Dysregulation of Retinal Melatonin Biosynthetic Pathway and Differential Expression of Retina-Specific Genes Following Blast-Induced Ocular Injury in Ferrets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blast Exposure and Sample Collection
2.3. RNA Extraction and Quantitative RT-PCR
3. Statistical Analysis
4. Results
4.1. Effect of Blast Exposure on Expression of Melatonin-Synthesizing Enzymes
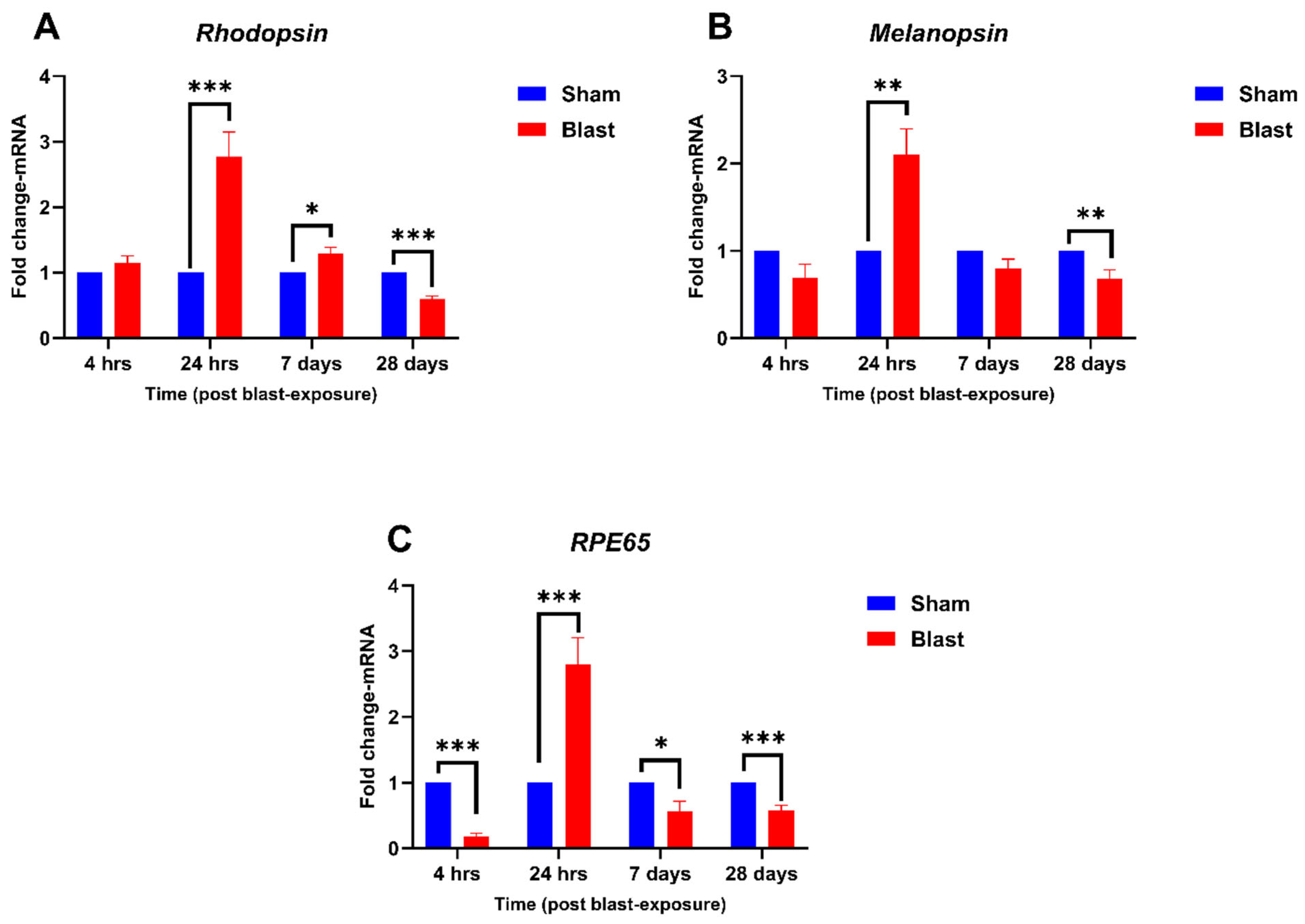
4.2. Effect of Blast Exposure on Expression of Retina-Specific Genes
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Disclaimer
Acknowledgments
Conflicts of Interest
References
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Public Health Approaches to Reduce Vision Impairment and Promote Eye Health; Welp, A.; Woodbury, R.B.; McCoy, M.A.; Teutsch, S.M. The Impact of Vision Loss. In Making Eye Health a Population Health Imperative: Vision for Tomorrow; National Academies Press (US): Washington, DC, USA, 2016. [Google Scholar]
- Ríos, J.D.; Choi, J.H.; McDaniel, J.S.; Becera, S.; Bice, L.; Johnson, P.; Cleland, J.M.; Glickman, R.D.; Reilly, M.A.; Gray, W.; et al. Altered Expression of Aquaporin 1 and Aquaporin 5 in the Cornea after Primary Blast Exposure. Mol. Vis. 2019, 25, 283–294. [Google Scholar] [PubMed]
