Harnessing Stevia rebaudiana for Zinc Oxide Nanoparticle Green Synthesis: A Sustainable Solution to Combat Multidrug-Resistant Bacterial Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and S. aureus Isolation
2.2. Antimicrobial Susceptibility Test
2.3. Molecular Characterization of the Selected Strains
2.4. Biosynthesis of Zinc Oxide Nanoparticles
2.5. Characterization of Zinc Oxide Nanoparticles
2.6. Assessment of the Bioactivity Efficacy of ZnO-NPs Against Virulent MDR S. aureus
2.6.1. Antibacterial Activity
2.6.2. Antibiofilm Activity
2.7. ZnO-NPs Mechanism as Antibacterial Agent
2.7.1. Cell Membrane Integrity
2.7.2. Changes in Bacterial Cell Morphology
2.7.3. Changes in Bacterial DNA Content
2.8. Data Analysis
3. Results and Discussion
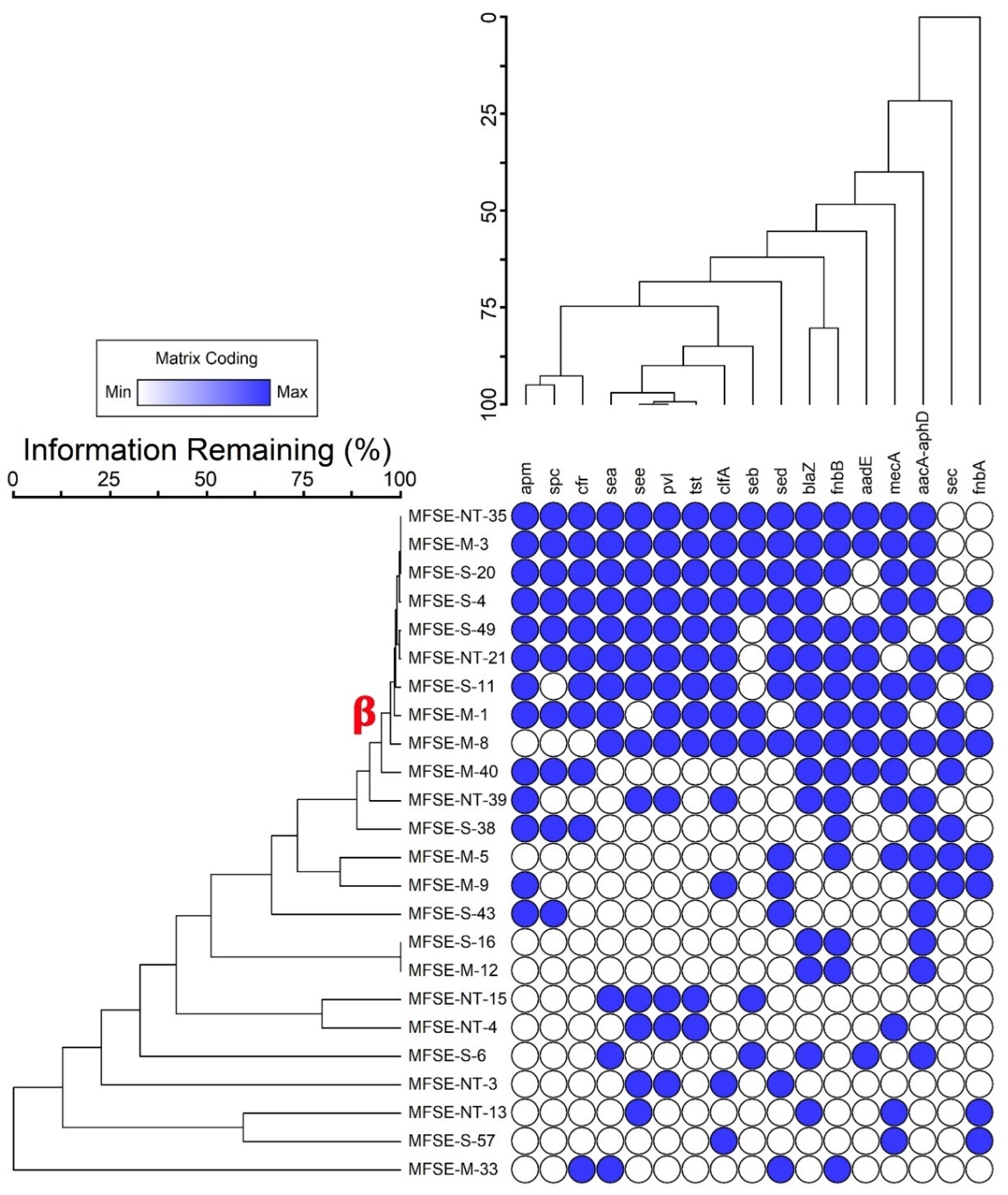
3.1. Prevalence of S. aureus in Fish Samples
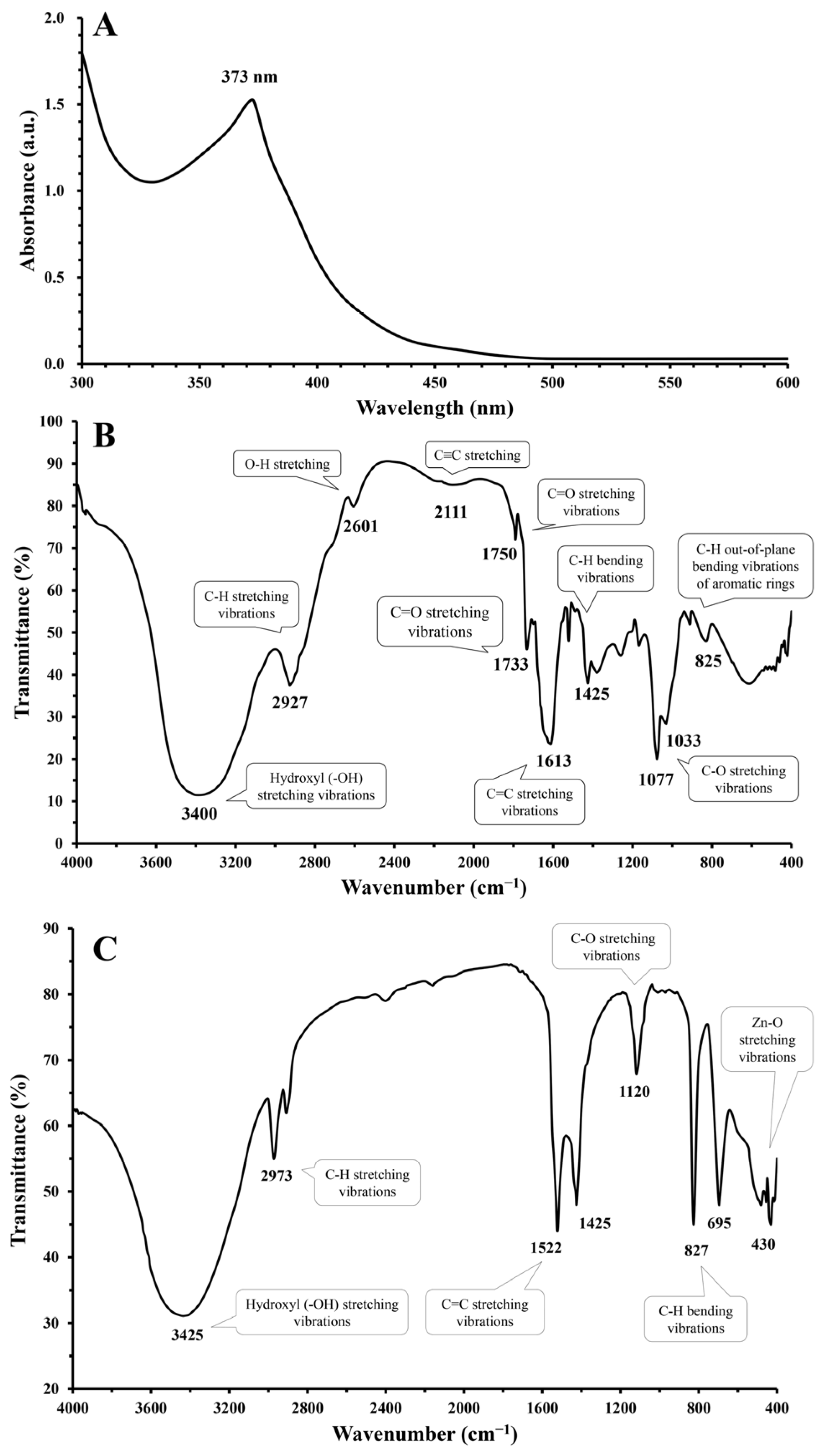
3.2. Characterization of the Green-Synthesized ZnO-NPs
3.3. ZnO-NPs Antibacterial and Antibiofilm Efficacy
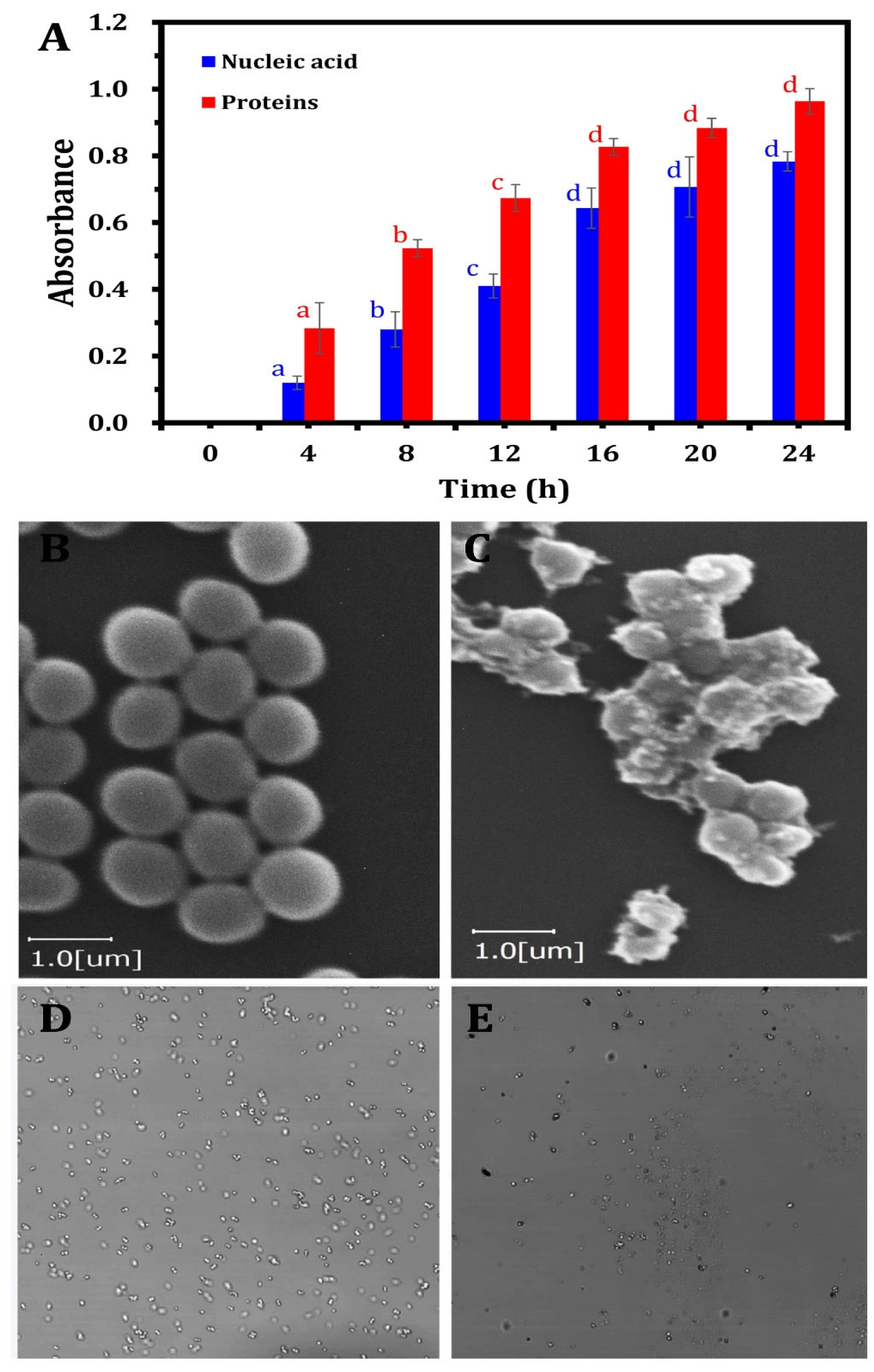
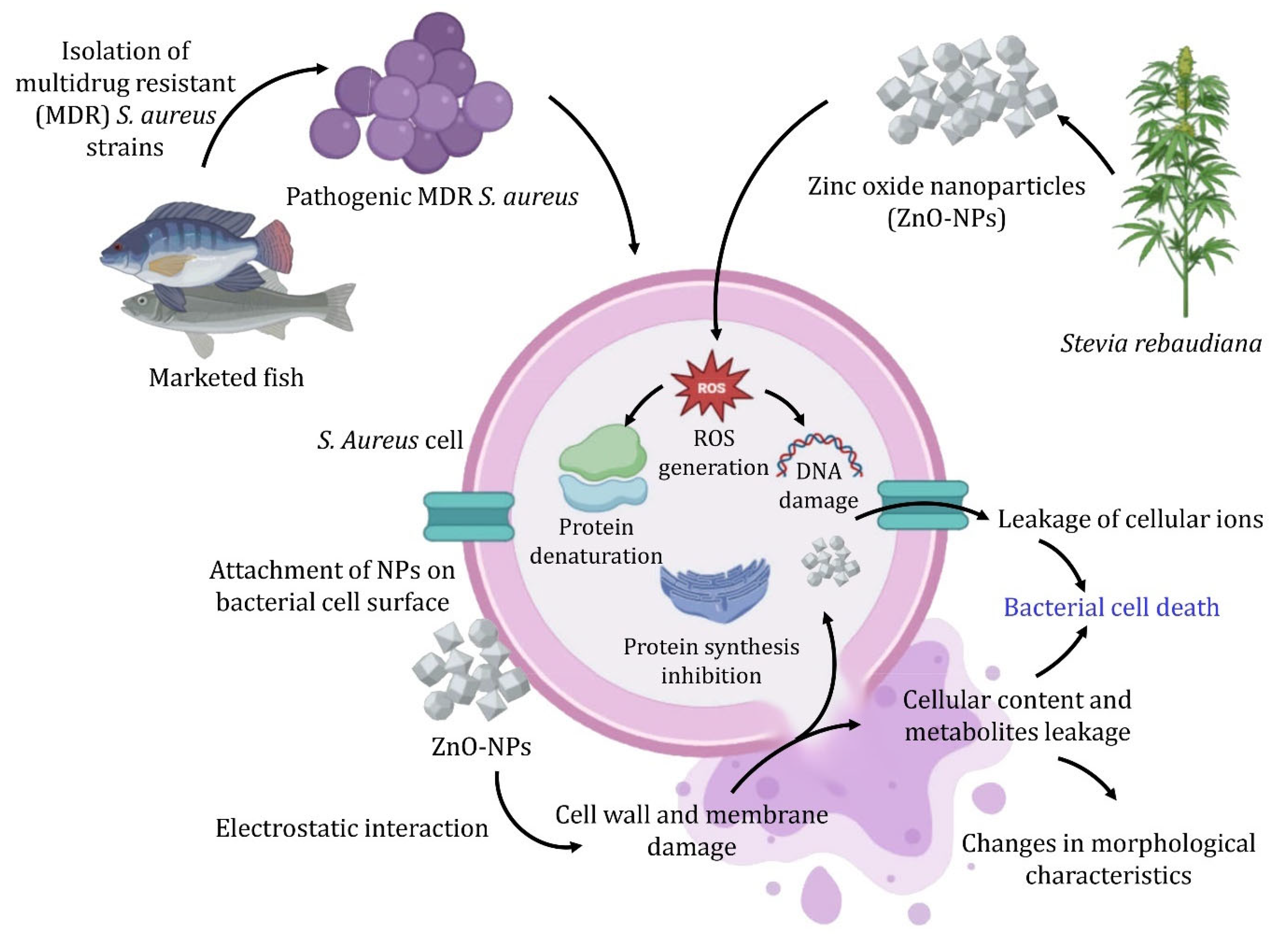
3.4. ZnO-NPs Mode of Action as Antibacterial Agents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nuryanto, N.; Afifah, D.N.; Sulchan, M.; Martosuyono, P.; Ihsani, K.; Kurniastuti, P.L. Potential of Nile Tilapia (Oreochromis Niloticus) as an Alternative Complementary Food Ingredient for Stunting Children. Open Access Maced. J. Med. Sci. 2022, 10, 1170–1177. [Google Scholar] [CrossRef]
- Furuya, W.M.; Cruz, T.P.d.; Gatlin, D.M., III. Amino Acid Requirements for Nile Tilapia: An Update. Animals 2023, 13, 900. [Google Scholar] [CrossRef] [PubMed]
- Sengun, I.Y.; Yildiz Turp, G.; Cicek, S.N.; Avci, T.; Ozturk, B.; Kilic, G. Assessment of the Effect of Marination with Organic Fruit Vinegars on Safety and Quality of Beef. Int. J. Food Microbiol. 2021, 336, 108904. [Google Scholar] [CrossRef]
