burg, M.B.; Boehm, M.; Dmitrieva, N.I. Suboptimal hydration remodels metabolism, promotes degenerative diseases, and shortens life. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Taveau, C.; Chollet, C.; Waeckel, L.; Desposito, D.; Bichet, D.G.; Arthus, M.F.; Magnan, C.; Philippe, E.; Paradis, V.; Foufelle, F.; et al. Vasopressin and hydration play a major role in the development of glucose intolerance and hepatic steatosis in obese rats. Diabetologia 2015, 58, 1081–1090. [Google Scholar] [CrossRef]
- Kavouras, S.A. Hydration, dehydration, underhydration, optimal hydration: Are we barking up the wrong tree? Eur. J. Nutr. 2019, 58, 471–473. [Google Scholar] [CrossRef]
- Perrier, E.T.; Armstrong, L.E.; Daudon, M.; Kavouras, S.; Lafontan, M.; Lang, F.; Péronnet, F.; Stookey, J.D.; Tack, I.; Klein, A. From state to process: Defining hydration. Obes. Facts 2014, 7, 6–12. [Google Scholar] [CrossRef]
- Armstrong, L.E. Assessing hydration status: The elusive gold standard. J. Am. Coll. Nutr. 2007, 26, 575S–584S. [Google Scholar] [CrossRef] [PubMed]
- Kratz, A.; Ferraro, M.; Sluss, P.M.; Lewandrowski, K.B. Laboratory reference values. N. Engl. J. Med. 2004, 351, 1548–1564. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Pumerantz, A.C.; Fiala, K.A.; Roti, M.W.; Kavouras, S.A.; Casa, D.J.; Maresh, C.M. Human hydration indices: Acute and longitudinal reference values. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Johnson, E.C.; Muñoz, C.X.; Swokla, B.; Le Bellego, L.; Jimenez, L.; Casa, D.J.; Maresh, C.M. Hydration biomarkers and dietary fluid consumption of women. J. Acad. Nutr. Diet. 2012, 112, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Shore, A.C.; Markandu, N.D.; Sagnella, G.A.; Singer, D.R.; Forsling, M.L.; Buckley, M.G.; Sugden, A.L.; MacGregor, G.A. Endocrine and renal response to water loading and water restriction in normal man. Clin. Sci. 1988, 75, 171–177. [Google Scholar] [CrossRef]
- Robertson, G.L.; Mahr, E.A.; Athar, S.; Sinha, T. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J. Clin. Investig. 1973, 52, 2340–2352. [Google Scholar] [CrossRef]
- Robertson, G.L.; Athar, S. The interaction of blood osmolality and blood volume in regulating plasma vasopressin in man. J. Clin. Endocrinol. Metab. 1976, 42, 613–620. [Google Scholar] [CrossRef]
- Thompson, C.J.; Bland, J.; Burd, J.; Baylis, P.H. The osmotic thresholds for thirst and vasopressin release are similar in healthy man. Clin. Sci. 1986, 71, 651–656. [Google Scholar] [CrossRef]
- Thompson, C.J.; Edwards, C.R.; Baylis, P.H. Osmotic and non-osmotic regulation of thirst and vasopressin secretion in patients with compulsive water drinking. Clin. Endocrinol. 1991, 35, 221–228. [Google Scholar] [CrossRef]
- Sagawa, S.; Miki, K.; Tajima, F.; Tanaka, H.; Choi, J.K.; Keil, L.C.; Shiraki, K.; Greenleaf, J.E. Effect of dehydration on thirst and drinking during immersion in men. J. Appl. Physiol. 1992, 72, 128–134. [Google Scholar] [CrossRef]
- Montain, S.J.; Laird, J.E.; Latzka, W.A.; Sawka, M.N. Aldosterone and vasopressin responses in the heat: Hydration level and exercise intensity effects. Med. Sci. Sports Exerc. 1997, 29, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Figaro, M.K.; Mack, G.W. Regulation of fluid intake in dehydrated humans: Role of oropharyngeal stimulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997, 272, R1740–R1746. [Google Scholar] [CrossRef] [PubMed]
- Jamison, R.L.; Gehrig, J.J. Urinary concentration and dilution: Physiology. In Handbook of Physiology, Renal Physiology; American Physiological Society: Bethesda, MD, USA, 1992; pp. 1219–1279. [Google Scholar]
- Hammer, M.; Ladefoged, J.; Olgaard, K. Relationship between plasma osmolality and plasma vasopressin in human subjects. Am. J. Physiol. Endocrinol. Metab. 1980, 238, E313–E317. [Google Scholar] [CrossRef] [PubMed]
- Inder, W.J.; Hellemans, J.; Swanney, M.P.; Prickett, T.C.R.; Donald, R.A. Prolonged exercise increases peripheral plasma ACTH, CRH, and AVP in male athletes. J. Appl. Physiol. 1998, 85, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.L.; Shelton, R.L.; Athar, S. The osmoregulation of vasopressin. Kidney Int. 1976, 10, 25–37. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E.; Zera, T.; Sosnowski, P.; Cudnoch-Jedrzejewska, A.; Puszko, A.; Misicka, A. Vasopressin and related peptides; potential value in diagnosis, prognosis and treatment of clinical disorders. Curr. Drug Metab. 2017, 18, 306–345. [Google Scholar] [CrossRef]
- Singh, A.; Petrides, J.S.; Gold, P.W.; Chrousos, G.P.; Deuster, P.A. Differential hypothalamic-pituitary-adrenal axis reactivity to psychological and physical stress. J. Clin. Endocrinol. Metab. 1999, 84, 1944–1948. [Google Scholar] [CrossRef]
- Katan, M.; Christ-Crain, M. The stress hormone copeptin: A new prognostic biomarker in acute illness. Swiss Med. Wkly. 2010, 140. [Google Scholar] [CrossRef]
- Katan, M.; Morgenthaler, N.; Widmer, I.; Puder, J.J.; Konig, C.; Muller, B.; Christ-Crain, M. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol. Lett. 2008, 29, 341–346. [Google Scholar]
- Dinan, T.G.; Scott, L.V. Anatomy of melancholia: Focus on hypothalamic-pituitary-adrenal axis overactivity and the role of vasopressin. J. Anat. 2005, 207, 259–264. [Google Scholar] [CrossRef]
