Nanoparticle-Mediated Therapeutic Agent Delivery for Treating Metastatic Breast Cancer—Challenges and Opportunities
Abstract
1. Introduction
2. Traditional Cytotoxic Chemotherapy and Targeted Therapy against MBC
3. Current Techniques Used for ncRNA Delivery in Cancer Therapy
3.1. Lipid-Based Vectors for Antineoplastic Gene Delivery
3.2. Polymer-Mediated Gene Delivery Systems
4. The Clinically Applied Drug-Based Nanomedicine against Cancer and Its Confronted Challenges
5. Biological Challenges Associated with Treating MBC
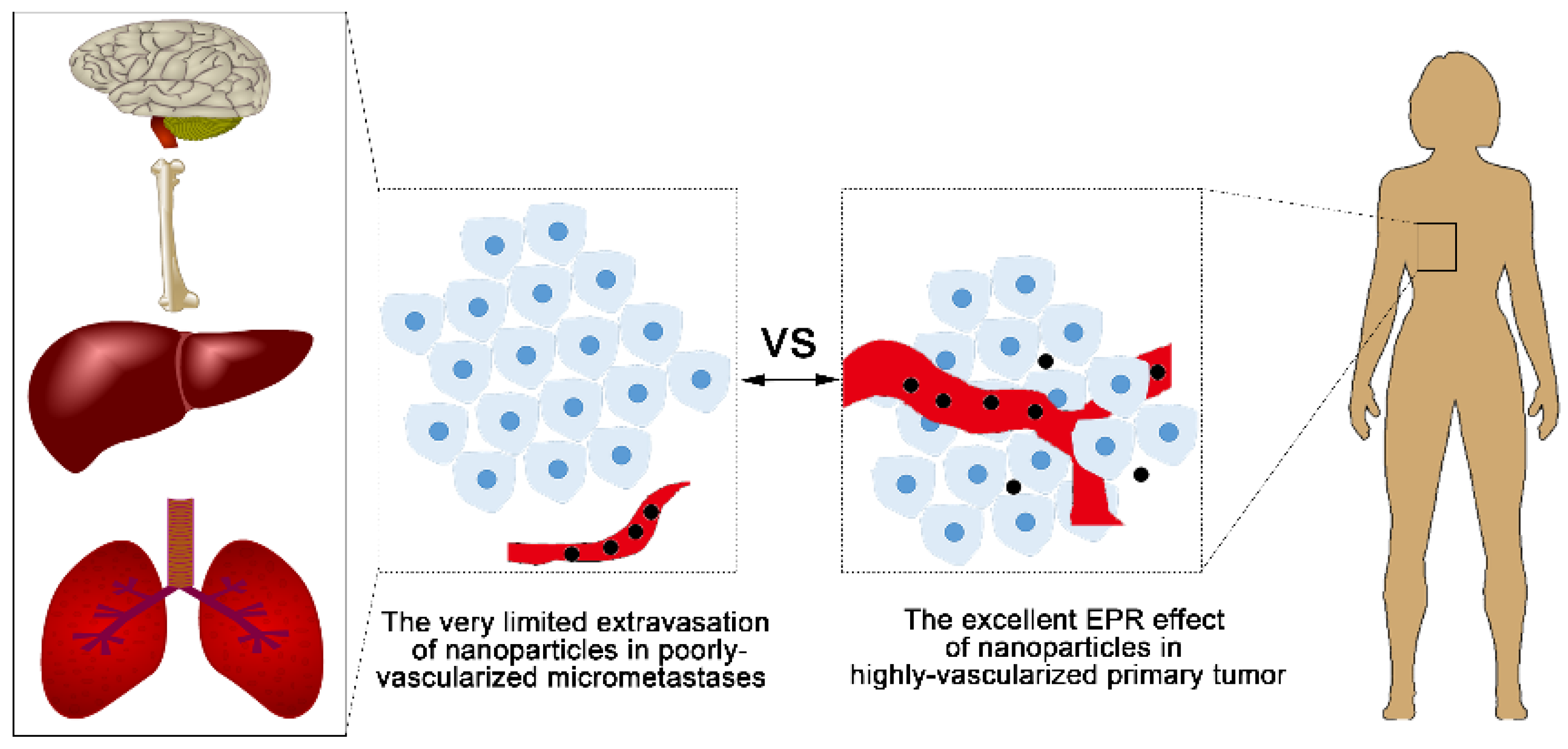
5.1. Challenge 1—Limited Access to the Dormant Cells or Micrometastases
5.2. Challenge 2—The Anti-Apoptosis Characteristic of Cancer Stem Cells (CSCs)
6. Designing Nanomedicine Strategies to Overcome the Limitations in MBC Metastasis
6.1. Preventing the Physical Translocation Away from Primary Tumor to Secondary Organs
6.1.1. Targeting Epithelial-to-Mesenchymal Transition (EMT) of Primary Tumor
6.1.2. Blocking the Spread through the Lymphatic System
6.2. Interfering with the Colonization of Distant Organ Sites
7. Summary and Future Perspectives
Author contributions
Acknowledgments
Conflicts of Interest
References
- DeSantis, C.E.; Ma, J.; Goding Sauer, A.; Newman, L.A.; Jemal, A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J. Clin. 2017, 67, 439–448. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist 2005, 10 (Suppl. S3), 20–29. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Parker, J.S.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.I.; He, X.; Perou, C.M. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010, 12, R68. [Google Scholar] [CrossRef] [PubMed]
- Neve, R.M.; Chin, K.; Fridlyand, J.; Yeh, J.; Baehner, F.L.; Fevr, T.; Clark, L.; Bayani, N.; Coppe, J.-P.; Tong, F. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006, 10, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. The first targeted delivery of sirna in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol. Pharm. 2009, 6, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, W.; Li, Y.; Liu, S.; Jin, M.; Wang, Y.; Jia, L.; Gao, Z. Anti-tumor effects in mice induced by survivin-targeted sirna delivered through polysaccharide nanoparticles. Biomaterials 2013, 34, 5689–5699. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil–based paclitaxel in women with breast cancer. J. Clin. Oncol. 2005, 23, 7794–7803. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Krasnojon, D.; Cheporov, S.; Makhson, A.N.; Manikhas, G.M.; Clawson, A.; Bhar, P. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J. Clin. Oncol. 