- Zhang, Y.; Kang, X.; Wu, Q.; Zheng, Z.; Ying, J.; Zhang, M.-N. Explosive Eye Injuries: Characteristics, Traumatic Mechanisms, and Prognostic Factors for Poor Visual Outcomes. Mil. Med. Res. 2023, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.F.; Raza, Z.; Cash, A.T.G.; Zampieri, T.; Mazzoli, R.A.; Kardon, R.H.; Gomes, R.S.M. Traumatic Brain Injury and Sight Loss in Military and Veteran Populations—A Review. Mil. Med. Res. 2021, 8, 42. [Google Scholar] [CrossRef]
- McMaster, D.; Clare, G. Incidence of Ocular Blast Injuries in Modern Conflict. Eye 2021, 35, 3451–3452. [Google Scholar] [CrossRef]
- Lee, I.; Davis, B.; Purt, B.; DesRosiers, T. Ocular Trauma and Traumatic Brain Injury on the Battlefield: A Systematic Review After 20 Years of Fighting the Global War on Terror. Mil. Med. 2023, 188, 2916–2923. [Google Scholar] [CrossRef]
- Edri, I.E. Blast Injury Risks to Humans within a Military Trench. Def. Technol. 2024, 40, 91–104. [Google Scholar] [CrossRef]
- Erdurman, F.C.; Hurmeric, V.; Gokce, G.; Durukan, A.H.; Sobaci, G.; Altinsoy, H.I. Ocular Injuries from Improvised Explosive Devices. Eye 2011, 25, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Brain Injury and Vision Loss from Blast Trauma. Available online: https://www.aao.org/eyenet/article/brain-injury-vision-loss-from-blast-trauma (accessed on 16 January 2025).