- Thunhorst, R.L.; Beltz, T.G.; Johnson, A.K. Glucocorticoids increase salt appetite by promoting water and sodium excretion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1444–R1451. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, R.; De Martino, M.C.; Iacuaniello, D.; Simeoli, C.; Muscogiuri, G.; Carlomagno, F.; De Leo, M.; Cozzolino, A.; Colao, A. Metabolic alterations and cardiovascular outcomes of cortisol excess. In Cortisol Excess and Insufficiency; Arvat, F., Florni, A., Eds.; Karger: Basel, Switzerland, 2016; Volume 46, pp. 54–65. [Google Scholar]
- Hunter, A.; Syed, A. Obesity, cortisol excess, and the hypothalamic-pituitary-adrenal axis. In Practical Guide to Obesity Medicine; Weaver, J., Ed.; Elsevier: Cambridge, MA, USA, 2018; pp. 37–48. [Google Scholar]
- Anagnostis, P.; Athyros, V.G.; Tziomalos, K.; Karagiannis, A.; Mikhailidis, D.P. The pathogenetic role of cortisol in the metabolic syndrome: A hypothesis. J. Clin. Endocrinol. Metab. 2009, 94, 2692–2701. [Google Scholar] [CrossRef] [PubMed]
- De Winter, R.F.; van Hemert, A.M.; DeRijk, R.H.; Zwinderman, K.H.; Frankhuijzen-Sierevogel, A.C.; Wiegant, V.M.; Goekoop, J.G. Anxious-retarded depression: Relation with plasma vasopressin and cortisol. Neuropsychopharmacology 2003, 28, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Ganio, M.S.; Armstrong, L.E.; Casa, D.J.; McDermott, B.P.; Lee, E.C.; Yamamoto, L.M.; Marzano, S.; Lopez, R.M.; Jimenez, L.; Le Bellego, L.; et al. Mild dehydration impairs cognitive performance and mood of men. Br. J. Nutr. 2011, 106, 1535–1543. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Ganio, M.S.; Casa, D.J.; Lee, E.C.; McDermott, B.P.; Klau, J.F.; Jimenez, L.; Le Bellego, L.; Chevillotte, E.; Lieberman, H.R. Mild dehydration affects mood in healthy young women. J. Nutr. 2012, 142, 382–388. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Krey, L.C.; McEwen, B.S. The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocr. Rev. 1986, 7, 284–301. [Google Scholar] [CrossRef]
- Aguilera, G.; Subburaju, S.; Young, S.; Chen, J. The parvocellular vasopressinergic system and responsiveness of the hypothalamic pituitary adrenal axis during chronic stress. Prog. Brain Res. 2008, 170, 29–39. [Google Scholar]
- Stookey, J.D.; Klein, A.; Hamer, J.; Chi, C.; Higa, A.; Ng, V.; Arieff, A.; Kuypers, F.A.; Larkin, S.; Perrier, E.; et al. RBC deformability and amino acid concentrations after hypo-osmotic challenge may reflect chronic cell hydration status in healthy young men. Physiol. Rep. 2013, 1, e00117. [Google Scholar] [CrossRef]
- Kirk, C.J.; Rodrigues, L.M.; Hems, D.A. The influence of vasopressin and related peptides on glycogen phosphorylase activity and phosphatidylinositol metabolism in hepatocytes. Biochem. J. 1979, 178, 493–496. [Google Scholar] [CrossRef]
- Oshikawa, S.; Tanoue, A.; Koshimizu, T.A.; Kitagawa, Y.; Tsujimoto, G. Vasopressin stimulates insulin release from islet cells through V1b receptors: A combined pharmacological/knockout approach. Mol. Pharmacol. 2004, 65, 623–629. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Hiroyama, M.; Sanbe, A.; Aoyagi, T.; Birumachi, J.I.; Yamauchi, J.; Tsujimoto, G.; Tanoue, A. Insulin hypersensitivity in mice lacking the V1b vasopressin receptor. J. Physiol. 2007, 584, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Stachenfeld, N.S.; Splenser, A.E.; Calzone, W.L.; Taylor, M.P.; Keefe, D.L. Sex differences in osmotic regulation of AVP and renal sodium handling. J. Appl. Physiol. 2001, 91, 1893–1901. [Google Scholar] [CrossRef]
- Robertson, G.L. Antidiuretic hormone. Normal and disordered function. Endocrinol. Metab. Clin. N. Am. 2001, 30, 671–694. [Google Scholar] [CrossRef]
- Perucca, J.; Bouby, N.; Valeix, P.; Bankir, L. Sex difference in urine concentration across differing ages, sodium intake, and level of kidney disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R700–R705. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.L.; Berl, T. Water metabolism. In The Kidney; WB Saunders: Philadelphia, PA, USA, 1986; pp. 385–432. [Google Scholar]
- Verney, E.B. Croonian lecture-the antidiuretic hormone and the factors which determine its release. Proc. R. Soc. Lond. B Biol. Sci. 1947, 135, 25–106. [Google Scholar] [PubMed]
- Robertson, G.L. The regulation of vasopressin function in health and disease. In Proceedings of the 1976 Laurentian Hormone Conference; Academic Press: New York, NY, USA, 1977; pp. 333–385. [Google Scholar]
- Feig, P.U.; McCurdy, D.K. The hypertonic state. N. Engl. J. Med. 1977, 297, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Perrier, E.T.; Buendia-Jimenez, I.; Vecchio, M.; Armstrong, L.E.; Tack, I.; Klein, A. Twenty-four-hour urine osmolality as a physiological index of adequate water intake. Dis. Markers 2015, 2015, 231063. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Johnson, E.C.; McKenzie, A.L.; Muñoz, C.X. An empirical method to determine inadequacy of dietary water. Nutrition 2016, 32, 79–82. [Google Scholar] [CrossRef]
- Ganong, W.F. Review of Medical Physiology, 10th ed.; Lange Medical Publications: Los Altos, CA, USA, 1981; pp. 18–20. [Google Scholar]
- Sterns, R.H. Disorders of plasma sodium—Causes, consequences, and correction. N. Engl. J. Med. 2015, 372, 55–65. [Google Scholar] [CrossRef]
- Gao, S.; Cui, X.; Wang, X.; Burg, M.B.; Dmitrieva, N.I. Cross-sectional positive association of serum lipids and blood pressure with serum sodium within the normal reference range of 135–145 mmol/L. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 598–606. [Google Scholar] [CrossRef]