- Gufe, C.; Canaan Hodobo, T.; Mbonjani, B.; Majonga, O.; Marumure, J.; Musari, S.; Jongi, G.; Makaya, P.V.; Machakwa, J. Antimicrobial Profiling of Bacteria Isolated from Fish Sold at Informal Market in Mufakose, Zimbabwe. Int. J. Microbiol. 2019, 2019, 8759636. [Google Scholar] [CrossRef]
- Okyere, A.; Bishoff, D.; Oyaro, M.O.; Ajami, N.J.; Darkoh, C. Analysis of Fish Commonly Sold in Local Supermarkets Reveals the Presence of Pathogenic and Multidrug-Resistant Bacterial Communities. Microbiol. Insights 2018, 11, 1178636118786925. [Google Scholar] [CrossRef]
- Darwish, W.; Othman, A.; Tharwat, A.E.; Eissa, K.M.; Nabawy, E.E.; Abd Elmoaty, A.M.; El-Wehedy, S.E. Prevalence of Multidrug-Resistant Enterotoxigenic Staphylococcus aureus in Pagrus and Saurus Fish Intended for Human Consumption. J. Adv. Vet. Res. 2023, 13, 1210–1213. [Google Scholar]
- Kulkarni, A.P.; Nagvekar, V.C.; Veeraraghavan, B.; Warrier, A.R.; Ts, D.; Ahdal, J.; Jain, R. Current Perspectives on Treatment of Gram-Positive Infections in India: What Is the Way Forward? Interdiscip. Perspect. Infect. Dis. 2019, 2019, 7601847. [Google Scholar] [CrossRef]
- Jayachandran, A.; Aswathy, T.R.; Nair, A.S. Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Cayratia pedata Leaf Extract. Biochem. Biophys. Rep. 2021, 26, 100995. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Athinarayanan, J.; Periyasamy, V.S.; Alshuniaber, M.A.; Alshammari, G.; Hakeem, M.J.; Ahmed, M.A.; Alshatwi, A.A. Antibacterial Mechanisms of Zinc Oxide Nanoparticle against Bacterial Food Pathogens Resistant to Beta-Lactam Antibiotics. Molecules 2022, 27, 2489. [Google Scholar] [CrossRef]