- Cipolla-Neto, J.; Amaral, F.G. do Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Cai, Y.; Yoshida, S.; Li, Y.; Zhou, Y. Melatonin: Unveiling the Functions and Implications in Ocular Health. Pharmacol. Res. 2024, 205, 107253. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal Melatonin: Sources, Regulation, and Potential Functions. Cell Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin Antioxidative Defense: Therapeutical Implications for Aging and Neurodegenerative Processes. Neurotox. Res. 2013, 23, 267–300. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, Q.; Wu, W.; Zeng, W.; Feng, Y. Therapeutic Effects of Melatonin on Ocular Diseases: Knowledge Map and Perspective. Front. Pharmacol. 2021, 12, 721869. [Google Scholar] [CrossRef] [PubMed]
- La Morgia, C.; Carelli, V.; Carbonelli, M. Melanopsin Retinal Ganglion Cells and Pupil: Clinical Implications for Neuro-Ophthalmology. Front. Neurol. 2018, 9, 1047. [Google Scholar] [CrossRef]
- Kiser, P.D. Retinal Pigment Epithelium 65 kDa Protein (RPE65): An Update. Prog. Retin. Eye Res. 2022, 88, 101013. [Google Scholar] [CrossRef]
- Paul, K.N.; Saafir, T.B.; Tosini, G. The Role of Retinal Photoreceptors in the Regulation of Circadian Rhythms. Rev. Endocr. Metab. Disord. 2009, 10, 271–278. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Patel, M.Y.; Wilder, D.M.; Krishnan, J.; LaValle, C.; Pandya, J.D.; Shear, D.A.; Hefeneider, S.H.; Long, J.B.; Arun, P. Upregulation of Multiple Toll-like Receptors in Ferret Brain after Blast Exposure: Potential Targets for Treatment. Neurosci. Lett. 2023, 810, 137364. [Google Scholar] [CrossRef]
- Escher, P.; Schorderet, D.F. Exploration of the Visual System: Part 1: Dissection of the Mouse Eye for RNA, Protein, and Histological Analyses. Curr. Protoc. Mouse Biol. 2011, 1, 445–462. [Google Scholar] [CrossRef]
- Chivite, M.; Leal, E.; Míguez, J.M.; Cerdá-Reverter, J.M. Distribution of Two Isoforms of Tryptophan Hydroxylase in the Brain of Rainbow Trout (Oncorhynchus Mykiss). An in Situ Hybridization Study. Brain Struct. Funct. 2021, 226, 2265–2278. [Google Scholar] [CrossRef]
- Liu, C.; Fukuhara, C.; Wessel, J.H.; Iuvone, P.M.; Tosini, G. Localization of Aa-Nat mRNA in the Rat Retina by Fluorescence in Situ Hybridization and Laser Capture Microdissection. Cell Tissue Res. 2004, 315, 197–201. [Google Scholar] [CrossRef]
- Chen, G.-L.; Miller, G.M. Advances in Tryptophan Hydroxylase-2 Gene Expression Regulation: New Insights into Serotonin–Stress Interaction and Clinical Implications. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2012, 159B, 152–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-L.; Miller, G.M. Tryptophan Hydroxylase-2: An Emerging Therapeutic Target for Stress Disorders. Biochem. Pharmacol. 2013, 85, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- DeMar, J.; Sharrow, K.; Hill, M.; Berman, J.; Oliver, T.; Long, J. Effects of Primary Blast Overpressure on Retina and Optic Tract in Rats. Front. Neurol. 2016, 7, 59. [Google Scholar] [CrossRef]
- Mohan, K.; Kecova, H.; Hernandez-Merino, E.; Kardon, R.H.; Harper, M.M. Retinal Ganglion Cell Damage in an Experimental Rodent Model of Blast-Mediated Traumatic Brain Injury. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3440–3450. [Google Scholar] [CrossRef]