- Zerbe, R.L.; Robertson, G.L. Osmoregulation of thirst and vasopressin secretion in human subjects: Effect of various solutes. Am. J. Physiol. Endocrinol. Metab. 1983, 244, E607–E614. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q. Dietary influence on body fluid acid-base and volume balance: The deleterious “norm” furthers and cloaks subclinical pathophysiology. Nutrients 2018, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.J.; Rolls, E.T. The control of normal drinking. In Thirst; Cambridge University Press: Cambridge, UK, 1982; pp. 152–166. [Google Scholar]
- Zerbe, R.L.; Miller, J.Z.; Robertson, G.L. The reproducibility and heritability of individual differences in osmoregulatory function in normal human subjects. J. Lab. Clin. Med. 1991, 117, 51–59. [Google Scholar] [PubMed]
- Baylis, P.H. Osmoregulation and control of vasopressin secretion in healthy humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1987, 253, R671–R678. [Google Scholar] [CrossRef]
- Fitzsimons, J.T. Angiotensin, thirst, and sodium appetite. Physiol. Rev. 1998, 78, 583–686. [Google Scholar] [CrossRef]
- Stricker, E.M.; Sved, A.F. Thirst. Nutrition 2000, 16, 821–826. [Google Scholar] [CrossRef]
- Wojszel, Z.B. What serum sodium concentration is suggestive for underhydration in geriatric patients? Nutrients 2020, 12, 496. [Google Scholar] [CrossRef]
- National Research Council. Recommended Dietary Allowances, 10th ed.; National Academy Press: Washington, DC, USA, 1989. [Google Scholar]
- Manz, F.; Wentz, A. Hydration status in the United States and Germany. Nutr. Rev. 2005, 63, S55–S62. [Google Scholar] [CrossRef]
- Manz, F.; Johner, S.A.; Wentz, A.; Boeing, H.; Remer, T. Water balance throughout the adult life span in a German population. Br. J. Nutr. 2012, 107, 1673–1681. [Google Scholar] [CrossRef]
- Geelen, G.; Keil, L.C.; Kravik, S.E.; Wade, C.E.; Thrasher, T.N.; Barnes, P.R.; Pyka, G.; Nesvig, C.; Greenleaf, J.E. Inhibition of plasma vasopressin after drinking in dehydrated humans. Am. J. Physiol. 1984, 247, R968–R971. [Google Scholar] [CrossRef]
- Thompson, C.J.; Burd, J.M.; Baylis, P.H. Acute suppression of plasma vasopressin and thirst after drinking in hypernatremic humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1987, 252, R1138–R1142. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, C.A.; Lin, Y.C.; Leib, D.E.; Guo, L.; Huey, E.L.; Daly, G.E.; Chen, Y.; Knight, Z.A. Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature 2016, 537, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.F. The role of the salivary glands in the thirst mechanism. Am. J. Physiol. 1931, 96, 221–227. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Herrera Soto, J.A.; Hacker, F.T.; Casa, D.J.; Kavouras, S.A.; Maresh, C.M. Urinary indices during dehydration, exercise, and rehydration. Int. J. Sport Nutr. 1998, 8, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Geelen, G.; Greenleaf, J.E.; Keil, L.C. Drinking-induced plasma vasopressin and norepinephrine changes in dehydrated humans. J. Clin. Endocrinol. Metab. 1996, 81, 2131–2135. [Google Scholar] [PubMed]
- Kovacs, E.M.; Senden, J.M.; Brouns, F.J. Urine color, osmolality and specific electrical conductance are not accurate measures of hydration status during postexercise rehydration. Sports Med. Phys. Fit. 1999, 39, 47–53. [Google Scholar]
- Popowski, L.A.; Oppliger, R.A.; Lambert, G.P.; Johnson, R.F.; Johnson, A.K.; Gisolfi, C.V. Blood and urinary measures of hydration status during progressive acute dehydration. Med. Sci. Sports Exerc. 2001, 33, 747–753. [Google Scholar] [CrossRef]
- Williams, T.D.; Seckl, J.R.; Lightman, S.L. Dependent effect of drinking volume on vasopressin but not atrial peptide in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1989, 257, R762–R764. [Google Scholar] [CrossRef]
- Brunstrom, J.M.; Macrae, A.W. Effects of temperature and volume on measures of mouth dryness, thirst and stomach fullness in males and females. Appetite 1997, 29, 31–42. [Google Scholar] [CrossRef]
- Kanbay, M.; Yilmaz, S.; Dincer, N.; Ortiz, A.; Sag, A.A.; Covic, A.; Sánchez-Lozada, L.G.; Lanaspa, M.A.; Cherney, D.Z.; Johnson, R.J.; et al. Antidiuretic hormone and serum osmolarity physiology and related outcomes: What is old, what is new, and what is unknown? J. Clin. Endocrinol. Metab. 2019, 104, 5406–5420. [Google Scholar] [CrossRef]
- Veilleux, J.C.; Caldwell, A.R.; Johnson, E.C.; Kavouras, S.; McDermott, B.P.; Ganio, M.S. Examining the links between hydration knowledge, attitudes and behavior. Eur. J. Nutr. 2019, 3. [Google Scholar] [CrossRef] [PubMed]
- Booth, D.A. Influences on human fluid consumption. In Thirst: Physiological and Psychological Aspects; Ramsay, D.J., Booth, D., Eds.; Springer: London, UK, 1991; pp. 53–75. [Google Scholar]
- Holland, P.C. Learning, thirst and drinking. In Thirst: Physiological and Psychological Aspects; Ramsay, D.J., Booth, D., Eds.; Springer: London, UK, 1991; pp. 291–295. [Google Scholar]
- Guelinckx, I.; Tavoularis, G.; König, J.; Morin, C.; Gharbi, H.; Gandy, J. Contribution of water from food and fluids to total water intake: Analysis of a French and UK population surveys. Nutrients 2016, 8, 630. [Google Scholar] [CrossRef]
- Gebhardt, S.E.; Thomas, R.G. Nutritive Value of Foods; U.S. Government Printing Office: Washington, DC, USA, 2002.
