Sodium Selenite Alleviates Breast Cancer-Related Lymphedema Independent of Antioxidant Defense System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design and Intervention
2.3. Clinical Diagnosis of Lymphedema Stage
2.4. Data Collection
2.5. Blood Collection
2.6. Whole Blood Selenium Level Measurement by ICP-MS
2.7. Thiobarbituric Acid (TBA) Reactivity
2.8. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.9. Glutathione Peroxidase (GSH-Px) Activity
2.10. Analysis of Glutathione (GSH) and Glutathione Disulfide (GSSG)
2.11. Statistical Analysis
3. Results
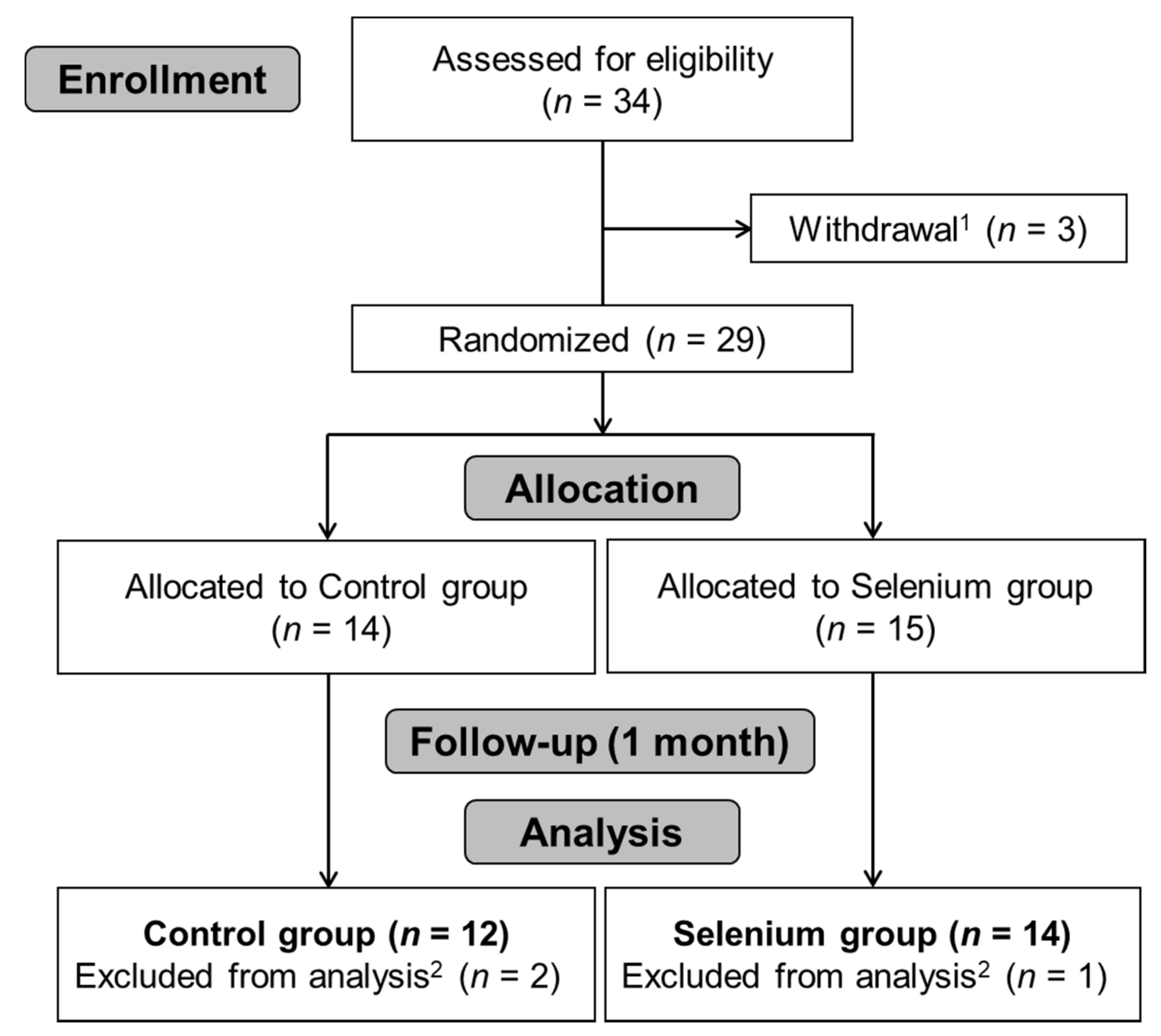
3.1. Participants
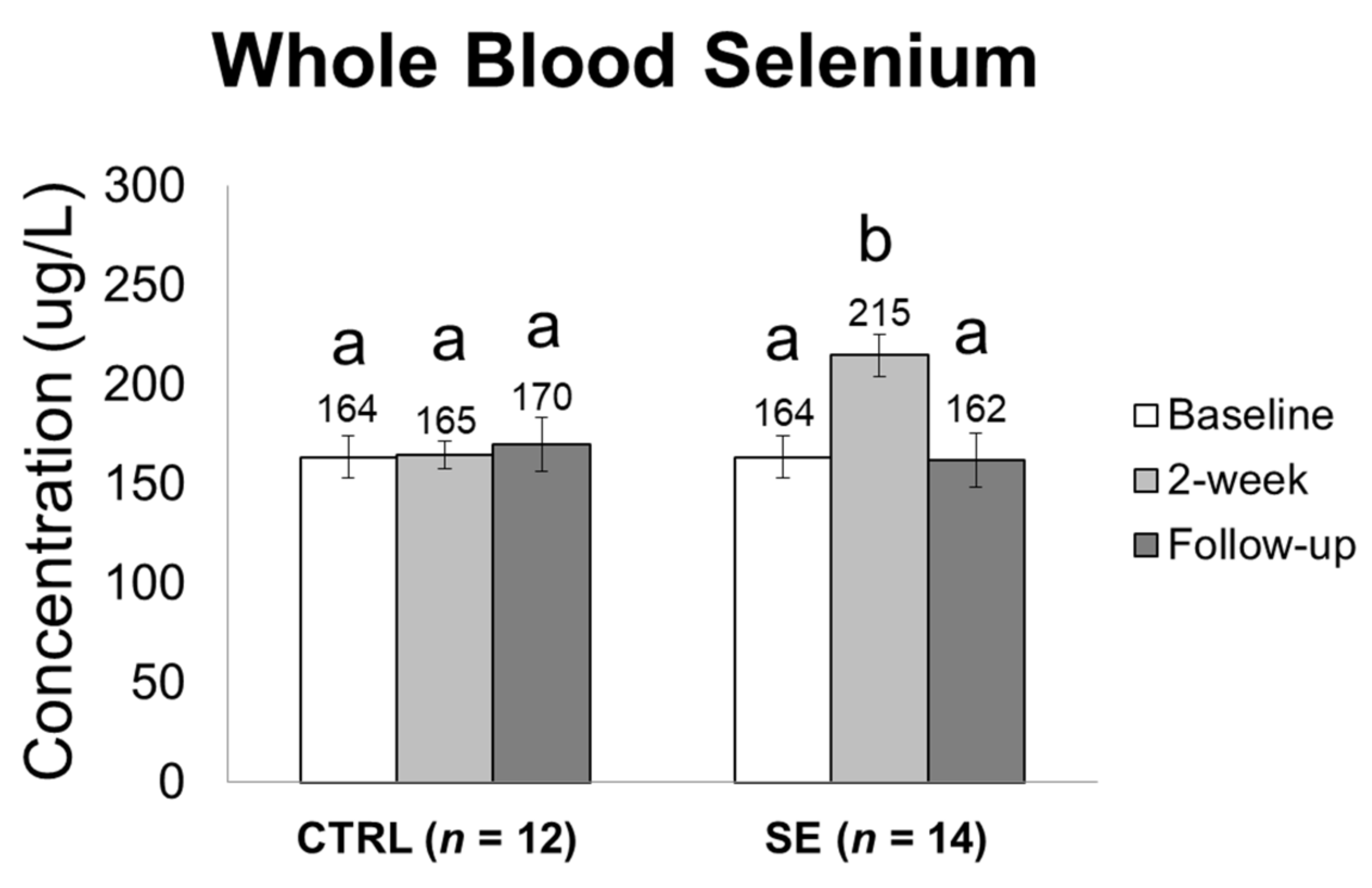
3.2. Effect of Sodium Selenite Supplementation on Blood Selenium Levels
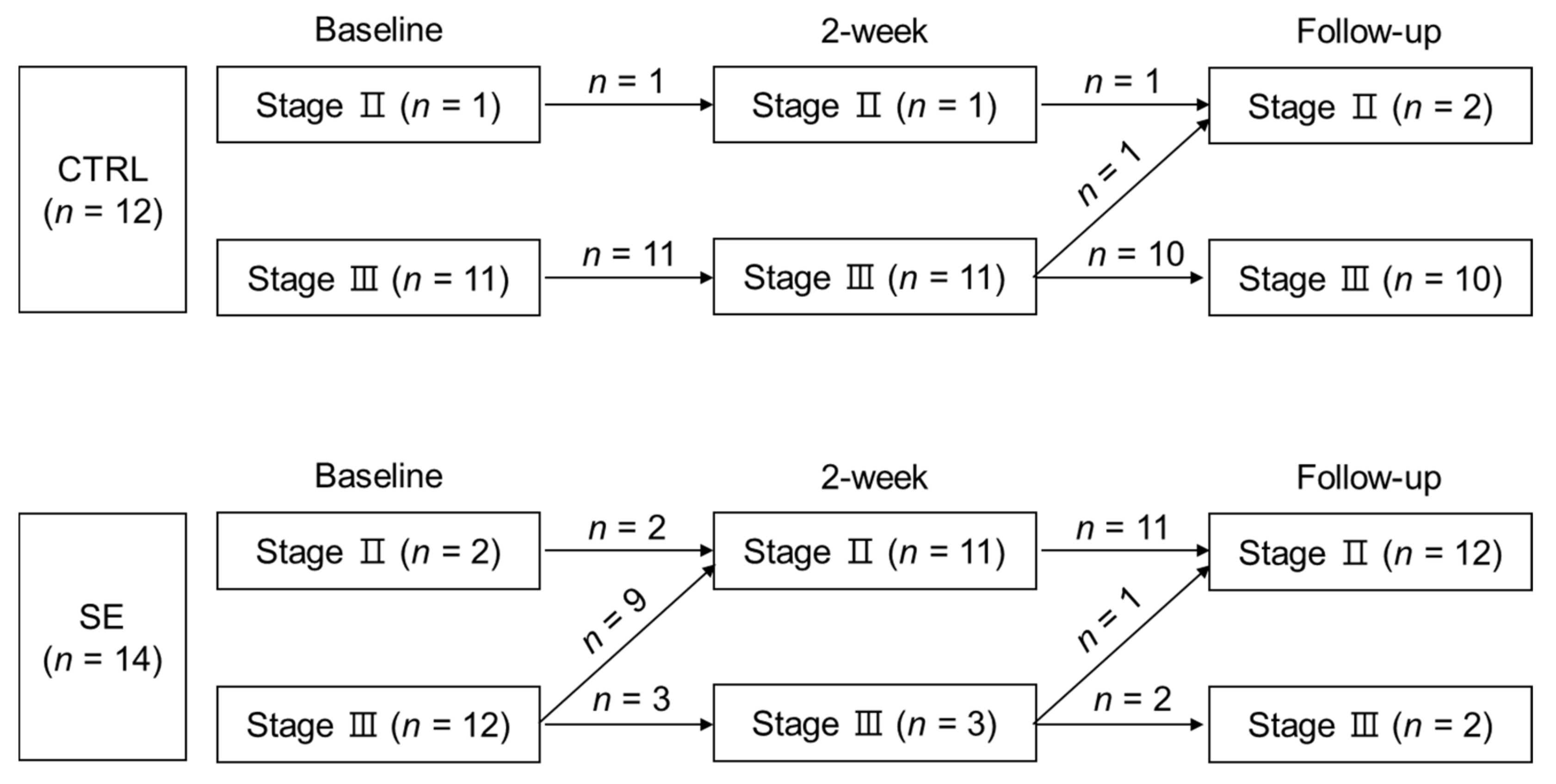
3.3. Effect of Sodium Selenite Supplementation on Lymphedema Clinical Stage
3.4. Effect of Sodium Selenite Supplementation on Bio-Impedance Measures in BCRL Patients
3.5. Effect of Sodium Selenite Supplementation on Blood Parameters is Indicative of Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fu, M.R. Breast cancer-related lymphedema: Symptoms, diagnosis, risk reduction, and management. World J. Clin. Oncol. 2014, 5, 241. [Google Scholar] [CrossRef]
- Ahmed, R.; Prizment, A.; Lazovich, D.; Schmitz, K.; Folsom, A. Lymphedema and quality of life in breast cancer survivors: The Iowa Women’s Health Study. J. Clin. Oncol. 2008, 26, 5689. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Charikleia, S.; Konstantinos, K. Lymphedema and breast cancer: A review of the literature. Breast Cancer 2011, 18, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Lacomba, M.T.; Sánchez, M.J.Y.; Goñi, Á.Z.; Merino, D.P.; del Moral, O.M.; Téllez, E.C.; Mogollón, E.M. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: Randomised, single blinded, clinical trial. BMJ 2010, 340, b5396. [Google Scholar] [CrossRef]
- Smoot, B.; Chiavola-Larson, L.; Lee, J.; Manibusan, H.; Allen, D.D. Effect of low-level laser therapy on pain and swelling in women with breast cancer-related lymphedema: A systematic review and meta-analysis. J. Cancer. Surviv. 2015, 9, 287–304. [Google Scholar] [CrossRef]
