The Course Of IGF-1 Levels and Nutrient Intake in Extremely and Very Preterm Infants During Hospitalisation
Abstract
1. Introduction
2. Methods
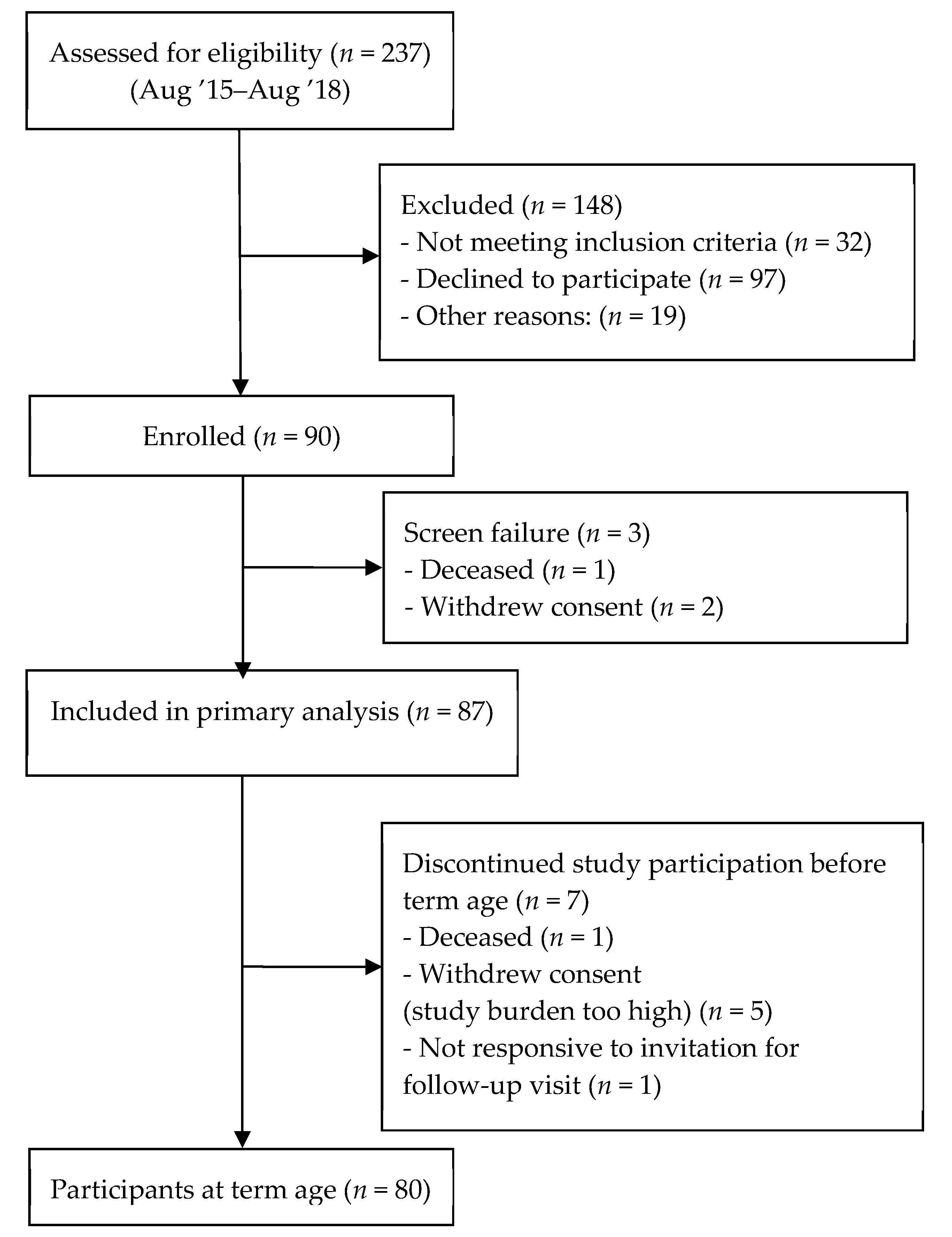
2.1. Study Population
2.2. Nutrition
2.3. Study Procedures
2.4. Growth
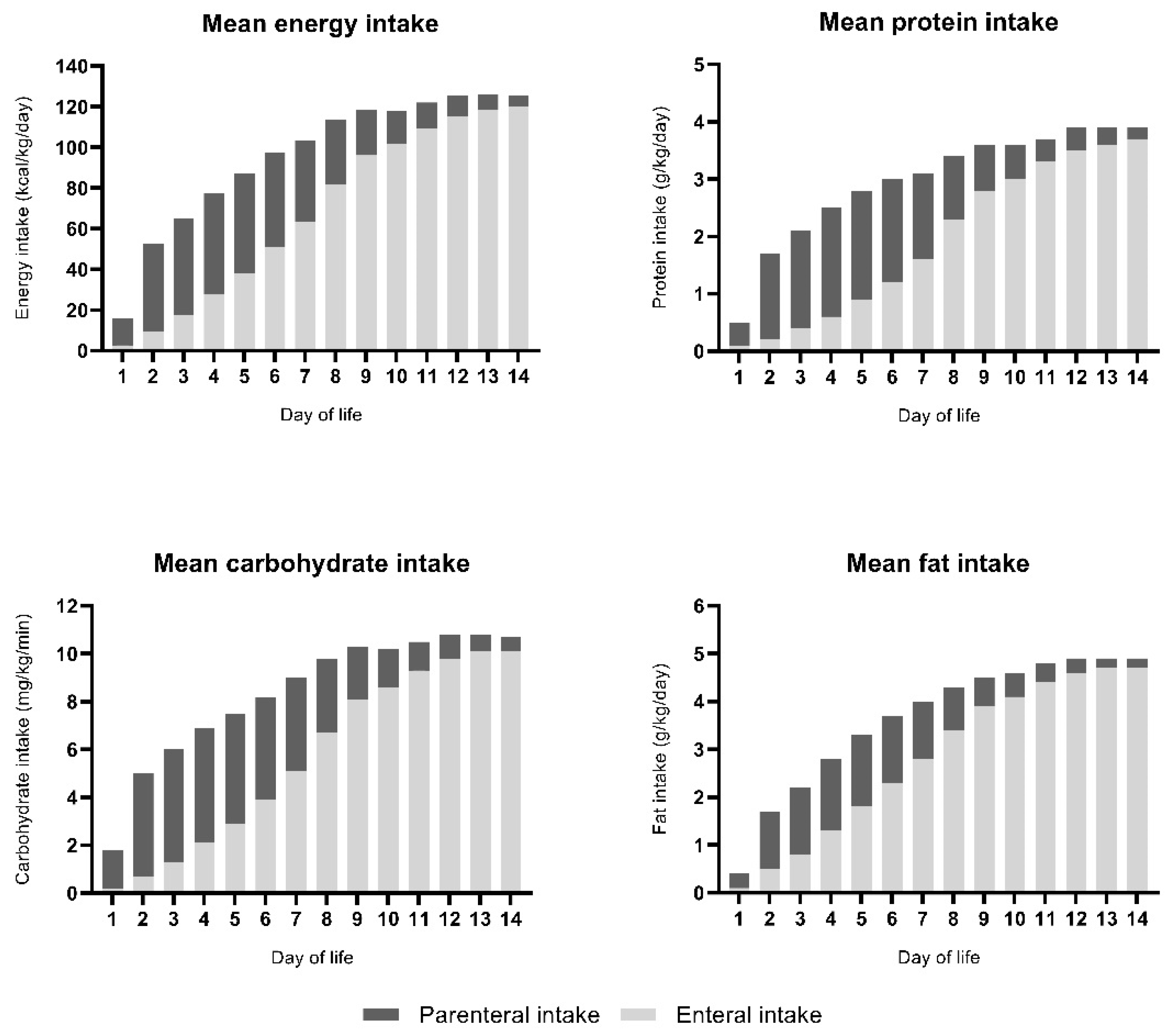
2.5. Intake
2.6. Endocrine Parameters
2.7. Potential Confounders
- -
- Bronchopulmonary dysplasia (BPD); defined as having had a need for supplemental oxygen for at least 28 days at 36 weeks PMA or discharge home (whichever came first) [17].
- -
- Necrotizing enterocolitis (NEC); classified according to the Modified Bell’s staging criteria [18].
- -
- Late-onset sepsis (LOS), defined as sepsis occurring 72 h after birth with a positive blood culture or a full course of antibiotic treatment [19].
- -
- Retinopathy of prematurity (ROP), classified according to the International Classification for Retinopathy of Prematurity [20].
- -
- Intraventricular hemorrhage (IVH), classified according to the Papile grading system [21].