2009, 27, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Krasnojon, D.; Cheporov, S.; Makhson, A.N.; Manikhas, G.M.; Clawson, A.; Bhar, P.; McGuire, J.R.; Iglesias, J. Phase II trial of nab-paclitaxel compared with docetaxel as first-line chemotherapy in patients with metastatic breast cancer: Final analysis of overall survival. Clin. Breast Cancer 2012, 12, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Humphries, B.; Wang, Z.; Lang, S.; Huang, X.; Xiao, H.; Jiang, Y.; Yang, C. Complex coacervation-integrated hybrid nanoparticles increasing plasmid DNA delivery efficiency in vivo. ACS Appl. Mater. Interfaces 2016, 8, 30735–30746. [Google Scholar] [CrossRef] [PubMed]
- O’brien, M.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.; Tomczak, P.; Ackland, S. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin hcl (caelyx™/doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.; Knight, R.A. Mir-34: From Bench to Bedside. Oncotarget 2014, 5, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-D.; Huang, L. Targeted delivery of antisense oligodeoxynucleotide and small interference rna into lung cancer cells. Mol. Pharm. 2006, 3, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Y.-C.; Tseng, Y.-C.; Mozumdar, S.; Huang, L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic sirna delivery. J. Controll. Release 2010, 142, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Y.; Huang, L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for sirna delivery to the tumor. J. Controll. Release 2012, 158, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kim, W.Y.; Huang, L. Systemic delivery of gemcitabine triphosphate via lcp nanoparticles for nsclc and pancreatic cancer therapy. Biomaterials 2013, 34, 3447–3458. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, Y.; Huang, L. Influence of polyethylene glycol density and surface lipid on pharmacokinetics and biodistribution of lipid-calcium-phosphate nanoparticles. Biomaterials 2014, 35, 3027–3034. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-C.; Xu, Z.; Guley, K.; Yuan, H.; Huang, L. Lipid–calcium phosphate nanoparticles for delivery to the lymphatic system and spect/ct imaging of lymph node metastases. Biomaterials 2014, 35, 4688–4698. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (abraxane® abi-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 4, 99–105. [Google Scholar]
- Friedman, R. Nano Dot Technology Enters Clinical Trials; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding rna networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Moulder, S.; Hortobagyi, G. Advances in the treatment of breast cancer. Clin. Pharmacol. Ther. 2008, 83, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Long, J.-P.; Li, X.-N.; Zhang, F. Targeting metabolism in breast cancer: How far we can go? World J. Clin. Oncol. 