- Thomas, C.N.; Courtie, E.; Bernardo-Colón, A.; Essex, G.; Rex, T.S.; Ahmed, Z.; Blanch, R.J. Assessment of Necroptosis in the Retina in a Repeated Primary Ocular Blast Injury Mouse Model. Exp. Eye Res. 2020, 197, 108102. [Google Scholar] [CrossRef]
- Wang, H.-C.H.; Choi, J.-H.; Greene, W.A.; Plamper, M.L.; Cortez, H.E.; Chavko, M.; Li, Y.; Dalle Lucca, J.J.; Johnson, A.J. Pathophysiology of Blast-Induced Ocular Trauma With Apoptosis in the Retina and Optic Nerve. Mil. Med. 2014, 179, 34–40. [Google Scholar] [CrossRef]
- Dutca, L.M.; Stasheff, S.F.; Hedberg-Buenz, A.; Rudd, D.S.; Batra, N.; Blodi, F.R.; Yorek, M.S.; Yin, T.; Shankar, M.; Herlein, J.A.; et al. Early Detection of Subclinical Visual Damage after Blast-Mediated TBI Enables Prevention of Chronic Visual Deficit by Treatment with P7C3-S243. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8330–8341. [Google Scholar] [CrossRef]
- Harper, M.M.; Woll, A.W.; Evans, L.P.; Delcau, M.; Akurathi, A.; Hedberg-Buenz, A.; Soukup, D.A.; Boehme, N.; Hefti, M.M.; Dutca, L.M.; et al. Blast Preconditioning Protects Retinal Ganglion Cells and Reveals Targets for Prevention of Neurodegeneration Following Blast-Mediated Traumatic Brian Injury. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4159–4170. [Google Scholar] [CrossRef]
- Harper, M.M.; Rudd, D.; Meyer, K.J.; Kanthasamy, A.G.; Anantharam, V.; Pieper, A.A.; Vázquez-Rosa, E.; Shin, M.-K.; Chaubey, K.; Koh, Y.; et al. Identification of Chronic Brain Protein Changes and Protein Targets of Serum Auto-Antibodies after Blast-Mediated Traumatic Brain Injury. Heliyon 2020, 6, e03374. [Google Scholar] [CrossRef]
- Allen, R.S.; Motz, C.T.; Feola, A.; Chesler, K.C.; Haider, R.; Ramachandra Rao, S.; Skelton, L.A.; Fliesler, S.J.; Pardue, M.T. Long-Term Functional and Structural Consequences of Primary Blast Overpressure to the Eye. J. Neurotrauma 2018, 35, 2104–2116. [Google Scholar] [CrossRef]
- Bricker-Anthony, C.; Hines-Beard, J.; D’Surney, L.; Rex, T.S. Exacerbation of Blast-Induced Ocular Trauma by an Immune Response. J. Neuroinflam. 2014, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Bricker-Anthony, C.; Hines-Beard, J.; Rex, T.S. Molecular Changes and Vision Loss in a Mouse Model of Closed-Globe Blast Trauma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4853–4862. [Google Scholar] [CrossRef] [PubMed]
- Bricker-Anthony, C.; Rex, T.S. Neurodegeneration and Vision Loss after Mild Blunt Trauma in the C57Bl/6 and DBA/2J Mouse. PLoS ONE 2015, 10, e0131921. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Howard, J.T.; Edsall, P.R.; Morris, R.B.; Lund, B.J.; Cleland, J.M. Blast Exposure Induces Ocular Functional Changes with Increasing Blast Over-Pressures in a Rat Model. Curr. Eye Res. 2019, 44, 770–780. [Google Scholar] [CrossRef]
- Felder-Schmittbuhl, M.P.; Hicks, D.; Ribelayga, C.P.; Tosini, G. Melatonin in the Mammalian Retina: Synthesis, Mechanisms of Action and Neuroprotection. J. Pineal Res. 2024, 76, e12951. [Google Scholar] [CrossRef]