- -
- Patent ductus arteriosus (PDA), which was defined as hemodynamically significant if treatment was prescribed [22].
2.8. Statistical Analysis
3. Results
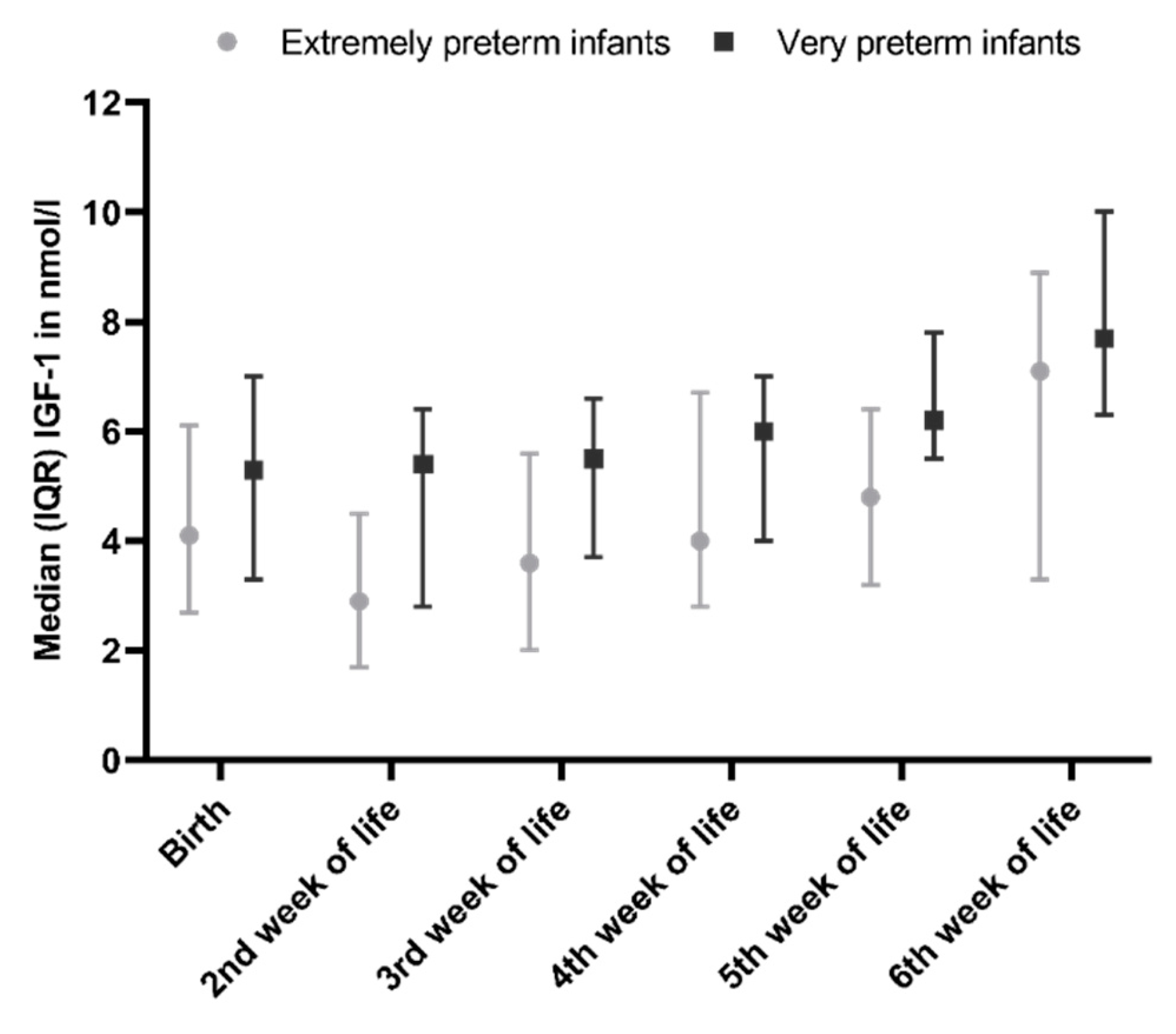
3.1. Changes in IGF-1 During Hospitalisation
3.2. IGF-1 Levels in Relation to Growth
3.3. IGF-1 Levels and Route of Administration
3.4. Nutrient Intake in Relation to Concurrent IGF-1 Levels
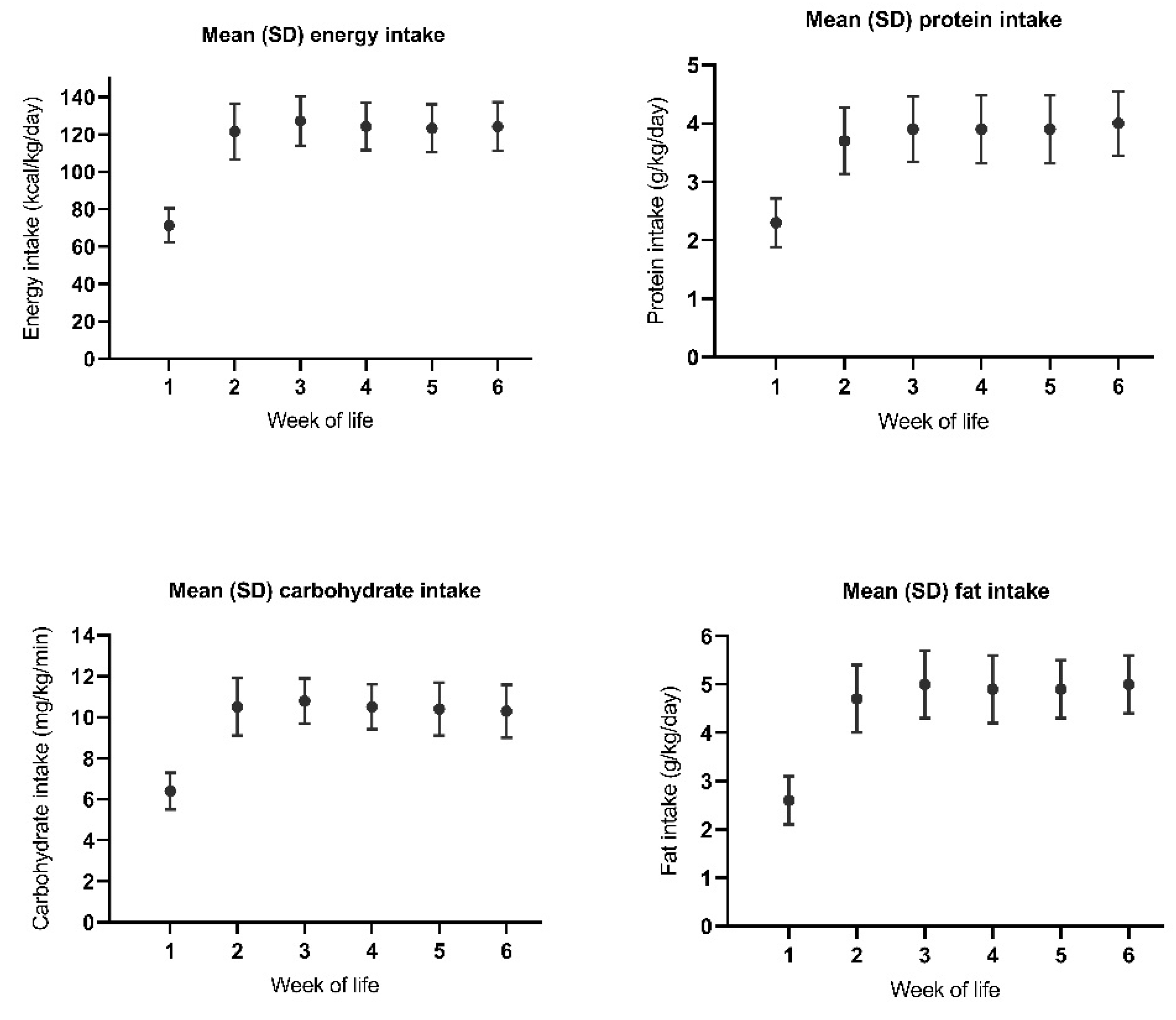
3.5. Nutrition in Relation to Changes in IGF-1 According to Postnatal Age
3.6. Nutrition in Relation to Changes in IGF-1 According to Postmenstrual Age
4. Discussion
4.1. The Effect of the Various Macronutrients on IGF-1 Levels
4.2. The Route of Nutrient Administration
4.3. Window of Effect of Nutrient Intake on IGF-1 Levels
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Larnkjaer, A.; Molgaard, C.; Michaelsen, K.F. Early nutrition impact on the insulin-like growth factor axis and later health consequences. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 285–292. [Google Scholar] [CrossRef]
- Yumani, D.F.; Lafeber, H.N.; van Weissenbruch, M.M. Dietary proteins and IGF I levels in preterm infants: Determinants of growth, body composition, and neurodevelopment. Pediatric Res. 2015, 77, 156–163. [Google Scholar] [CrossRef]
- Engstrom, E.; Niklasson, A.; Wikland, K.A.; Ewald, U.; Hellstrom, A. The role of maternal factors, postnatal nutrition, weight gain, and gender in regulation of serum IGF-I among preterm infants. Pediatric Res. 2005, 57, 605–610. [Google Scholar] [CrossRef]
- Hansen-Pupp, I.; Löfqvist, C.; Polberger, S.; Niklasson, A.; Fellman, V.; Hellström, A.; Ley, D. Influence of insulin-like growth factor I and nutrition during phases of postnatal growth in very preterm infants. Pediatric Res. 2011, 69, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.J.; Underwood, L.E.; Keyes, L.; Clemmons, D.R. Use of insulin-like growth factor I (IGF-I) and IGF-binding protein measurements to monitor feeding of premature infants. J. Clin. Endocrinol. Metab. 1997, 82, 3982–3988. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Pollak, M.; Liu, Y.; Platz, E.A.; Majeed, N.; Rimm, E.B.; Willett, W.C. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol. Biomark. Prev. 2003, 12, 84–89. [Google Scholar]