- Ferdowsian, H.R.; Barnard, N.D. Effects of plant-based diets on plasma lipids. Am. J. Cardiol. 2009, 104, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in US adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Bhanu, C.; Avgerinou, C.; Kharicha, K.; Bauernfreund, Y.; Croker, H.; Liljas, A.; Rea, J.; Kirby-Barr, M.; Hopkins, J.; Walters, K. ‘I’ve never drunk very much water and I still don’t, and I see no reason to do so’: A qualitative study of the views of community-dwelling older people and carers on hydration in later life. Age Ageing 2019, 49, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Wojszel, Z.B. Impending low intake dehydration at admission to a geriatric ward- prevalence and correlates in a cross-sectional study. Nutrients 2020, 12, 398. [Google Scholar] [CrossRef] [PubMed]
- Stookey, J.D.; Hamer, J.; Killilea, D.W. Change in hydration indices associated with an increase in total water intake of more than 0.5 L/day, sustained over 4 weeks, in healthy young men with initial total water intake below 2 L/day. Physiol. Rep. 2017, 5, e13356. [Google Scholar] [CrossRef]
- Borghi, L.; Meschi, T.; Amato, F.; Briganti, A.; Novarini, A.; Giannini, A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J. Urol. 1996, 155, 839–843. [Google Scholar] [CrossRef]
- McCauley, L.R.; Dyer, A.J.; Stern, K.; Hicks, T.; Nguyen, M.M. Factors influencing fluid intake behavior among kidney stone formers. J. Urol. 2012, 187, 1282–1286. [Google Scholar] [CrossRef]
- Manz, F.; Wentz, A. 24-h hydration status: Parameters, epidemiology and recommendations. Eur. J. Clin. Nutr. 2003, 57, S101–S108. [Google Scholar] [CrossRef]
- Martin, W.F.; Cerundolo, L.H.; Pikosky, M.A.; Gaine, P.C.; Maresh, C.M.; Armstrong, L.E.; Bolster, D.R.; Rodriguez, N.R. Effects of dietary protein intake on indexes of hydration. J. Am. Diet. Assoc. 2006, 106, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Amro, O.W.; Paulus, J.K.; Noubary, F.; Perrone, R.D. Low-osmolar diet and adjusted water intake for vasopressin reduction in autosomal dominant polycystic kidney disease: A pilot randomized controlled trial. Am. J. Kidney Dis. 2016, 68, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, B.; Velez, J.C.Q. Evaluation of polyuria: The roles of solute loading and water diuresis. Am. J. Kidney Dis. 2016, 67, 507–511. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blalock, T., Jr.; Gerron, G.; Quiter, E.; Rudman, D. Role of diet in the management of vasopressin-responsive and-resistant diabetes insipidus. Am. J. Clin. Nutr. 1977, 30, 1070–1076. [Google Scholar] [CrossRef]
- Bouby, N.A.; Trinh-Trang-Tan, M.M.; Coutaud, C.H.; Bankir, L.I. Vasopressin is involved in renal effects of high-protein diet: Study in homozygous Brattleboro rats. Am. J. Physiol. Ren. Physiol. 1991, 260, F96–F100. [Google Scholar] [CrossRef]
- Daniels, B.S.; Hostetter, T.H. Effects of dietary protein intake on vasoactive hormones. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1990, 258, R1095–R1100. [Google Scholar] [CrossRef]