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef]
- Armer, J.; Stewart, B. Post-breast cancer lymphedema: Incidence increases from 12 to 30 to 60 months. Lymphology 2010, 43, 118. [Google Scholar]
- Shah, C.; Vicini, F.A. Breast cancer-related arm lymphedema: Incidence rates, diagnostic techniques, optimal management and risk reduction strategies. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 907–914. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.; Arthur, J. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B. Rationale for the treatment of cancer with sodium selenite. Med. Hypotheses 2005, 64, 806–810. [Google Scholar] [CrossRef]
- Ogawa, H.; Taneda, A. Characteristic distribution of sulfhydryl groups in human epidermal cancer by the new histochemical staining method. Arch. Dermatol. Res. 1979, 264, 77–81. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B.; Błażejak, S. Application of sodium selenite in the prevention and treatment of cancers. Cells 2017, 6, 39. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B. Pathophysiological significance of protein hydrophobic interactions: An emerging hypothesis. Med. Hypotheses 2018, 110, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kiremidjian-Schumacher, L.; Roy, M.; Wishe, H.I.; Cohen, M.W.; Stotzky, G. Supplementation with selenium augments the functions of natural killer and lymphokine-activated killer cells. Biol. Trace. Elem. Res. 1996, 52, 227–239. [Google Scholar] [CrossRef]
- Kasseroller, R.G.; Schrauzer, G.N. Treatment of secondary lymphedema of the arm with physical decongestive therapy and sodium selenite: A review. Am. J. Ther. 2000, 7, 273–279. [Google Scholar] [CrossRef]
- Zimmermann, T.; Leonhardt, H.; Kersting, S.; Albrecht, S.; Range, U.; Eckelt, U. Reduction of postoperative lymphedema after oral tumor surgery with sodium selenite. Biol. Trace Elem. Res. 2005, 106, 193–203. [Google Scholar] [CrossRef]