- Kaklamani, V.G.; Linos, A.; Kaklamani, E.; Markaki, I.; Koumantaki, Y.; Mantzoros, C.S. Dietary fat and carbohydrates are independently associated with circulating insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 concentrations in healthy adults. J. Clin. Oncol. 1999, 17, 3291–3298. [Google Scholar] [CrossRef] [PubMed]
- Norat, T.; Dossus, L.; Rinaldi, S.; Overvad, K.; Grønbæk, H.; Tjønneland, A.; Olsen, A.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Boeing, H.; et al. Diet, serum insulin-like growth factor-I and IGF-binding protein-3 in European women. Eur. J. Clin. Nutr. 2007, 61, 91–98. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.J.; Xue, A.; Scarlett, C.J.; Sevette, A.; Kee, A.J.; Smith, R.C. Parenteral versus enteral nutrition: Effect on serum cytokines and the hepatic expression of mRNA of suppressor of cytokine signaling proteins, insulin-like growth factor-1 and the growth hormone receptor in rodent sepsis. Crit. Care (Lond. Engl.) 2007, 11, R79. [Google Scholar] [CrossRef]
- Wojnar, M.M.; Fan, J.; Li, Y.H.; Lang, C.H. Endotoxin-induced changes in IGF-I differ in rats provided enteral vs. parenteral nutrition. Am. J. Physiol. 1999, 276, E455–E464. [Google Scholar] [CrossRef]
- Cormack, B.E.; Harding, J.E.; Miller, S.P.; Bloomfield, F.H. The Influence of Early Nutrition on Brain Growth and Neurodevelopment in Extremely Preterm Babies: A Narrative Review. Nutrients 2019, 11, 2029. [Google Scholar] [CrossRef] [PubMed]
- Embleton, N.D.; Korada, M.; Wood, C.L.; Pearce, M.S.; Swamy, R.; Cheetham, T.D. Catch-up growth and metabolic outcomes in adolescents born preterm. Arch. Dis. Child. 2016, 101, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Lapillonne, A.; Griffin, I.J. Feeding preterm infants today for later metabolic and cardiovascular outcomes. J. Pediatr. 2013, 162, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- Boyce, C.; Watson, M.; Lazidis, G.; Reeve, S.; Dods, K.; Simmer, K.; McLeod, G. Preterm human milk composition: A systematic literature review. Br. J. Nutr. 2016, 116, 1033–1045. [Google Scholar] [CrossRef]