2016, 7, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Zeichner, S.B.; Terawaki, H.; Gogineni, K. A review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer Basic Clin. Res. 2016, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Ovcaricek, T.; Frkovic, S.; Matos, E.; Mozina, B.; Borstnar, S. Triple negative breast cancer-prognostic factors and survival. Radiol. Oncol. 2011, 45, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Pietenpol, J.A. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J. Pathol. 2014, 232, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, B.L.; Francis, P.A.; Parker, B.S.; Anderson, R.L. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat. Rev. Drug Discov. 2012, 11, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.A.; Dees, E.C.; Sawyer, L.; Gatti, L.; Moore, D.T.; Collichio, F.; Ollila, D.W.; Sartor, C.I.; Graham, M.L.; Perou, C.M. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin. Cancer Res. 2007, 13, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Lehár, J.; Krueger, A.S.; Avery, W.; Heilbut, A.M.; Johansen, L.M.; Price, E.R.; Rickles, R.J.; Short, G.F., III; Staunton, J.E.; Jin, X. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 2009, 27, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, M.; Ito, Y.; Tokudome, N.; Sugihara, T.; Miura, H.; Takahashi, S.; Seto, Y.; Iwase, T.; Hatake, K. Possible use of combination chemotherapy with mitomycin c and methotrexate for metastatic breast cancer pretreated with anthracycline and taxanes. Breast Cancer 2009, 16, 301. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Moghe, P.V.; Roth, C.M. Targeting tumor metastases: Drug delivery mechanisms and technologies. J. Controll. Release 2015, 219, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Hudziak, R.M.; Lewis, G.D.; Winget, M.; Fendly, B.M.; Shepard, H.M.; Ullrich, A. P185her2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol. Cell. Biol. 1989, 9, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Group, E.B.C.T.C. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar]
- Khvorova, A.; Watts, J.K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 2017, 35, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.; Judge, A.; MacLachlan, I. Sirna and innate immunity. Oligonucleotides 2009, 19, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Guenthner-Biller, M.; Bourquin, C.; Ablasser, A.; Schlee, M.; Uematsu, S.; Noronha, A.; Manoharan, M.; Akira, S.; de Fougerolles, A. Sequence-specific potent induction of ifn-α by short interfering rna in plasmacytoid dendritic cells through tlr7. Nat. Med. 2005, 11, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.K.; Deleavey, G.F.; Damha, M.J. Chemically modified sirna: Tools and applications. Drug Discov. Today 2008, 13, 842–855. [Google Scholar] [CrossRef] [PubMed]
- MiRBase: The microRNA Database. Available online: http://www.mirbase.org/cgi-bin/browse.pl?org=hsa (accessed on 5 May 2018).