- Sana, S.S.; Kumbhakar, D.V.; Pasha, A.; Pawar, S.C.; Grace, A.N.; Singh, R.P.; Nguyen, V.-H.; Van Le, Q.; Peng, W. Crotalaria verrucosa Leaf Extract Mediated Synthesis of Zinc Oxide Nanoparticles: Assessment of Antimicrobial and Anticancer Activity. Molecules 2020, 25, 4896. [Google Scholar] [CrossRef]
- Zare, M.; Namratha, K.; Ilyas, S.; Sultana, A.; Hezam, A.; Sunil, L.; Surmeneva, M.A.; Surmenev, R.A.; Nayan, M.B.; Ramakrishna, S.; et al. Emerging Trends for ZnO Nanoparticles and Their Applications in Food Packaging. ACS Food Sci. Technol. 2022, 2, 763–781. [Google Scholar] [CrossRef]
- Singh, R.; Dutt, S.; Sharma, P.; Sundramoorthy, A.K.; Dubey, A.; Singh, A.; Arya, S. Future of Nanotechnology in Food Industry: Challenges in Processing, Packaging, and Food Safety. Glob. Chall. 2023, 7, 2200209. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent Advances in Zinc Oxide Nanoparticles (ZnO NPs) for Cancer Diagnosis, Target Drug Delivery, and Treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Feng, Z.; Wang, J.; Zhao, F.; Li, C.; Ju, J. Application of Nano-ZnO in the Food Preservation Industry: Antibacterial Mechanisms, Influencing Factors, Intelligent Packaging, Preservation Film and Safety. Crit. Rev. Food Sci. Nutr. 2024, 1–27. [Google Scholar] [CrossRef]
- Mahato, A.; Chatterjee, P.N.; Sarkar, S.; Sen, A.R.; Pal, A.; Roy, S.; Patra, A.K. Effects of Chemically and Green Synthesized Zinc Oxide Nanoparticles on Shelf Life and Sensory Quality of Minced Fish (Pangasius Hypophthalmus). Foods 2024, 13, 2810. [Google Scholar] [CrossRef]
- Wei, X.; Li, X.; Wu, C.; Yi, S.; Zhong, K.; Sun, T.; Li, J. The Modification of In Situ SiO × Chitosan Coatings by ZnO/TiO2 NPs and Its Preservation Properties to Silver Carp Fish Balls. J. Food Sci. 2018, 83, 2992–3001. [Google Scholar] [CrossRef]