- Gidrewicz, D.A.; Fenton, T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014, 14, 216. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Kliegman, R.M.; Walsh, M.C. Neonatal necrotizing enterocolitis: Pathogenesis, classification, and spectrum of illness. Curr. Probl. Pediatr. 1987, 17, 213–288. [Google Scholar] [CrossRef]
- Bekhof, J.; Reitsma, J.B.; Kok, J.H.; Van Straaten, I.H. Clinical signs to identify late-onset sepsis in preterm infants. Eur. J. Pediatr. 2013, 172, 501–508. [Google Scholar] [CrossRef]
- International Committee for the Classification of Retinopathy of P. The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [CrossRef]
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Jain, A.; Shah, P.S. Diagnosis, Evaluation, and Management of Patent Ductus Arteriosus in Preterm Neonates. JAMA Pediatr. 2015, 169, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, J.I.; Blanco, A.M.; Canosa, L.F.; Unniappan, S. Direct actions of macronutrient components on goldfish hepatopancreas in vitro to modulate the expression of ghr-I, ghr-II, igf-I and igf-II mRNAs. Gen. Comp. Endocrinol. 2017, 250, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hansen-Pupp, I.; Hellstrom-Westas, L.; Cilio, C.M.; Andersson, S.; Fellman, V.; Ley, D. Inflammation at birth and the insulin-like growth factor system in very preterm infants. Acta Paediatr. 2007, 96, 830–836. [Google Scholar] [CrossRef]
- MohanKumar, K.; Namachivayam, K.; Ho, T.T.; Torres, B.A.; Ohls, R.K.; Maheshwari, A. Cytokines and growth factors in the developing intestine and during necrotizing enterocolitis. Semin. Perinatol. 2017, 41, 52–60. [Google Scholar] [CrossRef]
- Alzaree, F.A.; AbuShady, M.M.; Atti, M.A.; Fathy, G.A.; Galal, E.M.; Ali, A.; Elias, T.R. Effect of Early Breast Milk Nutrition on Serum Insulin-Like Growth Factor-1 in Preterm Infants. Open Access Maced. J. Med Sci. 2019, 7, 77–81. [Google Scholar] [CrossRef]

| Variables | OMM | OMM + BMF (4.4g/100 mL) | DHM | DHM + BMF |
|---|---|---|---|---|
| Energy (kcal) | 68.5 | 83.8 | 60 | 75 |
| Protein (g) | 1.5 | 2.6 | 0.8 | 1.9 |
| Protein/energy ratio (g/100 kcal) | 2.2/100 | 1.3/100 | ||