- Micke, O.; Bruns, F.; Mücke, R.; Schäfer, U.; Glatzel, M.; DeVries, A.F.; Schönekaes, K.; Kisters, K.; Büntzel, J. Selenium in the treatment of radiation-associated secondary lymphedema. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 40–49. [Google Scholar] [CrossRef]
- Bruns, F.; Büntzel, J.; Mücke, R.; Schönekaes, K.; Kisters, K.; Micke, O. Selenium in the treatment of head and neck lymphedema. Med. Princ. Pract. 2004, 13, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Pfister, C.; Dawzcynski, H.; Schingale, F. Sodium selenite and cancer related lymphedema: Biological and pharmacological effects. J. Trace Elem. Med. Biol. 2016, 37, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Brenke, R.; Siems, W.; Grune, T. Therapieoptimierungsmaßnahmen beim chronischen Lymphödem. Z. Für Lymphol. 1997, 1, 97. [Google Scholar]
- Ortega, A.L.; Mena, S.; Estrela, J.M. Glutathione in cancer cell death. Cancers 2011, 3, 1285–1310. [Google Scholar] [CrossRef]
- ISL, I. The Diagnosis and Treatment of Peripheral Lymphedema 2009 Consensus Document of the International Society of Lymphology. Lymphology 2009, 42, 51–60. [Google Scholar]
- Amin, M.B.; Edge, S.B. AJCC Cancer Staging Manual; Springer: New York, NY, USA, 2017. [Google Scholar]
- Wang, T.; Song, Y.; Wang, H.; Zhang, J.; Yu, S.; Gu, Y.; Chen, T.; Wang, Y.; Shen, H.; Jia, G. Oxidative DNA damage and global DNA hypomethylation are related to folate deficiency in chromate manufacturing workers. J. Hazard. Mater. 2012, 213, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, H.S.; Stump, D.D.; Roth, E.F., Jr. A method to correct for errors caused by generation of interfering compounds during erythrocyte lipid peroxidation. Anal. Biochem. 1984, 137, 282–286. [Google Scholar] [CrossRef]