- Lauridsen, T.G.; Vase, H.; Starklint, J.; Bech, J.N.; Pedersen, E.B. Protein-enriched diet increases water absorption via the aquaporin-2 water channels in healthy humans. Nephrol. Dial. Transplant. 2010, 25, 2502–2510. [Google Scholar] [CrossRef][Green Version]
- Bankir, L.; Bichet, D.G.; Morgenthaler, N.G. Vasopressin: Physiology, assessment and osmosensation. J. Intern. Med. 2017, 282, 284–297. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for Sodium and Potassium; The National Academies Press: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- McCarron, D.A.; Kazaks, A.G.; Geerling, J.C.; Stern, J.S.; Graudal, N.A. Normal range of human dietary sodium intake: A perspective based on 24-hour urinary sodium excretion worldwide. Am. J. Hypertens. 2013, 26, 1218–1223. [Google Scholar] [CrossRef]
- Jacobson, M.F.; Havas, S.; McCarter, R. Changes in sodium levels in processed and restaurant foods, 2005 to 2011. JAMA Intern. Med. 2013, 173, 1285–1291. [Google Scholar] [CrossRef]
- Institute of Medicine. Sodium Intake in Populations: Assessment of Evidence; National Academies Press: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Mattes, R.D.; Donnelly, D. Relative contributions of dietary sodium sources. J. Am. Coll. Nutr. 1991, 10, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Kavouras, S. Assessing hydration status. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Cheuvront, S.N.; Carter, R.; Montain, S.J.; Sawka, M.N. Daily body mass variability and stability in active men undergoing exercise-heat stress. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 532–540. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Ganio, M.S.; Klau, J.F.; Johnson, E.C.; Casa, D.J.; Maresh, C.M. Novel hydration assessment techniques employing thirst and a water intake challenge in healthy men. Appl. Physiol. Nutr. Metab. 2014, 39, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Burchfield, J.M.; Ganio, M.S.; Kavouras, S.A.; Adams, J.D.; Gonzalez, M.A.; Ridings, C.B.; Moyen, N.E.; Tucker, M.A. 24-h Void number as an indicator of hydration status. Eur. J. Clin. Nutr. 2015, 69, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.A.; Gonzalez, M.A.; Adams, J.D.; Burchfield, J.M.; Moyen, N.E.; Robinson, F.B.; Schreiber, B.A.; Ganio, M.S. Reliability of 24-h void frequency as an index of hydration status when euhydrated and hypohydrated. Eur. J. Clin. Nutr. 2016, 70, 908–911. [Google Scholar] [CrossRef]
- Perrier, E.T.; Johnson, E.C.; Ganio, M.S.; McKenzie, A.L.; Ellis, L.A.; Armstrong, L.E. Urine colour change as an indicator of change in daily water intake: A quantitative analysis. Eur. J. Nutr. 2016, 55, 1943–1949. [Google Scholar] [CrossRef]
- McKenzie, A.L.; Muñoz, C.X.; Armstrong, L.E. Accuracy of urine color to detect equal to or greater than 2% body mass loss in men. J. Athl. Train. 2016, 50, 1306–1309. [Google Scholar] [CrossRef]
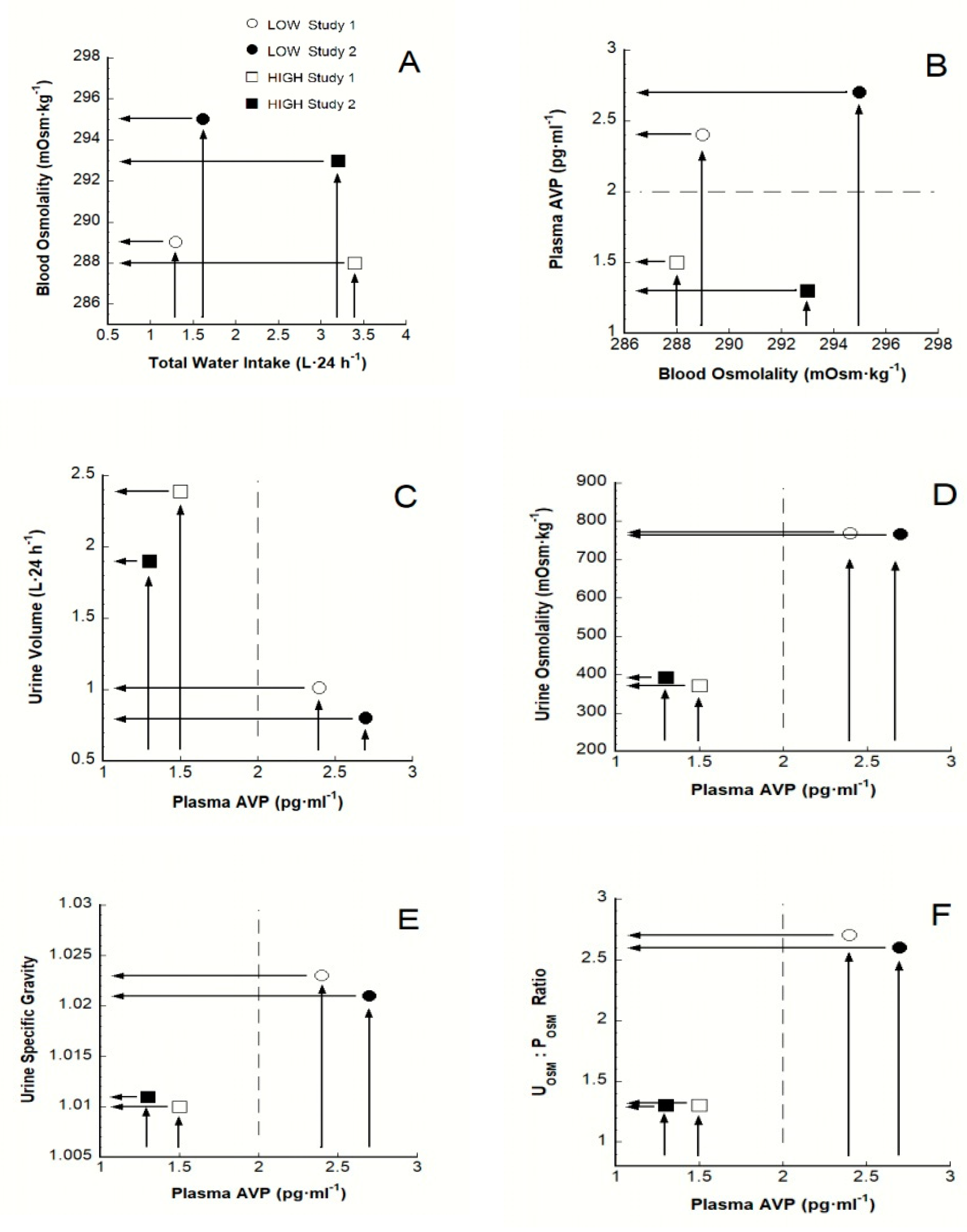
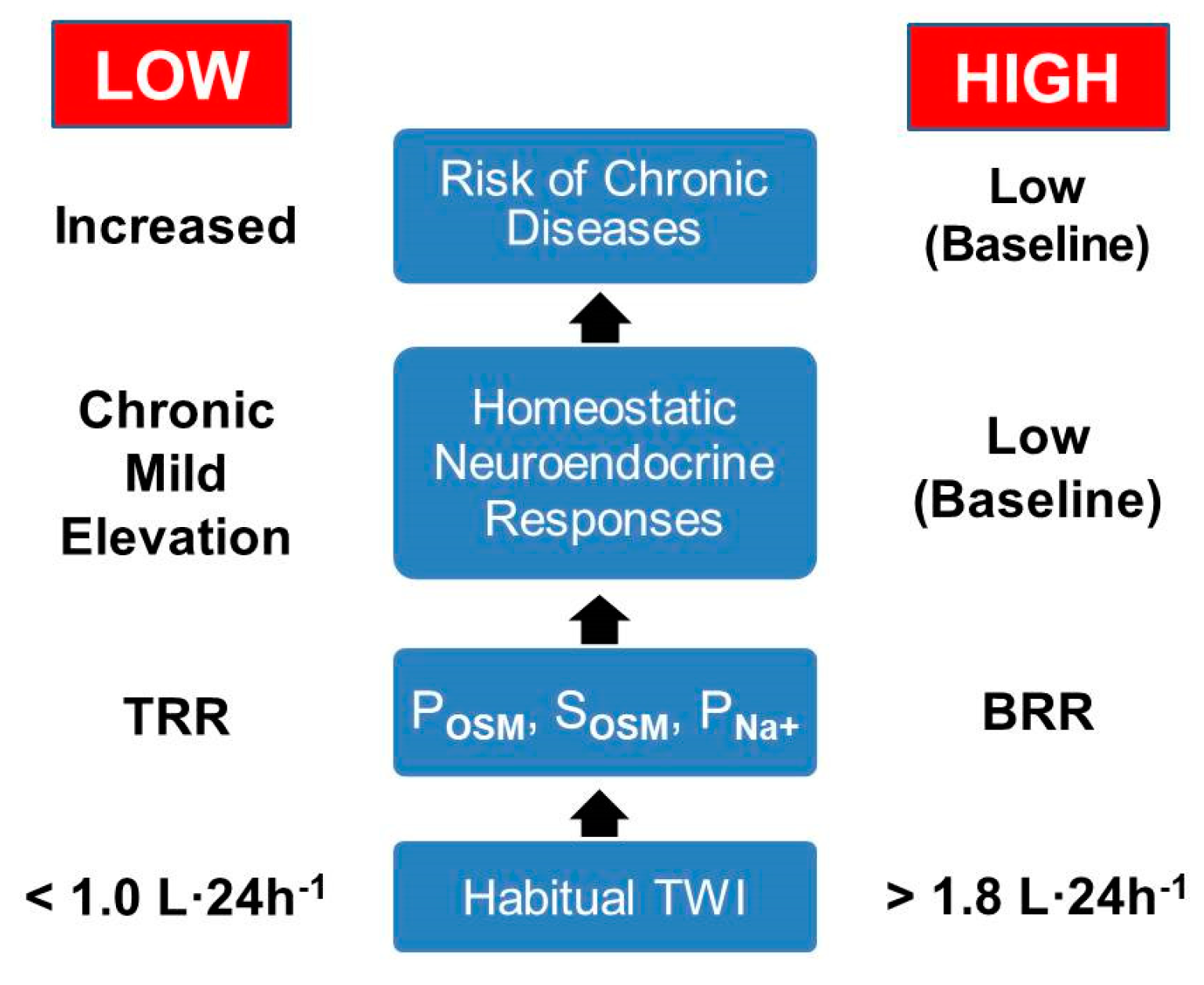
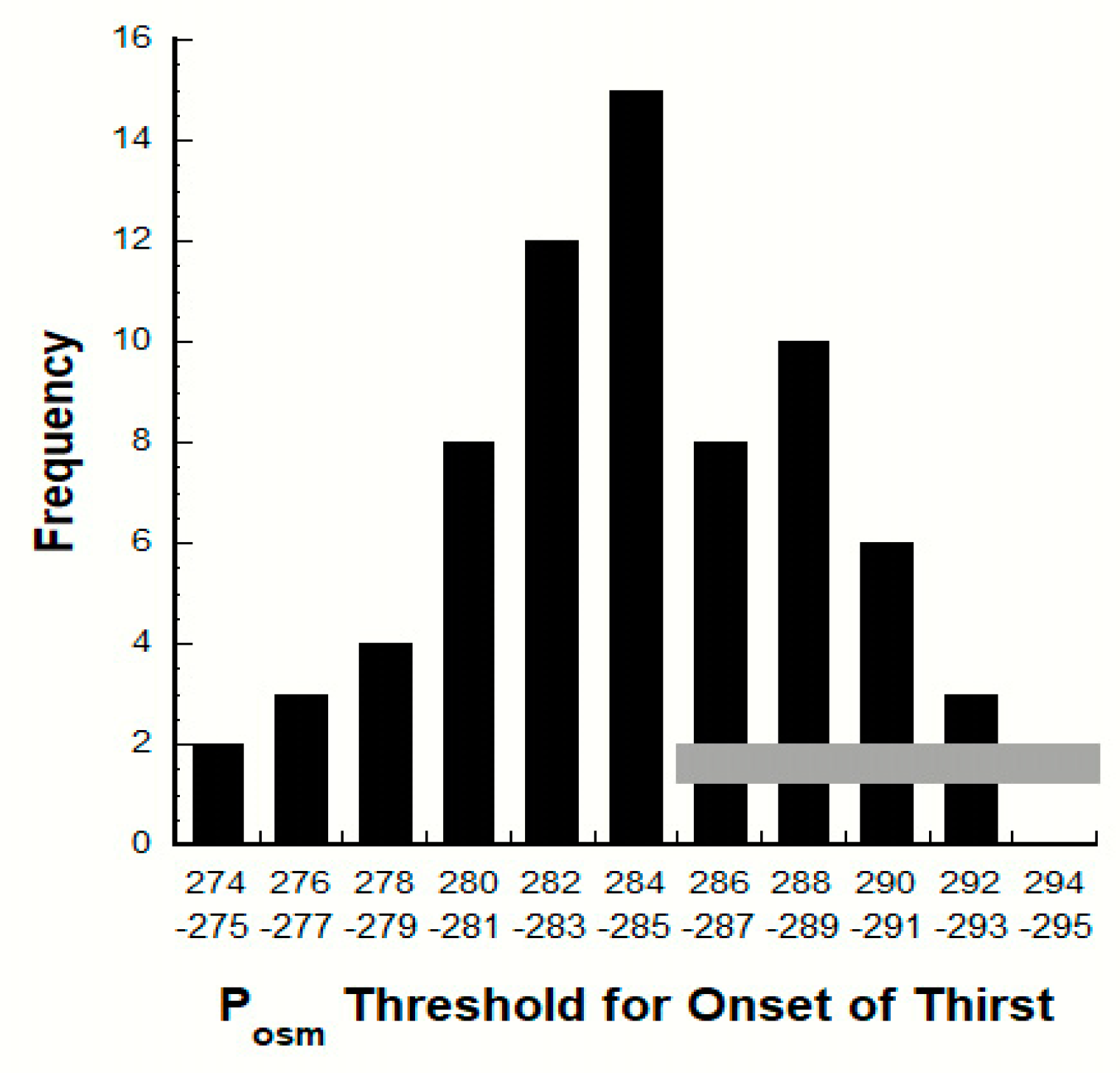
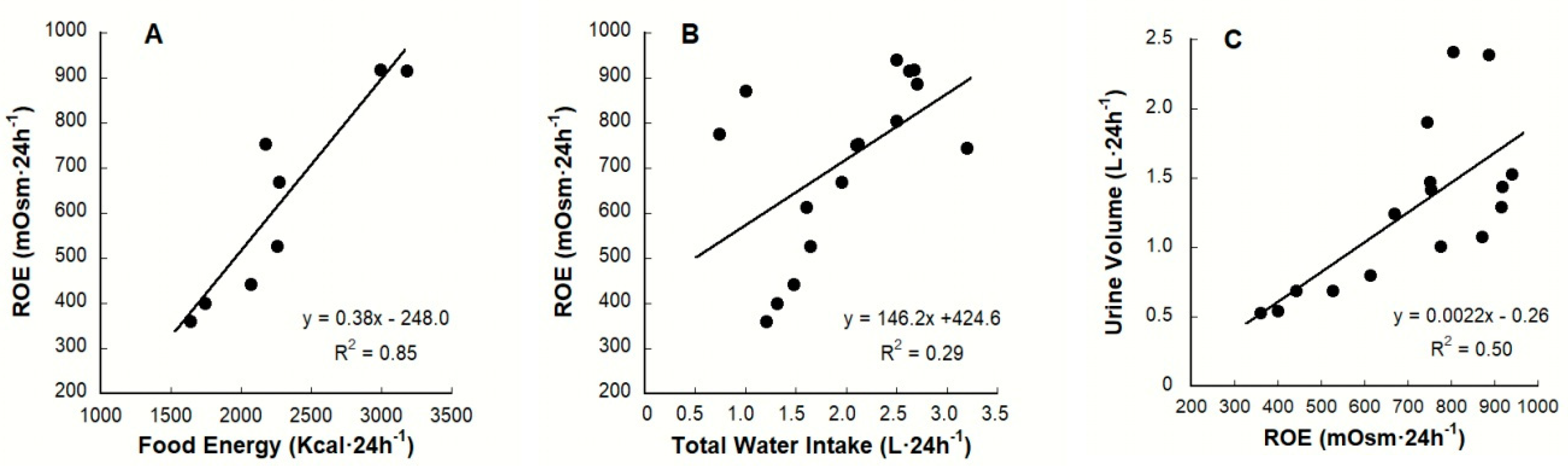
| Variable (unit) | Baseline Mean Values | Statistical Significance: Low Versus HIGH | Laboratory Reference Ranges for Healthy Adults a | References | |
|---|---|---|---|---|---|