| Carbohydrates (g) | 7.3 | 10.0 | 7.5 | 10.2 |
| Fat (g) | 3.3 | 3.3 | 2.9 | 2.9 |
| PMA | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N Total | 2 | 3 | 7 | 17 | 21 | 33 | 30 | 34 | 25 | 33 | 25 | 26 | 29 |
| N Postnatal | 0 | 0 | 1 | 3 | 6 | 12 | 16 | 22 | 25 | 33 | 25 | 26 | 29 |
| Variables | (n = 87) |
|---|---|
| Gender, n (%) | |
| Male | 44 (50.6) |
| Female | 43 (49.4) |
| Ethnicity, n (%) | |
| White | 66 (75.9) |
| Other | 21 (24.1) |
| Gestational age (weeks), mean (SD) | 29.0 (1.8) |
| Extremely preterm, n (%) | 25 (28.7) |
| Very preterm, n (%) | 62 (71.3) |
| Birthweight (g), mean (SD) | 1210 (216) |
| Birthweight SDS, mean (SD) | 0.0 (0.7) |
| Birthweight SDS < −1.3, n (%) | 3 (3.4) |
| BPD, n (%) | 30 (34.5) |
| NEC, n (%) | 8 (9.2) |
| LOS, n (%) | 30 (34.5) |
| PDA, n (%) | |
| Hemodynamically Insignificant PDA | 11 (12.6) |
| Hemodynamically Significant PDA | 8 (9.2) |
| ROP, n (%) | |
| ROP stage I | 4 (4.6) |
| ROP stage III | 1 (1.1) |
| IVH, n (%) | |
| IVH grade I | 8 (9.2) |
| IVH grade II | 11 (12.6) |
| IVH grade III | 4 (4.6) |
| Variables | B (SE) | β | p-Value |
|---|---|---|---|
| Included variables | |||
| Constant | 1.482 (1.359) | 0.281 | |
| Percentage parenteral intake on day 8 | −0.027 (0.011) | −0.234 | 0.019 |
| Weight on day 8 (grams) | 0.004 (0.001) | 0.478 | <0.001 |
| BPD | −1.134 (0.516) | −0.233 | 0.032 |
| Hemodynamic significant PDA | −1.350 (0.793) | −0.159 | 0.095 |
| Variables | Model R² | Model p-Value | B (SE) | β | p-Value |
|---|---|---|---|---|---|
| Energy intake model 1: | 0.605 | 0.006 | |||
| Constant | 11.8 (6.7) | 0.106 | |||
| Energy intake (kcal/day) | 0.05 (0.02) | 0.6 | 0.015 | ||
| Gestational age (weeks) | −0.6 (0.2) | −0.5 | 0.029 | ||
| Energy intake model 2: | 0.640 | 0.014 | |||
| Constant | 9.4 (7.1) | 0.215 | |||
| Energy intake (kcal/day) | 0.03 (0.03) | 0.3 | 0.395 | ||
| Gestational age (weeks) | −0.5 (0.2) | −0.4 | 0.073 | ||
| Weight (grams) | 0.003 (0.003) | 0.36 | 0.348 | ||
| Protein intake model 1: | 0.578 | 0.009 | |||
| Constant | 14.5 (6.7) | 0.053 | |||
| Protein intake (g/day) | 1.2 (0.4) | 0.6 | 0.013 | ||
| Gestational age (weeks) | −0.6 (0.2) | −0.5 | 0.025 | ||