- Kim, J.; Paik, H.; Shin, M.; Park, E. Eight weeks of conjugated linoleic acid supplementation has no effect on antioxidant status in healthy overweight/obese Korean individuals. Eur. J. Nutr. 2012, 51, 135–141. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Milzani, A.; Fanti, P.; Rossi, R. Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat. Protoc. 2013, 8, 1660. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.E.; Yu, J.; Paik, H.J.; Ryu, J.M.; Kim, I.; Bae, S.Y.; Lee, S.K.; Kim, S.W.; Nam, S.J. Risk Factors Affecting Breast Cancer-related Lymphedema: Serial Body Weight Change During Neoadjuvant Anthracycline Plus Cyclophosphamide Followed by Taxane. Clin. Breast Cancer 2018, 18, e49–e54. [Google Scholar] [CrossRef]
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef]
- Morris, J.; Crane, S. Selenium toxicity from a misformulated dietary supplement, adverse health effects, and the temporal response in the nail biologic monitor. Nutrients 2013, 5, 1024–1057. [Google Scholar] [CrossRef]
- Berger, M.M. Can oxidative damage be treated nutritionally? Clin. Nutr. 2005, 24, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Brodin, O.; Eksborg, S.; Wallenberg, M.; Asker-Hagelberg, C.; Larsen, E.H.; Mohlkert, D.; Lenneby-Helleday, C.; Jacobsson, H.; Linder, S.; Misra, S.; et al. Pharmacokinetics and Toxicity of Sodium Selenite in the Treatment of Patients with Carcinoma in a Phase I Clinical Trial: The SECAR Study. Nutrients 2015, 7, 4978–4994. [Google Scholar] [Green Version]
- Forceville, X.; Laviolle, B.; Annane, D.; Vitoux, D.; Bleichner, G.; Korach, J.-M.; Cantais, E.; Georges, H.; Soubirou, J.-L.; Combes, A.; et al. Effects of high doses of selenium, as sodium selenite, in septic shock: A placebo-controlled, randomized, double-blind, phase II study. Crit. Care 2007, 11, R73. [Google Scholar] [CrossRef] [PubMed]
- Bruns, F.; Micke, O.; Bremer, M. Current status of selenium and other treatments for secondary lymphedema. J. Support. Oncol. 2003, 1, 121–130. [Google Scholar]
- Song, J.H.; Lee, S.W.; Kim, G.A.; Kim, M.-J. Measurement of fluid shift in CAPD patients using segmental bioelectrical impedance analysis. Perit. Dial. Int. 1999, 19, 386–390. [Google Scholar]
- Cha, K.; Chertow, G.; Gonzalez, J.; Lazarus, J.; Wilmore, D. Multifrequency bioelectrical impedance estimates the distribution of body water. J. Appl. Physiol. 1995, 79, 1316–1319. [Google Scholar] [CrossRef]