| LOW | HIGH | ||||
| TWI (L·24 h−1) | 0.74 | 2.70 | IV | [9] b | |
| POSM (mOsm·kg−1) | 289 + | 288 + | NS | 285–295 | |
| PAVP (pmol·L−1) | 2.4 + | 1.5 + | p < 0.001 | 0.3–3.3 | |
| Hematocrit (%) | 41.2 | 40.8 | NS | 41–53 ♂, 36–46 ♀ | |
| PCORT (nmol·L−1) | 545 | 459 | p = 0.012 | 138–690 | |
| UVOL (L) c | 1.01 | 2.39 | p < 0.001 | 0.68–3.00 ♂, 1.17–2.41 ♀ | |
| UOSM (mOsm·kg−1) c | 767 | 371 | p < 0.001 | 300–900 | |
| USG c | 1.023 + | 1.010 + | p < 0.001 | 1.001–1.035 | |
| TWI (L·24 h−1) | 1.0 | 2.5 | IV | [20] d | |
| POSM (mOsm·kg−1) | 292, 291 e | 289, 287 e | NS | 285–295 | |
| UVOL (L) c | 1.08, 0.97 e | 2.41, 2.48 e | § | 0.68–3.00 ♂, 1.17–2.41 ♀ | |
| UOSM (mOsm·kg−1) c | 807, 875 e | 334, 331 e | § | 300–900 | |
| USG c | 1.021, 1.022 e | 1.010, 1.009 e | § | 1.001–1.035 | |
| TWI (L·24 h−1) | 1.6 | 3.2 | IV | [18,21] f | |
| SOSM (mOsm·kg−1) | 295 | 293 | NS | 285–295 | |
| PAVP (pg·mL−1) | 2.7 + | 1.3 + | p < 0.001 | 0.3–3.3 | |
| Hematocrit (%) | 42 | 41 | NS | 41–53 ♂, 36–46 ♀ | |
| SNa+ (mmol·L−1) | 142 | 141 | NS | 136–145 | |
| TPP (g·dL−1) | 7.3 | 7.0 | p < 0.05 | 5.5–8.0 | |
| UVOL (L) c | 0.8 | 1.9 | p < 0.05 | 0.68–3.00 ♂, 1.17–2.41 ♀ | |
| UOSM (mOsm·kg−1) c | 766 | 392 | p < 0.05 | 300–900 | |
| USG c | 1.021 | 1.011 | p < 0.05 | 1.001–1.035 | |
| Variable (unit) | Days of Modified 24-h TWI Intervention | Physiological Changes After 24 h TWI Was Modified | Authors | |
|---|---|---|---|---|
| LOW → HIGH | HIGH → LOW | |||
| POSM (mOsm·kg−1) | 3 a | 0 | +3 | [20] b |
| UVOL (L) c | 3 a | +1.355 | +1.450 | |
| UOSM (mOsm·kg−1) c | 3 a | −486 | +417 | |
| USG c | 3 a | −0.012 | +0.010 | |
| SOSM (mOsm·kg−1) | 1–4 d | −2 | +1 | [18,21] e |
| Hematocrit (%) | 1–4 d | 0 | +1.0 | |
| TPP (mg·dL−1) | 1–4 d | +0.2 | −0.1 | |
| PAVP (pg·mL−1) | 1–4 d | −1.2 | +1.3 | |
| SNa+ (mmol·L−1) | 1–4 d | 0 | +1 | |
| UVOL (L) c | 1–4 d | +1.4 | −0.7 | |
| UOSM (mOsm·kg−1) c | 1–4 d | −492 | +201 | |
| USG c | 1–4 d | −0.013 | +0.006 | |
| Mean ROE (mOsm·24 h−1) a | Study Participants (n) | Characteristics | References |
|---|---|---|---|
| 401 | Boys (n = 189) | 4–6.9 y | [101] |
| 527 | Boys (n = 174) | 7–10.9 y | |
| 359 | Girls (n = 181) | 4–6.9 y | |
| 443 | Girls (n = 174) | 7-–10.9 y | |
| 941 | Men (n = 507) | Mean age, 47 y | |
| 752 b | Women (n = 682) | Mean age, 43 y | |
| 669 | Women (n = 101) | 18–24 y | [102] |
| 754 | Women (n = 468) | 25–49 y | |
| 915 | Men (n = 70) | 18–24 y | |
| 918 | Men (n = 308) | 25–49 y | |
| 656–1,222 | Men (n = 639) | Reference Range c | |
| 283–1,215 | Women (n = 889) | Reference Range c | |
| 775 | Test subjects (22♂, 17♀) | LOW consuming 0.74 L·24 h−1; mean age, 31 y | [9] d |
| 887 | Test subjects (10♂, 20♀) | HIGH consuming 2.70 L·24 h−1; mean age, 32 y | |
| 872 | Test subjects (11♂, 19♀) | LOW consuming 1.0 L·24 h−1; mean age, 25 y | [20] d |
| 805 | Test subjects (22♀) | HIGH consuming 2.5 L·24 h−1; mean age, 25 y | |
| 613 | Test subjects (14♀) | LOW consuming 1.6 L·24 h−1; mean age, 20 y | [18,21] d |
| 745 | Test subjects (14♀) | HIGH consuming 3.2 L·24 h−1; mean age, 21 y |