- Srisuwan, Y.; Srihanam, P.; Rattanasuk, S.; Baimark, Y. Preparation of Poly(L-Lactide)-b-Poly(Ethylene Glycol)-b-Poly(L-Lactide)/Zinc Oxide Nanocomposite Bioplastics for Potential Use as Flexible and Antibacterial Food Packaging. Polymers 2024, 16, 1660. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges. Food Rev. Int. 2022, 38, 537–565. [Google Scholar] [CrossRef]
- Gökmen, G.G.; Mirsafi, F.S.; Leißner, T.; Akan, T.; Mishra, Y.K.; Kışla, D. Zinc Oxide Nanomaterials: Safeguarding Food Quality and Sustainability. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70051. [Google Scholar] [CrossRef]
- Islam, F.; Shohag, S.; Uddin, M.J.; Islam, M.R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Emran, T.B.; Cavalu, S. Exploring the Journey of Zinc Oxide Nanoparticles (ZnO-NPs) toward Biomedical Applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Wu, Z.; Zhou, Y.; Pei, K.; Song, W.; Li, S.; Zhang, J. The Applications of Flower-Shaped ZnO-UHMWPE Fibers in Photocatalysis and Composites. J. Ind. Eng. Chem. 2023, 125, 95–104. [Google Scholar] [CrossRef]
- Guirguis, H.A.; Youssef, N.; William, M.; Abdel-Dayem, D.; El-Sayed, M.M.H. Bioinspired Stevia rebaudiana Green Zinc Oxide Nanoparticles for the Adsorptive Removal of Antibiotics from Water. ACS Omega 2024, 9, 12881–12895. [Google Scholar] [CrossRef] [PubMed]
- Moffo, F.; Ndebé, M.M.F.; Tangu, M.N.; Noumedem, R.N.G.; Awah-Ndukum, J.; Mouiche, M.M.M. Antimicrobial Use, Residues and Resistance in Fish Production in Africa: Systematic Review and Meta-Analysis. BMC Vet. Res. 2024, 20, 307. [Google Scholar] [CrossRef] [PubMed]
- Perveen, R.; Shujaat, S.; Qureshi, Z.; Nawaz, S.; Khan, M.I.; Iqbal, M. Green versus Sol-Gel Synthesis of ZnO Nanoparticles and Antimicrobial Activity Evaluation against Panel of Pathogens. J. Mater. Res. Technol. 2020, 9, 7817–7827. [Google Scholar] [CrossRef]