- Berlit, S.; Brade, J.; Tuschy, B.; Hornemann, A.; Leweling, H.; Eghardt, V.; Suetterlin, M. Comparing bioelectrical impedance values in assessing early upper limb lymphedema after breast cancer surgery. In Vivo 2012, 26, 863–867. [Google Scholar] [PubMed]
- Takeuchi, M.; Yoshizawa, T.; Kusaka, Y.; Furusawa, Y.; Nakamura, Y.; Atogami, F.; Niikura, H. Detecting subclinical secondary lymphoedema using bioimpedance: A preliminary study. J. Lymphoedema 2013, 8, 16–20. [Google Scholar]
- Rockson, S. Bioimpedance analysis in the assessment of lymphoedema diagnosis and management. J. Lymphoedema 2007, 2, 44. [Google Scholar] [CrossRef]
- Kasseroller, R. Sodium selenite in the treatment of lymphedema. Der. Allg. 1995, 13, 1396–1404. [Google Scholar]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Salen, P. Selenium and antioxidant defenses as major mediators in the development of chronic heart failure. Heart Fail. Rev. 2006, 11, 13–17. [Google Scholar] [CrossRef]
- Neve, J. Human selenium supplementation as assessed by changes in blood selenium concentration and glutathione peroxidase activity. J. Trace Elem. Med. Biol. 1995, 9, 65–73. [Google Scholar] [CrossRef]
- Bermingham, E.; Hesketh, J.; Sinclair, B.; Koolaard, J.; Roy, N. Selenium-enriched foods are more effective at increasing glutathione peroxidase (GPx) activity compared with selenomethionine: A meta-analysis. Nutrients 2014, 6, 4002–4031. [Google Scholar] [CrossRef]
- Thomson, C. Assessment of requirements for selenium and adequacy of selenium status: A review. Eur. J. Clin. Nutr. 2004, 58, 391. [Google Scholar] [CrossRef]
- Levander, O.A.; Alfthan, G.; Arvilommi, H.; Gref, C.; Huttunen, J.; Kataja, M.; Koivistoinen, P.; Pikkarainen, J. Bioavailability of selenium to Finnish men as assessed by platelet glutathione peroxidase activity and other blood parameters. Am. J. Clin. Nutr. 1983, 37, 887–897. [Google Scholar] [CrossRef] [Green Version]