- Feng, C.; Neumeister, V.; Ma, W.; Xu, J.; Lu, L.; Bordeaux, J.; Maihle, N.J.; Rimm, D.L.; Huang, Y. Lin28 regulates her2 and promotes malignancy through multiple mechanisms. Cell Cycle 2012, 11, 2486–2494. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, J.; Qian, X.; Han, L.; Zhang, K.; Chen, L.; Liu, J.; Ren, Y.; Yang, M.; Zhang, A. Ac1mmyr2, an inhibitor of dicer-mediated biogenesis of oncomir mir-21, reverses epithelial–mesenchymal transition and suppresses tumor growth and progression. Cancer Res. 2013, 73, 5519–5531. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, N.; Talcott, S.; Safe, S.; Mertens-Talcott, S.U. Cytotoxicity of pomegranate polyphenolics in breast cancer cells in vitro and vivo: Potential role of mirna-27a and mirna-155 in cell survival and inflammation. Breast Cancer Res. Treat. 2012, 136, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz, M.; Huppi, K.; Pitt, J.J.; Dorsey, T.H.; Ambs, S.; Caplen, N.J. Identification of the receptor tyrosine kinase axl in breast cancer as a target for the human mir-34a microrna. Breast Cancer Res. Treat. 2011, 130, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; He, L.; Richards, E.; Challa, S.; Xu, C.; Permuth-Wey, J.; Lancaster, J.; Coppola, D.; Sellers, T.; Djeu, J. Upregulation of mirna-155 promotes tumour angiogenesis by targeting vhl and is associated with poor prognosis and triple-negative breast cancer. Oncogene 2014, 33, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, J.; Deng, H. 17β-estradiol up-regulates mir-155 expression and reduces tp53inp1 expression in mcf-7 breast cancer cells. Mol. Cell. Biochem. 2013, 379, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Y.; Tsuyada, A.; Ren, X.; Wu, X.; Stubblefield, K.; Rankin-Gee, E.K.; Wang, S.E. Transforming growth factor-β regulates the sphere-initiating stem cell-like feature in breast cancer through mirna-181 and atm. Oncogene 2011, 30, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Humphries, B.; Wang, Z.; Oom, A.L.; Fisher, T.; Tan, D.; Cui, Y.; Jiang, Y.; Yang, C. Microrna-200b targets protein kinase cα and suppresses triple-negative breast cancer metastasis. Carcinogenesis 2014, 35, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Nassirpour, R.; Mehta, P.P.; Baxi, S.M.; Yin, M.-J. Mir-221 promotes tumorigenesis in human triple negative breast cancer cells. PLoS ONE 2013, 8, e62170. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zhang, J.; Zhong, L.; Wang, L.; Liu, Y.; Wang, Y.; Peng, L.; Guo, B. Upregulated microrna-301a in breast cancer promotes tumor metastasis by targeting pten and activating wnt/β-catenin signaling. Gene 2014, 535, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Santel, A.; Aleku, M.; Keil, O.; Endruschat, J.; Esche, V.; Fisch, G.; Dames, S.; Löffler, K.; Fechtner, M.; Arnold, W. A novel sirna-lipoplex technology for rna interference in the mouse vascular endothelium. Gene Ther. 2006, 13, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; McMillan, N.A. Lipidic systems for in vivo sirna delivery. AAPS J. 2009, 11, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Bellocq, N.C.; Pun, S.H.; Jensen, G.S.; Davis, M.E. Transferrin-containing, cyclodextrin polymer-based particles for tumor-targeted gene delivery. Bioconjug. Chem. 2003, 14, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.J.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of rnai in humans from systemically administered sirna via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin—More than just a serum protein. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Stylianopoulos, T.; Cui, J.; Martin, J.; Chauhan, V.P.; Jiang, W.; Popović, Z.; Jain, R.K.; Bawendi, M.G.; Fukumura, D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 2426–2431. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Volk, L.D.; Flister, M.J.; Chihade, D.; Desai, N.; Trieu, V.; Ran, S. Synergy of nab-paclitaxel and bevacizumab in eradicating large orthotopic breast tumors and preexisting metastases. Neoplasia 2011, 13, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Goss, P.E.; Chambers, A.F. Does tumour dormancy offer a therapeutic target? Nat. Rev. Cancer 2010, 10, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Naumov, G.N.; Townson, J.L.; MacDonald, I.C.; Wilson, S.M.; Bramwell, V.H.; Groom, A.C.; Chambers, A.F. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res. Treat. 2003, 82, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Matsumura, Y. Epr effect based drug design and clinical outlook for enhanced cancer chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Heller, D.A.; Winslow, M.M.; Dahlman, J.E.; Pratt, G.W.; Langer, R.; Jacks, T.; Anderson, D.G. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer 2012, 12, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Peiris, P.M.; Toy, R.; Doolittle, E.; Pansky, J.; Abramowski, A.; Tam, M.; Vicente, P.; Tran, E.; Hayden, E.; Camann, A. Imaging metastasis using an integrin-targeting chain-shaped nanoparticle. ACS Nano 2012, 6, 8783–8795. [Google Scholar] [CrossRef] [PubMed]
- Ailles, L.E.; Weissman, I.L. Cancer stem cells in solid tumors. Curr. Opin. Biotechnol. 2007, 18, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Fillmore, C.M.; Kuperwasser, C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, A.M.; Salcido, C.D.; Gillet, J.-P.; Wu, C.-P.; Fostel, J.M.; Mumau, M.D.; Gottesman, M.M.; Varticovski, L.; Ambudkar, S.V. Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. J. Natl. Cancer Inst. 2010, 102, 1637–1652. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Buck, E.; Eyzaguirre, A.; Barr, S.; Thompson, S.; Sennello, R.; Young, D.; Iwata, K.K.; Gibson, N.W.; Cagnoni, P.; Haley, J.D. Loss of homotypic cell adhesion by epithelial-mesenchymal transition or mutation limits sensitivity to epidermal growth factor receptor inhibition. Mol. Cancer Ther. 2007, 6, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Creighton, C.J.; Li, X.; Landis, M.; Dixon, J.M.; Neumeister, V.M.; Sjolund, A.; Rimm, D.L.; Wong, H.; Rodriguez, A.; Herschkowitz, J.I. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl. Acad. Sci. USA 2009, 106, 13820–13825. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.A.; Langer, R. Promoting convergence in biomedical science. Science 2011, 333, 527. [Google Scholar] [CrossRef] [PubMed]
- Korpal, M.; Lee, E.S.; Hu, G.; Kang, Y. The mir-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of e-cadherin transcriptional repressors zeb1 and zeb2. J. Biol. Chem. 2008, 283, 14910–14914. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Gaur, A.B.; Lengyel, E.; Peter, M.E. The mir-200 family determines the epithelial phenotype of cancer cells by targeting the e-cadherin repressors zeb1 and zeb2. Genes Dev. 2008, 22, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Gjerdrum, C.; Tiron, C.; Høiby, T.; Stefansson, I.; Haugen, H.; Sandal, T.; Collett, K.; Li, S.; McCormack, E.; Gjertsen, B.T. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc. Natl. Acad. Sci. USA 2010, 107, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.J.; Pan, A.; Franci, C.; Hu, Y.; Chang, B.; Li, W.; Duan, M.; Torneros, A.; Yu, J.; Heckrodt, T.J.; et al. R428, a selective small molecule inhibitor of axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010, 70, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Romano, G.; Di Leva, G.; Nuovo, G.; Jeon, Y.-J.; Ngankeu, A.; Sun, J.; Lovat, F.; Alder, H.; Condorelli, G. Egfr and met receptor tyrosine kinase–altered microrna expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat. Med. 2012, 18, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.J.; Morton, S.W.; Ben-Akiva, E.; Dreaden, E.C.; Shopsowitz, K.E.; Hammond, P.T. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and sirna for potential triple-negative breast cancer treatment. Acs Nano 2013, 7, 9571–9584. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, R.-T.; Qian, H.-Q.; Wei, J.; Xie, L.; Shen, J.; Yang, M.; Qian, X.-P.; Yu, L.-X.; Jiang, X.-Q. Targeted delivery of mir-200c/doc to inhibit cancer stem cells and cancer cells by the gelatinases-stimuli nanoparticles. Biomaterials 2013, 34, 7191–7203. [Google Scholar] [CrossRef] [PubMed]
- Stacker, S.A.; Williams, S.P.; Karnezis, T.; Shayan, R.; Fox, S.B.; Achen, M.G. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 2014, 14, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Raz, A.; Bucana, C.; Fogler, W.E.; Poste, G.; Fidler, I.J. Biochemical, morphological, and ultrastructural studies on the uptake of liposomes by murine macrophages. Cancer Res. 1981, 41, 487–494. [Google Scholar] [PubMed]
- Tassa, C.; Shaw, S.Y.; Weissleder, R. Dextran-coated iron oxide nanoparticles: A versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc. Chem. Res. 2011, 44, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Keliher, E.; Marinelli, B.; Leuschner, F.; Robbins, C.S.; Gerszten, R.E.; Pittet, M.J.; Swirski, F.K.; Weissleder, R. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography–computed tomography. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Harisinghani, M.G.; Barentsz, J.; Hahn, P.F.; Deserno, W.M.; Tabatabaei, S.; van de Kaa, C.H.; de la Rosette, J.; Weissleder, R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N. Eng. J. Med. 2003, 348, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Saaristo, A.; Holopainen, T.; Ylä-Herttuala, S.; Andersson, L.C.; Virolainen, S.; Immonen, I.; Alitalo, K. Photodynamic ablation of lymphatic vessels and intralymphatic cancer cells prevents metastasis. Sci. Transl. Med. 2011, 3, 69ra11. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R. The Biology of Cancer; Garland Science: New York, NY, USA, 2013; pp. 695–699. [Google Scholar]
- Tang, N.; Du, G.; Wang, N.; Liu, C.; Hang, H.; Liang, W. Improving penetration in tumors with nanoassemblies of phospholipids and doxorubicin. J. Natl. Cancer Inst. 2007, 99, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhang, F.; Lu, X.; Wei, X.; Wang, J.; Fang, X.; Si, D.; Wang, Y.; Zhang, C.; Yang, R. Polymeric micelles for enhanced lymphatic drug delivery to treat metastatic tumors. J. Controll. Release 2013, 171, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Rafi, M.; Cabral, H.; Kano, M.; Mi, P.; Iwata, C.; Yashiro, M.; Hirakawa, K.; Miyazono, K.; Nishiyama, N.; Kataoka, K. Polymeric micelles incorporating (1, 2-diaminocyclohexane) platinum (II) suppress the growth of orthotopic scirrhous gastric tumors and their lymph node metastasis. J. Controll. Release 2012, 159, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, M.; Shao, S.; Huang, W.; Yang, F.; Chen, W.; Zhang, S.; Xia, G.; Gao, Z. Small-sized polymeric micelles incorporating docetaxel suppress distant metastases in the clinically-relevant 4t1 mouse breast cancer model. BMC Cancer 2014, 14, 329. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Flatow, U.; King, C.R.; Sandeen, M.A.; Margulies, I.M.K.; Liotta, L.A.; Steeg, P.S. Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm3-transfected melanoma cells. Cell 1991, 65, 25–35. [Google Scholar] [CrossRef]