- Zeghoud, S.; Hemmami, H.; Ben Seghir, B.; Ben Amor, I.; Kouadri, I.; Rebiai, A.; Messaoudi, M.; Ahmed, S.; Pohl, P.; Simal-Gandara, J. A Review on Biogenic Green Synthesis of ZnO Nanoparticles by Plant Biomass and Their Applications. Mater. Today Commun. 2022, 33, 104747. [Google Scholar] [CrossRef]
- Elabbasy, M.T.; El Bayomi, R.M.; Abdelkarim, E.A.; Hafez, A.E.-S.E.; Othman, M.S.; Ghoniem, M.E.; Samak, M.A.; Alshammari, M.H.; Almarshadi, F.A.; Elsamahy, T.; et al. Antibacterial and Antibiofilm Activity of Green-Synthesized Zinc Oxide Nanoparticles Against Multidrug-Resistant Escherichia coli Isolated from Retail Fish. Molecules 2025, 30, 768. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Liang, L.; Xu, X.; Zhou, G. Prevalence, Genetic Characterization and Biofilm Formation in Vitro of Staphylococcus aureus Isolated from Raw Chicken Meat at Retail Level in Nanjing, China. Food Control 2018, 86, 11–18. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI Supplement M10; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Pérez-Etayo, L.; González, D.; Leiva, J.; Vitas, A.I. Multidrug-Resistant Bacteria Isolated from Different Aquatic Environments in the North of Spain and South of France. Microorganisms 2020, 8, 1425. [Google Scholar] [CrossRef]
- Sandhu, R.; Dahiya, S.; Sayal, P. Evaluation of Multiple Antibiotic Resistance (MAR) Index and Doxycycline Susceptibility of Acinetobacter Species among Inpatients. Indian. J. Microbiol. Res. 2016, 3, 299. [Google Scholar] [CrossRef]
- Gumaa, M.A.; Idris, A.B.; Bilal, N.E.; Hassan, M.A. First Insights into Molecular Basis Identification of 16 s Ribosomal RNA Gene of Staphylococcus aureus Isolated from Sudan. BMC Res. Notes 2021, 14, 240. [Google Scholar] [CrossRef]