- Żmijewski, M.A.; Sweatman, T.W.; Slominski, A.T. The Melatonin-Producing System Is Fully Functional in Retinal Pigment Epithelium (ARPE-19). Mol. Cell. Endocrinol. 2009, 307, 211–216. [Google Scholar] [CrossRef]
- Kuhn, D.M.; Hasegawa, H. Chapter 12—Tryptophan Hydroxylase and Serotonin Synthesis Regulation. In Handbook of Behavioral Neuroscience; Müller, C.P., Cunningham, K.A., Eds.; Handbook of the Behavioral Neurobiology of Serotonin; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 239–256. [Google Scholar]
- Chong, N.W.; Cassone, V.M.; Bernard, M.; Klein, D.C.; Iuvone, P.M. Circadian Expression of Tryptophan Hydroxylase mRNA in the Chicken Retina1. Mol. Brain Res. 1998, 61, 243–250. [Google Scholar] [CrossRef]
- Green, C.B.; Besharse, J.C. Tryptophan Hydroxylase Expression Is Regulated by a Circadian Clock in Xenopus Laevis Retina. J. Neurochem. 1994, 62, 2420–2428. [Google Scholar] [CrossRef]
- Kawa, L.; Arborelius, U.P.; Yoshitake, T.; Kehr, J.; Hökfelt, T.; Risling, M.; Agoston, D. Neurotransmitter Systems in a Mild Blast Traumatic Brain Injury Model: Catecholamines and Serotonin. J. Neurotrauma 2015, 32, 1190–1199. [Google Scholar] [CrossRef]
- Kawa, L.; Kamnaksh, A.; Long, J.B.; Arborelius, U.P.; Hökfelt, T.; Agoston, D.V.; Risling, M. A Comparative Study of Two Blast-Induced Traumatic Brain Injury Models: Changes in Monoamine and Galanin Systems Following Single and Repeated Exposure. Front. Neurol. 2018, 9, 479. [Google Scholar] [CrossRef]
- Kawa, L. Experimental, Mild Blast-Induced Traumatic Brain Injury: Focus on the Monoamine and Galanin Systems. Ph.D. Thesis, Karolinska Institutet, Stockholm, Sweden, 2017. [Google Scholar]
- Govindarajulu, M.; Patel, M.Y.; Wilder, D.M.; Long, J.B.; Arun, P. Blast Exposure Dysregulates Nighttime Melatonin Synthesis and Signaling in the Pineal Gland: A Potential Mechanism of Blast-Induced Sleep Disruptions. Brain Sci. 2022, 12, 1340. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.J.; Henderson, L.A.; Keay, K.A. Hypotensive but Not Normotensive Haemorrhage Increases Tryptophan Hydroxylase-2 mRNA in Caudal Midline Medulla. Neurosci. Lett. 2006, 398, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Shishkina, G.T.; Kalinina, T.S.; Dygalo, N.N. Serotonergic Changes Produced by Repeated Exposure to Forced Swimming: Correlation with Behavior. Ann. N. Y. Acad. Sci. 2008, 1148, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Boehme, N.A.; Hedberg-Buenz, A.; Tatro, N.; Bielecki, M.; Castonguay, W.C.; Scheetz, T.E.; Anderson, M.G.; Dutca, L.M. Axonopathy Precedes Cell Death in Ocular Damage Mediated by Blast Exposure. Sci. Rep. 2021, 11, 11774. [Google Scholar] [CrossRef]
- Ha, Y.; Palacios, E.; Luo, B.; Li, S.; Xia, F.; Liu, H.; Zhang, W. Alterations of Retinal Pathophysiology Following Repeated Blast Injury Resulting from Reproduced Open-Field Blast Environment. Investig. Ophthalmol. Vis. Sci. 2022, 63, 4138-F0375. [Google Scholar]
- Pomianowski, K.; Gozdowska, M.; Burzyński, A.; Kalamarz-Kubiak, H.; Sokołowska, E.; Kijewska, A.; Kulczykowska, E. A Study of Aanat and Asmt Expression in the Three-Spined Stickleback Eye and Skin: Not Only “on the Way to Melatonin”. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 241, 110635. [Google Scholar] [CrossRef]