- Van Der Torre, H.W.; Van Dokkum, W.; Schaafsma, G.; Wedel, M.; Ockhuizen, T. Effect of various levels of selenium in wheat and meat on blood Se status indices and on Se balance in Dutch men. Br. J. Nutr. 1991, 65, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Neve, J.; Vertongen, F.; Capel, P. Selenium supplementation in healthy Belgian adults: Response in platelet glutathione peroxidase activity and other blood indices. Am. J. Clin. Nutr. 1988, 48, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Alfthan, G.; Aro, A.; Arvilommi, H.; Huttunen, J.K. Selenium metabolism and platelet glutathione peroxidase activity in healthy Finnish men: Effects of selenium yeast, selenite, and selenate. Am. J. Clin. Nutr. 1991, 53, 120–125. [Google Scholar] [CrossRef]
- Foldi, E.; Sauerwald, A.; Hennig, B. Effect of complex decongestive physiotherapy on gene expression for the inflammatory response in peripheral lymphedema. Lymphology 2000, 33, 19–23. [Google Scholar]
- Paskett, E.D.; Dean, J.A.; Oliveri, J.M.; Harrop, J.P. Cancer-related lymphedema risk factors, diagnosis, treatment, and impact: A review. J. Clin. Oncol. 2012, 30, 3726–3733. [Google Scholar] [CrossRef]
- Kiremidjian-Schumacher, L.; Roy, M.; Wishe, H.; Cohen, M.; Stotzky, G. Selenium and immune cell functions. I. Effect on lymphocyte proliferation and production of interleukin 1 and interleukin 2. Proc. Soc. Exp. Biol. Med. 1990, 193, 136–142. [Google Scholar] [CrossRef]
- Kiremidjian-Schumacher, L.; Roy, M.; Wishe, H.I.; Cohen, M.W.; Stotzky, G. Supplementation with selenium and human immune cell functions. II. Effect on cytotoxic lymphocytes and natural killer cells. Biol. Trace Elem. Res. 1994, 41, 115. [Google Scholar] [CrossRef]
- Meltzer, H.; Norheim, G.; Bibow, K.; Myhre, K.; Holm, H. The form of selenium determines the response to supplementation in a selenium replete population. Eur. J. Clin. Nutr. 1990, 44, 435–446. [Google Scholar]