- Mehrotra, M.; Wang, G.; Johnson, W.M. Multiplex PCR for Detection of Genes for Staphylococcus aureus Enterotoxins, Exfoliative Toxins, Toxic Shock Syndrome Toxin 1, and Methicillin Resistance. J. Clin. Microbiol. 2000, 38, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Tristan, A.; Ying, L.; Bes, M.; Etienne, J.; Vandenesch, F.; Lina, G. Use of Multiplex PCR to Identify Staphylococcus aureus Adhesins Involved in Human Hematogenous Infections. J. Clin. Microbiol. 2003, 41, 4465–4467. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, F.; Zhao, J.; Lou, N.; Li, C.; Huang, T.; Li, Y. Molecular Characteristics and Virulence Gene Profiles of Staphylococcus aureus Causing Bloodstream Infection. Braz. J. Infect. Dis. 2018, 22, 487–494. [Google Scholar] [CrossRef]
- Argudín, M.A.; Mendoza, M.C.; González-Hevia, M.A.; Bances, M.; Guerra, B.; Rodicio, M.R. Genotypes, Exotoxin Gene Content, and Antimicrobial Resistance of Staphylococcus aureus Strains Recovered from Foods and Food Handlers. Appl. Environ. Microbiol. 2012, 78, 2930–2935. [Google Scholar] [CrossRef] [PubMed]
- Strommenger, B.; Kettlitz, C.; Werner, G.; Witte, W. Multiplex PCR Assay for Simultaneous Detection of Nine Clinically Relevant Antibiotic Resistance Genes in Staphylococcus aureus. J. Clin. Microbiol. 2003, 41, 4089–4094. [Google Scholar] [CrossRef]
- Azimian, A.; Havaei, S.A.; Fazeli, H.; Naderi, M.; Ghazvini, K.; Samiee, S.M.; Soleimani, M.; Peerayeh, S.N. Genetic Characterization of a Vancomycin-Resistant Staphylococcus aureus Isolate from the Respiratory Tract of a Patient in a University Hospital in Northeastern Iran. J. Clin. Microbiol. 2012, 50, 3581–3585. [Google Scholar] [CrossRef]