- Tosini, G.; Chaurasia, S.S.; Iuvone, P.M. Regulation of Arylalkylamine N-Acetyltransferase (AANAT) in the Retina. Chronobiol. Int. 2006, 23, 381–391. [Google Scholar] [CrossRef]
- Haghighi, F.; Ge, Y.; Chen, S.; Xin, Y.; Umali, M.U.; De Gasperi, R.; Gama Sosa, M.A.; Ahlers, S.T.; Elder, G.A. Neuronal DNA Methylation Profiling of Blast-Related Traumatic Brain Injury. J. Neurotrauma 2015, 32, 1200–1209. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, R.; Zhang, S.; Wu, J.; Sun, X. Activation of 5-HT1A Receptors Promotes Retinal Ganglion Cell Function by Inhibiting the cAMP-PKA Pathway to Modulate Presynaptic GABA Release in Chronic Glaucoma. J. Neurosci. 2019, 39, 1484–1504. [Google Scholar] [CrossRef]
- Zhou, X.; Li, G.; Zhang, S.; Wu, J. 5-HT1A Receptor Agonist Promotes Retinal Ganglion Cell Function by Inhibiting OFF-Type Presynaptic Glutamatergic Activity in a Chronic Glaucoma Model. Front. Cell. Neurosci. 2019, 13, 167. [Google Scholar] [CrossRef]
- Liang, H.; Liu, N.; Wang, R.; Zhang, Y.; Chen, J.; Dai, Z.; Yang, Y.; Wu, G.; Wu, Z. N-Acetyl Serotonin Alleviates Oxidative Damage by Activating Nuclear Factor Erythroid 2-Related Factor 2 Signaling in Porcine Enterocytes. Antioxidants 2020, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Biswal, M.R.; Paulson, R.J.; Vichare, R.; Lewin, A.S. Buspirone Enhances Cell Survival and Preserves Structural Integrity during Oxidative Injury to the Retinal Pigment Epithelium. Antioxidants 2023, 12, 2129. [Google Scholar] [CrossRef] [PubMed]
- Wirth, A.; Holst, K.; Ponimaskin, E. How Serotonin Receptors Regulate Morphogenic Signalling in Neurons. Prog. Neurobiol. 2017, 151, 35–56. [Google Scholar] [CrossRef]
- Masson, J. Serotonin in Retina. Biochimie 2019, 161, 51–55. [Google Scholar] [CrossRef]
- Hayreh, S.S.; Piegors, D.J.; Heistad, D.D. Serotonin-Induced Constriction of Ocular Arteries in Atherosclerotic Monkeys. Implications for Ischemic Disorders of the Retina and Optic Nerve Head. Arch. Ophthalmol. 1997, 115, 220–228. [Google Scholar] [CrossRef]
- Vanhoutte, P.M. Serotonin and the Vascular Wall. Int. J. Cardiol. 1987, 14, 189–203. [Google Scholar] [CrossRef]
- Maddaloni, G.; Barsotti, N.; Migliarini, S.; Giordano, M.; Nazzi, S.; Picchi, M.; Errico, F.; Usiello, A.; Pasqualetti, M. Impact of Serotonin Deficiency on Circadian Dopaminergic Rhythms. Int. J. Mol. Sci. 2024, 25, 6475. [Google Scholar] [CrossRef]
- Meyer-Bernstein, E.L.; Morin, L.P. Electrical Stimulation of the Median or Dorsal Raphe Nuclei Reduces Light-Induced FOS Protein in the Suprachiasmatic Nucleus and Causes Circadian Activity Rhythm Phase Shifts. Neuroscience 1999, 92, 267–279. [Google Scholar] [CrossRef]
- Ramkisoensing, A.; Meijer, J.H. Synchronization of Biological Clock Neurons by Light and Peripheral Feedback Systems Promotes Circadian Rhythms and Health. Front. Neurol. 2015, 6, 128. [Google Scholar] [CrossRef]
- Menon, S.T.; Han, M.; Sakmar, T.P. Rhodopsin: Structural Basis of Molecular Physiology. Physiol. Rev. 2001, 81, 1659–1688. [Google Scholar] [CrossRef]