| Avenues of Water. Gain & Loss (L·24 h−1 or kg·24 h−1) | LOW | HIGH | References |
|---|---|---|---|
| Fluids Consumed a,b | 0.74 | 2.70 | [9] |
| Moisture in food b | 0.64 c | 0.78 c | |
| 24-h Total Water Intake d | 1.38 | 3.48 | |
| Percent of TWI from moisture in food | 46 | 22 | |
| Fluids Consumed a,b | 1.1 | 2.5 | [18] |
| Moisture in food b | 0.5 | 0.7 | |
| 24-h Total Water Intake d | 1.6 | 3.2 | |
| Percent of TWI from moisture in food | 31 | 22 |
| Food Item | Serving Size and Weight (g) | Water (%) | Energy (Kcal) | Protein (g) | Sodium (mg) |
|---|---|---|---|---|---|
| Dairy Products | |||||
| Cottage Cheese | 1 cup (210) | 79 | 217 | 26 | 850 |
| American Cheese | 1 oz (28) | 39 | 106 | 6 | 406 |
| Swiss Cheese | 1 oz (28) | 42 | 95 | 7 | 388 |
| Frozen Yogurt | ½ cup (72) | 64 | 115 | 3 | 71 |
| Ice Cream | ½ cup (66) | 61 | 133 | 2 | 53 |
| Milk (Whole) | 1 cup (244) | 88 | 150 | 8 | 120 |
| Milk (2%) | 1 cup (244) | 90 | 102 | 8 | 122 |
| Yogurt (Fruited) | 8 oz (227) | 74 | 231 | 10 | 133 |
| Yogurt (Plain) | 8 oz (227) | 85 | 144 | 12 | 159 |
| Eggs | |||||
| Hard boiled | 1 Large (50) | 75 | 78 | 6 | 62 |
| Scrambled | 1 Large (61) | 73 | 101 | 7 | 171 |
| Fats and Oils | |||||
| Butter (salted) | 1 stick (113) | 16 | 813 | 1 | 937 |
| Margarine (soft) | 1 cup (227) | 16 | 1626 | 2 | 2449 |
| Canola Oil | 1 cup (218) | 0 | 1927 | 0 | 0 |
| Olive Oil | 1 cup (216) | 0 | 1909 | 0 | 0 |
| French Dressing | 1 Tbsp (16) | 38 | 67 | Trace | 214 |
| Italian Dressing | 1 Tbsp (15) | 38 | 69 | Trace | 116 |
| Mayonnaise | 1 Tbsp (14) | 15 | 99 | Trace | 78 |
| Fish | |||||
| Baked Haddock | 3 oz (85) | 74 | 95 | 21 | 74 |
| Baked Salmon | 3 oz (85) | 62 | 184 | 23 | 56 |
| Scallops | 3 oz (85) | 73 | 95 | 20 | 225 |
| Shrimp (canned) | 3 oz (85) | 73 | 102 | 20 | 144 |
| Tuna (water pk.) | 3 oz (85) | 73 | 109 | 20 | 320 |
| Fruits and Fruit Juices | |||||
| Apple (Raw) | 1 medium (138) | 84 | 81 | Trace | 0 |
| Apple Juice | 1 cup (248) | 88 | 117 | Trace | 7 |
| Applesauce | 1 cup (244) | 88 | 105 | Trace | 5 |
| Pear (Raw) | 1 medium (122) | 88 | 51 | 1 | 0 |
| Avocado | 1 oz (28) | 80 | 32 | Trace | 3 |
| Banana (Raw) | 1 medium (118) | 74 | 109 | 1 | 1 |
| Cherries (Raw) | 10 cherries (68) | 81 | 49 | 1 | 0 |
| Grapefruit (Raw) | ½ grapefruit (123) | 91 | 37 | 1 | 0 |
| Green Grapes | 10 grapes (50) | 81 | 36 | Trace | 1 |
| Mango | 1 cup (165) | 82 | 107 | 1 | 3 |
| Cantaloupe | 1 cup (160) | 90 | 56 | 1 | 14 |
| Honeydew | 1 cup (170) | 90 | 60 | 1 | 17 |
| Orange (Raw) | 1 medium (131) | 87 | 62 | 1 | 0 |
| Orange Juice | 1 cup (248) | 88 | 112 | 2 | 2 |
| Peach (Raw) | 1 medium (98) | 88 | 73 | 1 | 0 |
| Pineapple | 1 cup (155) | 87 | 76 | 1 | 2 |
| Plum (Raw) | 1 medium (66) | 85 | 36 | 1 | 0 |
| Raspberries | 1 cup (123) | 87 | 60 | 1 | 0 |
| Strawberries | 1 cup (166) | 92 | 50 | 1 | 2 |
| Watermelon | 1 cup (152) | 92 | 49 | 1 | 3 |
| Grain Products | |||||
| Corn Grits | 1 cup (242) | 85 | 145 | 3 | 0 |
| Cream of Wheat | 1 cup (251) | 87 | 133 | 4 | 3 |
| Macaroni | 1 cup cooked (140) | 66 | 197 | 7 | 1 |
| Egg Noodles | 1 cup cooked (160) | 69 | 213 | 8 | 11 |