- Osman, K.M.; Badr, J.; Orabi, A.; Elbehiry, A.; Saad, A.; Ibrahim, M.D.S.; Hanafy, M.H. Poultry as a Vector for Emerging Multidrug Resistant Enterococcus spp.: First Report of Vancomycin (van) and the Chloramphenicol–florfenicol (Cat-Fex-Cfr) Resistance Genes from Pigeon and Duck Faeces. Microb. Pathog. 2019, 128, 195–205. [Google Scholar] [CrossRef]
- Martineau, F.; Picard, F.J.; Lansac, N.; Ménard, C.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Correlation between the Resistance Genotype Determined by Multiplex PCR Assays and the Antibiotic Susceptibility Patterns of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2000, 44, 231–238. [Google Scholar] [CrossRef]
- Fessler, A.; Scott, C.; Kadlec, K.; Ehricht, R.; Monecke, S.; Schwarz, S. Characterization of Methicillin-Resistant Staphylococcus aureus ST398 from Cases of Bovine Mastitis. J. Antimicrob. Chemother. 2010, 65, 619–625. [Google Scholar] [CrossRef]
- Fessler, A.T.; Kadlec, K.; Schwarz, S. Novel Apramycin Resistance Gene ApmA in Bovine and Porcine Methicillin-Resistant Staphylococcus aureus ST398 Isolates. Antimicrob. Agents Chemother. 2011, 55, 373–375. [Google Scholar] [CrossRef]
- Essa, H.L.; Abdelfattah, M.S.; Marzouk, A.S.; Guirguis, H.A.; El-Sayed, M.M.H. Nano-Formulations of Copper Species Coated with Sulfated Polysaccharide Extracts and Assessment of Their Phytotoxicity on Wheat (Triticum Aestivum L.) Seedlings in Seed Germination, Foliar and Soil Applications. Appl. Sci. 2020, 10, 6302. [Google Scholar] [CrossRef]
- Yang, C.-C.; Hung, C.-F.; Chen, B.-H. Preparation of Coffee Oil-Algae Oil-Based Nanoemulsions and the Study of Their Inhibition Effect on UVA-Induced Skin Damage in Mice and Melanoma Cell Growth. Int. J. Nanomed. 2017, 12, 6559–6580. [Google Scholar] [CrossRef] [PubMed]