- Okada, T.; Ernst, O.P.; Palczewski, K.; Hofmann, K.P. Activation of Rhodopsin: New Insights from Structural and Biochemical Studies. Trends Biochem. Sci. 2001, 26, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Adil, M.Y.; Chang, K.; Yu, Z.; Yang, L.; Utheim, T.P.; Chen, D.F.; Cho, K.-S. Visual Contrast Sensitivity Correlates to the Retinal Degeneration in Rhodopsin Knockout Mice. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4196–4204. [Google Scholar] [CrossRef] [PubMed]
- Cideciyan, A.V.; Jacobson, S.G.; Aleman, T.S.; Gu, D.; Pearce-Kelling, S.E.; Sumaroka, A.; Acland, G.M.; Aguirre, G.D. In Vivo Dynamics of Retinal Injury and Repair in the Rhodopsin Mutant Dog Model of Human Retinitis Pigmentosa. Proc. Natl. Acad. Sci. USA 2005, 102, 5233–5238. [Google Scholar] [CrossRef] [PubMed]
- Lenahan, C.; Sanghavi, R.; Huang, L.; Zhang, J.H. Rhodopsin: A Potential Biomarker for Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 326. [Google Scholar] [CrossRef]
- Xiong, B.; Bellen, H.J. Rhodopsin Homeostasis and Retinal Degeneration: Lessons from the Fly. Trends Neurosci. 2013, 36, 652–660. [Google Scholar] [CrossRef]
- Di Pierdomenico, J.; García-Ayuso, D.; Pinilla, I.; Cuenca, N.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Villegas-Pérez, M.P. Early Events in Retinal Degeneration Caused by Rhodopsin Mutation or Pigment Epithelium Malfunction: Differences and Similarities. Front. Neuroanat. 2017, 11, 14. [Google Scholar] [CrossRef]
- Parsons, T.; Morris, A.; Zhu, J.; Arja, R.; Arjona, K.; Wang, K.; Yang, Z. Changes In Retinal Opsins Reflect Neurological Abnormalities in Multiple Models of Traumatic Brain Injury. J. Neurotrauma 2021, 38, A76. [Google Scholar]
- García-Ayuso, D.; Galindo-Romero, C.; Di Pierdomenico, J.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Villegas Pérez, M.P. Light-Induced Retinal Degeneration Causes a Transient Downregulation of Melanopsin in the Rat Retina. Exp. Eye Res. 2017, 161, 10–16. [Google Scholar] [CrossRef]
- Nadal-Nicolás, F.M.; Madeira, M.H.; Salinas-Navarro, M.; Jiménez-López, M.; Galindo-Romero, C.; Ortín-Martínez, A.; Santiago, A.R.; Vidal-Sanz, M.; Agudo-Barriuso, M. Transient Downregulation of Melanopsin Expression After Retrograde Tracing or Optic Nerve Injury in Adult Rats. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4309–4323. [Google Scholar] [CrossRef]
- Honig, M.G.; Del Mar, N.A.; Henderson, D.L.; Ragsdale, T.D.; Doty, J.B.; Driver, J.H.; Li, C.; Fortugno, A.P.; Mitchell, W.M.; Perry, A.M.; et al. Amelioration of Visual Deficits and Visual System Pathology after Mild TBI via the Cannabinoid Type-2 Receptor Inverse Agonism of Raloxifene. Exp. Neurol. 2019, 322, 113063. [Google Scholar] [CrossRef]
- Cui, Q.; Ren, C.; Sollars, P.J.; Pickard, G.E.; So, K.-F. The Injury Resistant Ability of Melanopsin-Expressing Intrinsically Photosensitive Retinal Ganglion Cells. Neuroscience 2015, 284, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Shen, S.Q.; Corbo, J.C.; Kefalov, V.J. Circadian and Light-Driven Regulation of Rod Dark Adaptation. Sci. Rep. 2015, 5, 17616. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Conley, S.M.; Naash, M.I. RPE65: Role in the Visual Cycle, Human Retinal Disease, and Gene Therapy. Ophthalmic Genet. 2009, 30, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kaczurowski, M.I. The Pigment Epithelium of the Human Eye. Am. J. Ophthalmol. 1962, 53, 79–92. [Google Scholar] [CrossRef]