- Lipinski, B. Sodium selenite as an anticancer agent. Anti-Cancer Agents Med. Chem. 2017, 17, 658–661. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Li, W.-G.; Huang, Q.-S.; Zhi-Huang, C.; Hou, C. A preliminary report on the intervention trials of primary liver cancer in high-risk populations with nutritional supplementation of selenium in China. Biol. Trace Elem. Res. 1991, 29, 289–294. [Google Scholar] [CrossRef]
| CTRL (n = 12) | SE (n = 14) | p | |
|---|---|---|---|
| Age (years) | 55.2 ± 13.9 | 48.0 ± 11.1 | 0.158 a |
| BMI (kg/m²) | 23.4 ± 2.3 | 25.2 ± 2.4 | 0.077 a |
| Weight (kg) | 59.0 ± 5.8 | 63.7 ± 7.6 | 0.145 a |
| Affected site n, (%) | 0.075 | ||
| Left | 10 (83.3) | 7 (50.0) | |
| Right | 2 (16.7) | 7 (50.0) | |
| Pathological stage group n, (%) | 0.416 | ||
| II A | 1 (8.3) | 2 (14.3) | |
| II B | 3 (25.0) | 5 (35.7) | |
| III A | 3 (25.0) | 6 (42.9) | |
| III B | 1 (8.3) | 0 (0.0) | |
| III C | 4 (33.3) | 1 (7.1) | |
| Local recurrence n, (%) | 0.449 | ||
| Yes | 2 (16.7) | 1 (7.1) | |
| No | 10 (83.3) | 13 (92.9) | |
| Breast surgery method n, (%) | 0.902 | ||
| SLNB | 1 (8.3) | 2 (14.3) | |
| ALND | 10 (83.3) | 11 (78.6) | |
| Unknown | 1 (8.3) | 1 (7.1) | |
| Number of dissected lymph nodes | 22.8 ± 9.1 | 24.8 ± 13.9 | 0.705 a |
| Unknown n, (%) | 3 (25.0) | 1 (7.1) | |
| Post-surgery time n, (%) | 0.914 | ||
| 1 < year | 2 (16.7) | 2 (14.3) | |
| 1–3 years | 5 (41.7) | 5 (35.7) | |
| <3 years | 5 (41.7) | 7 (50.0) | |
| Radiation therapy n, (%) | 1.000 | ||
| Yes | 12 (100) | 12 (85.7) | |
| No | 0 (0.0) | 0 (0.0) | |
| Unknown | 2 (14.3) | ||
| Chemotherapy n, (%) | 0.327 | ||
| Yes | 12 (100) | 11 (78.6) | |
| No | 0 (0.0) | 1 (7.1) | |
| Unknown | 2 (14.3) |
| Parameters | Time Point | Time Point Comparison | p-Value | ||
|---|---|---|---|---|---|
| CTRL (n = 12) | SE (n = 14) | Baseline Comparison | Time × Group | ||
| TBW ratio | Baseline | 1.31 ± 0.29 | 1.25 ± 0.20 | 0.705 | 0.129 |
| Δ 2-week | −0.04 | −0.01 | |||
| Δ Follow-up | −0.01 | −0.04 | |||
| ECW ratio | Baseline | 1.37 ± 0.32 | 1.29 ± 0.23 | 0.494 | 0.122 |
| Δ 2-week | −0.05 | −0.03 * | |||
| Δ Follow-up | −0.01 | −0.05 † | |||
| 1 kHz SFBIA ratio | Baseline | 1.40 ± 0.34 | 1.32 ± 0.25 | 0.595 | 0.307 |