- Marshall, J.-C.A.; Collins, J.W.; Nakayama, J.; Horak, C.E.; Liewehr, D.J.; Steinberg, S.M.; Albaugh, M.; Vidal-Vanaclocha, F.; Palmieri, D.; Barbier, M. Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer. J. Natl. Cancer Inst. 2012, 104, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Glinsky, G.V.; Glinsky, V.V.; Ivanova, A.B.; Hueser, C.J. Apoptosis and metastasis: Increased apoptosis resistance of metastatic cancer cells is associated with the profound deficiency of apoptosis execution mechanisms. Cancer Lett. 1997, 115, 185–193. [Google Scholar] [CrossRef]
- Del Bufalo, D.; Biroccio, A.; Leonetti, C.; Zupi, G. Bcl-2 overexpression enhances the metastatic potential of a human breast cancer line. FASEB J. 1997, 11, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, R.; Daidone, M.G.; Benini, E.; Faranda, A.; Tomasic, G.; Boracchi, P.; Salvadori, B.; Veronesi, U. Validation of p53 accumulation as a predictor of distant metastasis at 10 years of follow-up in 1400 node-negative breast cancers. Clin. Cancer Res. 1996, 2, 2007–2013. [Google Scholar] [PubMed]
- Ko, J.; Shin, S.M.; Oh, Y.M.; Lee, Y.S.; Ryoo, Z.Y.; Lee, Y.H.; Na, D.S.; Kim, J.W. Transgenic mouse model for breast cancer: Induction of breast cancer in novel oncogene hccr-2 transgenic mice. Oncogene 2004, 23, 1950–1953. [Google Scholar] [CrossRef] [PubMed]
- Inbal, B.; Cohen, O.; Polak-Charcon, S.; Kopolovic, J.; Vadai, E.; Eisenbach, L.; Kimchi, A. Dap kinase links the control of apoptosis to metastasis. Nature 1997, 390, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Um, J.H.; Kwon, J.K.; Kang, C.-D.; Kim, M.J.; Ju, D.S.; Bae, J.H.; Kim, D.W.; Chung, B.S.; Kim, S.H. Relationship between antiapoptotic molecules and metastatic potency and the involvement of DNA-dependent protein kinase in the chemosensitization of metastatic human cancer cells by epidermal growth factor receptor blockade. J. Pharmacol. Exp. Ther. 2004, 311, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Moriai, R.; Tsuji, N.; Moriai, M.; Kobayashi, D.; Watanabe, N. Survivin plays as a resistant factor against tamoxifen-induced apoptosis in human breast cancer cells. Breast Cancer Res. Treat. 2009, 117, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.; Shukla, M.; Pandey, M. Survivin expression and targeting in breast cancer. Surg. Oncol. 2012, 21, 125–131. [Google Scholar] [CrossRef] [PubMed]


| Name | Agent | Delivery System | Indications | Company | Current Status | Identifier |
|---|---|---|---|---|---|---|
| EphA2 targeting DOPC-encapsulated siRNA | siRNA | LNP | Advanced cancers | MD Anderson Cancer Center | phase 1 | NCT 01591356 |
| Atu027 | siRNA | LNP | Advanced solid tumors | Silence Therapeutics GmbH | phase 2 | NCT 00938574 |
| PRO-040201 | siRNA | LNP | Hypercholesterolemia | Tekmira | phase 1 | NCT 00927459 |
| TKM-080301 | siRNA | LNP | Multiple cancers | Tekmira | phase 1 | NCT 01437007 |
| ALN-VSP02 | siRNA | LNP | Solid tumors | Alnylam | phase 1 | NCT 01158079 |
| TKM-100201 | siRNA | LNP | Ebola-virus infection | Tekmira | phase 1 | NCT 01518881 |
| ALN-PCS02 | siRNA | LNP | Elevated LDL-Cholesterol | Alnylam | phase 1 | NCT 01437059 |
| ALN-TTR02 | siRNA | LNP | Amyloidosis | Alnylam | phase 3 | NCT 02510261 |
| DCR-MYC | siRNA | LNP | Hepatocellular Carcinoma | Dicerna | phase 2 | NCT 02314052 |
| MRX34 | miRNA mimic | liposome | various solid tumor | Mirna Therapeutics | phase 1 | NCT 01829971 |
| TargomiRs | miRNA mimic | minicells | Malignant Pleural Mesothelioma Non-Small Cell Lung Cancer | Asbestos Diseases Research Foundation | phase 1 | NCT 02369198 |
| CALAA-01 | siRNA | cyclodextrin polymer-based nanoparticle | Various solid tumors | Calando | Phase 2 | NCT 00689065 |
| siG12D LODER | siRNA | LODER polymer | Pancreatic cancer | Silenseed Ltd. | phase 2 | NCT 01676259 |
| Brand | Delivery System | Indications | Company | Current Status |
|---|---|---|---|---|
| NanoTherm® | Iron oxide nanoparticle | Local treatment of glioblastomas | MagForce AG | Approved in Germany |