| Oat Bran | 1 cup cooked (219) | 84 | 88 | 7 | 2 |
| White Rice | 1 cup cooked (158) | 68 | 205 | 4 | 2 |
| Spaghetti | 1 cup cooked (140) | 66 | 197 | 7 | 1 |
| Legumes, Nuts, Beans | |||||
| Black Beans | 1 cup cooked (172) | 66 | 227 | 15 | 2 |
| Red Kidney Beans | 1 cup cooked (177) | 67 | 225 | 15 | 4 |
| Lima Beans | 1 cup cooked (188) | 70 | 216 | 15 | 4 |
| Pinto Beans | 1 cup cooked (171) | 64 | 234 | 14 | 3 |
| Hummus | 1 Tbsp (14) | 67 | 23 | 1 | 53 |
| Peanut Butter | 1 Tbsp (16) | 1 | 95 | 4 | 75 |
| Soups, Sauces, Gravies | |||||
| Clam Chowder | 1 cup (248) | 85 | 164 | 9 | 992 |
| Tomato Soup | 1 cup (248) | 85 | 161 | 6 | 744 |
| Chicken & Rice Soup | 1 cup (241) | 94 | 60 | 4 | 815 |
| Lentil Soup | 1 cup (242) | 88 | 126 | 8 | 443 |
| Minestrone Soup | 1 cup (241) | 87 | 123 | 5 | 470 |
| Vegetable Soup | 1 cup (238) | 91 | 81 | 4 | 466 |
| Turkey Gravy | ¼ cup (60) | 89 | 31 | 2 | 346 |
| Sugar and Sweets | |||||
| Fruit Juice Bar | 1 solid bar (77) | 78 | 63 | 1 | 3 |
| Gelatin | ½ cup (135) | 85 | 80 | 2 | 57 |
| Vanilla Pudding | ½ cup (142) | 75 | 148 | 4 | 406 |
| Tapioca | ½ cup (113) | 74 | 134 | 2 | 180 |
| Vegetables | |||||
| Asparagus | 1 cup cooked (180) | 92 | 43 | 5 | 20 |
| Green Beans | 1 cup cooked (125) | 89 | 44 | 2 | 4 |
| Beets | 1 cup cooked (170) | 87 | 75 | 3 | 131 |
| Broccoli | 1 cup cooked (156) | 91 | 44 | 5 | 41 |
| Cabbage | 1 cup cooked (150) | 94 | 33 | 2 | 12 |
| Carrots | 1 cup cooked (156) | 87 | 70 | 2 | 103 |
| Cauliflower | 1 cup cooked (124) | 93 | 29 | 2 | 19 |
| Celery | 1 stalk (40) | 95 | 6 | Trace | 35 |
| Corn | 1 cup cooked (164) | 77 | 131 | 5 | 8 |
| Cucumber | 1 cup peeled (119) | 96 | 14 | 1 | 2 |
| Kale | 1 cup cooked (130) | 91 | 36 | 2 | 30 |
| Mushrooms | 1 cup cooked (156) | 91 | 42 | 3 | 3 |
| Okra | 1 cup cooked (160) | 90 | 51 | 3 | 8 |
| Onion (Raw) | 1 cup (160) | 90 | 61 | 2 | 5 |
| Green Peas | 1 cup cooked (160) | 89 | 67 | 5 | 6 |
| Green Pepper | 1 cup raw (149) | 92 | 40 | 1 | 3 |
| Potato | 1 baked (202) | 71 | 220 | 5 | 16 |
| Spinach | 1 cup cooked (180) | 91 | 41 | 5 | 126 |
| Summer Squash | 1 cup cooked (180) | 94 | 36 | 2 | 2 |
| Butternut Squash | 1 cup cooked (240) | 88 | 99 | 3 | 5 |
| Tomato (Raw) | 1 cup (180) | 99 | 38 | 2 | 16 |
| Tomato Sauce | 1 cup (245) | 89 | 74 | 3 | 1482 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armstrong, L.E.; Muñoz, C.X.; Armstrong, E.M. Distinguishing Low and High Water Consumers—A Paradigm of Disease Risk. Nutrients 2020, 12, 858. https://doi.org/10.3390/nu12030858
Armstrong LE, Muñoz CX, Armstrong EM. Distinguishing Low and High Water Consumers—A Paradigm of Disease Risk. Nutrients. 2020; 12(3):858. https://doi.org/10.3390/nu12030858
Chicago/Turabian StyleArmstrong, Lawrence E., Colleen X. Muñoz, and Elizabeth M. Armstrong. 2020. "Distinguishing Low and High Water Consumers—A Paradigm of Disease Risk" Nutrients 12, no. 3: 858. https://doi.org/10.3390/nu12030858
APA StyleArmstrong, L. E., Muñoz, C. X., & Armstrong, E. M. (2020). Distinguishing Low and High Water Consumers—A Paradigm of Disease Risk. Nutrients, 12(3), 858. https://doi.org/10.3390/nu12030858