| Δ 2-week | −0.04 | −0.02 | |||
| Δ Follow-up | −0.01 | −0.04 | |||
| 5 kHz SFBIA ratio | Baseline | 1.40 ± 0.33 | 1.31 ± 0.25 | 0.595 | 0.123 |
| Δ 2-week | −0.06 | −0.02 | |||
| Δ Follow-up | −0.02 | −0.04 | |||
| 50 kHz SFBIA ratio | Baseline | 1.36 ± 0.33 | 1.28 ± 0.23 | 0.860 | 0.129 |
| Δ 2-week | −0.04 | −0.02 | |||
| Δ Follow-up | −0.01 | −0.03 | |||
| Parameters | Time Point | Time Point Comparison | p-Value | ||
|---|---|---|---|---|---|
| CTRL (n = 12) | SE (n = 14) | Baseline Comparison | Time × Group | ||
| MDA (μM) | Baseline | 12.0 ± 3.75 | 10.3 ± 3.94 | 0.212 | 0.115 |
| Δ 2-week | −2.97 ** | −0.23 | |||
| Δ Follow-up | −3.80 †† | −2.47 † | |||
| GSH (nmol/g Hb) | Baseline | 6468 ± 2426 | 5751 ± 2437 | 0.462 | 0.869 |
| Δ 2-week | −172 | −26.8 | |||
| Δ Follow-up | +138 | +600 | |||
| GSSG (nmol/g Hb) | Baseline | 85.9 ± 16.5 | 85.4 ± 7.34 | 0.940 | 0.311 |
| Δ 2-week | −2.97 | −0.23 | |||
| Δ Follow-up | −3.80 | −2.47 | |||
| GSH/GSSG ratio | Baseline | 75.9 ± 29.5 | 68.8 ± 28.2 | 0.462 | 0.743 |
| Δ 2-week | −0.77 | −3.81 | |||
| Δ Follow-up | +5.10 | +6.36 | |||
| GSH-Px activity | Baseline | 72.1 ± 31.2 | 92.1 ± 38.0 | 0.860 | 0.742 |
| Δ 2-week | −4.00 | +3.13 | |||
| Δ Follow-up | −3.27 | +2.55 | |||
| ORAC (TE) | Baseline | 1025 ± 275 | 1027 ± 181 | 0.176 | 0.079 |
| Δ 2-week | +67.2 | −40.9 | |||
| Δ Follow-up | +26.6 | −66.4 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.W.; Yang, E.J.; Lee, S.-M. Sodium Selenite Alleviates Breast Cancer-Related Lymphedema Independent of Antioxidant Defense System. Nutrients 2019, 11, 1021. https://doi.org/10.3390/nu11051021
Han HW, Yang EJ, Lee S-M. Sodium Selenite Alleviates Breast Cancer-Related Lymphedema Independent of Antioxidant Defense System. Nutrients. 2019; 11(5):1021. https://doi.org/10.3390/nu11051021
Chicago/Turabian StyleHan, Hye Won, Eun Joo Yang, and Seung-Min Lee. 2019. "Sodium Selenite Alleviates Breast Cancer-Related Lymphedema Independent of Antioxidant Defense System" Nutrients 11, no. 5: 1021. https://doi.org/10.3390/nu11051021