| Protein intake model 2: | 0.625 | 0.017 | |||
| Constant | 10.2 (7.6) | 0.209 | |||
| Protein intake (g/day) | 0.5 (0.8) | 0.2 | 0.561 | ||
| Gestational age (weeks) | −0.5 (0.2) | −0.4 | 0.089 | ||
| Weight (grams) | 0.004 (0.003) | 0.4 | 0.289 | ||
| Carbohydrate intake model 1: | 0.593 | 0.014 | |||
| Constant | 9.1 (7.8) | 0.268 | |||
| Carbohydrate intake (g/day) | 0.3 (0.1) | 0.6 | 0.022 | ||
| Gestational age (weeks) | −0.4 (0.3) | −0.4 | 0.111 | ||
| Carbohydrate intake model 2: | 0.690 | 0.007 | |||
| Constant | 5.5 (6.9) | 0.444 | |||
| Carbohydrate intake (g/day) | 0.2 (0.1) | 0.3 | 0.144 | ||
| Gestational age (weeks) | −0.4 (0.2) | −0.3 | 0.113 | ||
| Weight (grams) | 0.004 (0.002) | 0.5 | 0.052 | ||
| Fat intake model 1: | 0.581 | 0.008 | |||
| Constant | 12.3 (6.9) | 0.102 | |||
| Fat intake (g/day) | 1.1 (0.4) | 0.6 | 0.012 | ||
| Gestational age (weeks) | −0.6 (0.2) | −0.5 | 0.034 | ||
| Fat intake model 2: | 0.631 | 0.015 | |||
| Constant | 9.4 (7.2) | 0.225 | |||
| Fat intake (g/day) | 0.5 (0.7) | 0.3 | 0.494 | ||
| Gestational age (weeks) | −0.5 (0.3) | −0.4 | 0.083 | ||
| Weight (grams) | 0.003 (0.003) | 0.4 | 0.273 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yumani, D.F.J.; Calor, A.K.; van Weissenbruch, M.M. The Course Of IGF-1 Levels and Nutrient Intake in Extremely and Very Preterm Infants During Hospitalisation. Nutrients 2020, 12, 675. https://doi.org/10.3390/nu12030675
Yumani DFJ, Calor AK, van Weissenbruch MM. The Course Of IGF-1 Levels and Nutrient Intake in Extremely and Very Preterm Infants During Hospitalisation. Nutrients. 2020; 12(3):675. https://doi.org/10.3390/nu12030675
Chicago/Turabian StyleYumani, Dana F.J., Alexandra K. Calor, and Mirjam. M. van Weissenbruch. 2020. "The Course Of IGF-1 Levels and Nutrient Intake in Extremely and Very Preterm Infants During Hospitalisation" Nutrients 12, no. 3: 675. https://doi.org/10.3390/nu12030675
APA StyleYumani, D. F. J., Calor, A. K., & van Weissenbruch, M. M. (2020). The Course Of IGF-1 Levels and Nutrient Intake in Extremely and Very Preterm Infants During Hospitalisation. Nutrients, 12(3), 675. https://doi.org/10.3390/nu12030675