| Aurimmune® | Colloidal gold-bound recombinant human tumor necrosis factor | Pancreatic cancer | CytImmune Sciences | Phase 2 clinical trial |
| Doxil®(US) [Caelyx®(Europe)] | PEGylated liposome doxorubicin | Ovarian/BC | Orthobiotech, Schering-Plough | FDA approved |
| Abraxane® | Albumin-bound Paclitaxel nanoparticles | Various cancer therapy | Abraxis Bioscience | FDA approved |
| Nab paclitaxel in combination with gemcitabine | Metastatic pancreatic cancer | Celgene | FDA approved | |
| Myocet® | Non-PEGylated liposome of Doxorubicin | BC therapy | Elan Pharmaceuticals/Sopherion Therapeutics | Approved in Europe and Canada |
| DaunoXome® | Liposome-encapsulated Daunorubicin | Advanced HIV-associated Kaposi sarcoma | Gilead Science | FDA approved |
| DepoCyt® | Liposomal Cytarabine | Lymphomatous meningitis | Pacira Pharms Inc. | FDA approved |
| Oncaspar® | PEGylated L-asparaginase | Acute Lymphocytic Leukemia | Sigma Tau | FDA approved |
| Onco-TCS® | Liposomal Vincristine | Non-Hodgkin Lymphoma | Inex | Phase 1/2 clinical trial |
| LEP-ETU® | Liposomal Paclitaxel | Ovarian/breast/lung cancers | Neopharma | Phase 1/2 clinical trial |
| Aroplatin® | Liposomal Cisplatin analog | Colorectal cancer | Antigenics, Inc. | Phase 1/2 clinical trial |
| OSI-211 | Liposomal Lurtotecan | Lung cancer/recurrent ovarian cancer | OSI | Phase 2 clinical trial |
| SPI-77 | PEGylated liposomal Cisplatin | Head and Neck cancer/Lung cancer | Alza | Phase 3 clinical trial |
| EndoTAG-1 | Paclitaxel embedded in liposomal membranes | BC/Pancreatic cancer | Medigene/SynCore Biotechnology | Phase 2 clinical trial |
| Marqibo® | Vincristine | Philadelphia chromosome-negative lymphoblastic leukemia | Talon Therapeutics | FDA approved |
| ThemoDox® | Doxorubicin | Hepatocellular carcinoma | Celsion Corporation | Phase 3 clinical trial |
| Atragen® | Liposomal all trans-retinoic acid | Acute promyelocytic leukemia | Aronex Pharmaceuticals | Phase 2 clinical trial |
| Lipoplatin® | Liposomal Cisplatin | Pancreatic/Head and Neck/BC | Regulon | Phase 3 clinical trial |
| Aurimmune® (CYT-6091) | TNF-α bound to colloidal gold nanoparticles | Head and Neck cancer | Cytimmune Sciences | Phase 2 clinical trial |
| Auroshell® | Gold nanoshell | Thermally destroy the tumor tissue | Nanospectra Bioscience | Phase 1 clinical trial |
| Genexol-PM® | Polymeric micelle loaded with paclitaxel | BC/small cell lung cancer | Samyang | Approved in Europe and Korea |
| Paclical® | Paclitaxel micelles | Ovarian cancer | Oasmia Pharmaceutical AB | Phase 3 clinical trial |
| Narekt-102 | PEGylated liposome loaded with Irinotecan | Breast/Colorectal cancer | Nektar Therapeutics | Phase 3 clinical trial |
| NKTR-105 | PEG-Docetaxel conjugate | Solid tumors | Nektar Therapeutics | Phase 1 clinical trial |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Humphries, B.; Yang, C.; Wang, Z. Nanoparticle-Mediated Therapeutic Agent Delivery for Treating Metastatic Breast Cancer—Challenges and Opportunities. Nanomaterials 2018, 8, 361. https://doi.org/10.3390/nano8060361
Li Y, Humphries B, Yang C, Wang Z. Nanoparticle-Mediated Therapeutic Agent Delivery for Treating Metastatic Breast Cancer—Challenges and Opportunities. Nanomaterials. 2018; 8(6):361. https://doi.org/10.3390/nano8060361
Chicago/Turabian StyleLi, Yunfei, Brock Humphries, Chengfeng Yang, and Zhishan Wang. 2018. "Nanoparticle-Mediated Therapeutic Agent Delivery for Treating Metastatic Breast Cancer—Challenges and Opportunities" Nanomaterials 8, no. 6: 361. https://doi.org/10.3390/nano8060361
APA StyleLi, Y., Humphries, B., Yang, C., & Wang, Z. (2018). Nanoparticle-Mediated Therapeutic Agent Delivery for Treating Metastatic Breast Cancer—Challenges and Opportunities. Nanomaterials, 8(6), 361. https://doi.org/10.3390/nano8060361