- Yi, C.; Pan, X.; Yan, H.; Guo, M.; Pierpaoli, W. Effects of Melatonin in Age-Related Macular Degeneration. Ann. N. Y. Acad. Sci. 2005, 1057, 384–392. [Google Scholar] [CrossRef]
- Fujishiro, T.; Kawasaki, H.; Aihara, M.; Saeki, T.; Ymagishi, R.; Atarashi, T.; Mayama, C.; Araie, M. Establishment of an Experimental Ferret Ocular Hypertension Model for the Analysis of Central Visual Pathway Damage. Sci. Rep. 2014, 4, 6501. [Google Scholar] [CrossRef]
- Ito, Y.; Shimazawa, M.; Chen, Y.-N.; Tsuruma, K.; Yamashima, T.; Araie, M.; Hara, H. Morphological Changes in the Visual Pathway Induced by Experimental Glaucoma in Japanese Monkeys. Exp. Eye Res. 2009, 89, 246–255. [Google Scholar] [CrossRef]
- Shimazawa, M.; Tomita, G.; Taniguchi, T.; Sasaoka, M.; Hara, H.; Kitazawa, Y.; Araie, M. Morphometric Evaluation of Changes with Time in Optic Disc Structure and Thickness of Retinal Nerve Fibre Layer in Chronic Ocular Hypertensive Monkeys. Exp. Eye Res. 2006, 82, 427–440. [Google Scholar] [CrossRef]


| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
| TPH1 | GCCGATCATCCTGGCTTCAA | CTGCAGGCATGGGTTGGGTA |
| TPH2 | ATGCCGACCACCCAGGATTT | AACACGACACCCCACGTCTT |
| AANAT | TCGAGCGCGAAGCCTTCAT | GGTCCCAAAGCGAACCGATG |
| ASMT | ACGACGTACCTGTGTTGGGG | CGCTCACCCTCGGATCTGTA |
| Rhodopsin | GTGGTGGTGTGTAAGCCCAT | CCTCTGGGATGTACCTGGACC |
| Melanopsin | TCTATACCTTCTGCAGGACCAG | CTTATGGAGGCTGCTGACGA |
| RPE65 | CCTCTGAATATTGACAAGGCTGAC | ACACGCTTAGGAAAACTCTGAA |
| 18S rRNA | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pundkar, C.; Thanapaul, R.J.R.S.; Govindarajulu, M.; Phuyal, G.; Long, J.B.; Arun, P. Dysregulation of Retinal Melatonin Biosynthetic Pathway and Differential Expression of Retina-Specific Genes Following Blast-Induced Ocular Injury in Ferrets. Neurol. Int. 2025, 17, 42. https://doi.org/10.3390/neurolint17030042
Pundkar C, Thanapaul RJRS, Govindarajulu M, Phuyal G, Long JB, Arun P. Dysregulation of Retinal Melatonin Biosynthetic Pathway and Differential Expression of Retina-Specific Genes Following Blast-Induced Ocular Injury in Ferrets. Neurology International. 2025; 17(3):42. https://doi.org/10.3390/neurolint17030042
Chicago/Turabian StylePundkar, Chetan, Rex Jeya Rajkumar Samdavid Thanapaul, Manoj Govindarajulu, Gaurav Phuyal, Joseph B. Long, and Peethambaran Arun. 2025. "Dysregulation of Retinal Melatonin Biosynthetic Pathway and Differential Expression of Retina-Specific Genes Following Blast-Induced Ocular Injury in Ferrets" Neurology International 17, no. 3: 42. https://doi.org/10.3390/neurolint17030042
APA StylePundkar, C., Thanapaul, R. J. R. S., Govindarajulu, M., Phuyal, G., Long, J. B., & Arun, P. (2025). Dysregulation of Retinal Melatonin Biosynthetic Pathway and Differential Expression of Retina-Specific Genes Following Blast-Induced Ocular Injury in Ferrets. Neurology International, 17(3), 42. https://doi.org/10.3390/neurolint17030042