- Atapakala, S.; Sana, S.S.; Kuppam, B.; Varma, R.S.; Aly Saad Aly, M.; Kim, S.-C.; Vadde, R. Honey Mediated Synthesis of Zinc Oxide Nanoparticles, and Evaluation of Antimicrobial, Antibiofilm Activities against Multidrug Resistant Clinical Bacterial Isolates. J. Ind. Eng. Chem. 2024, 135, 110–121. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Biswas, K.; Jena, S.K.; Hashem, A.; Abd Allah, E.F.; Mohanta, T.K. Anti-Biofilm and Antibacterial Activities of Silver Nanoparticles Synthesized by the Reducing Activity of Phytoconstituents Present in the Indian Medicinal Plants. Front. Microbiol. 2020, 11, 1143. [Google Scholar] [CrossRef]
- Abdelghafar, A.; Yousef, N.; Askoura, M. Zinc Oxide Nanoparticles Reduce Biofilm Formation, Synergize Antibiotics Action and Attenuate Staphylococcus aureus Virulence in Host; an Important Message to Clinicians. BMC Microbiol. 2022, 22, 244. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial Activity and Mechanism of Cinnamon Essential Oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial Mechanism of Oregano Essential Oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Kumar, L.R.G.; Kasim, A.K.; Lekshmi, M.; Nayak, B.B.; Kumar, S. Incidence of Methicillin-Resistant Staphylococci in Fresh Seafood. Adv. Microbiol. 2016, 6, 399–406. [Google Scholar] [CrossRef]
- Sivaraman, G.K.; Gupta, S.S.; Visnuvinayagam, S.; Muthulakshmi, T.; Elangovan, R.; Perumal, V.; Balasubramanium, G.; Lodha, T.; Yadav, A. Prevalence of S. Aureus and/or MRSA from Seafood Products from Indian Seafood Products. BMC Microbiol. 2022, 22, 233. [Google Scholar] [CrossRef]
- Mohamed, G.; Thabet, H. Prevalence of Methicillin Resistant Staphylococcus Aureus (MRSA) in Molouha (Salted Fish). Assiut Vet. Med. J. 2016, 62, 107–114. [Google Scholar] [CrossRef]
- Kukułowicz, A.; Steinka, I.; Siwek, A. Presence of Antibiotic-Resistant Staphylococcus aureus in Fish and Seafood Originating from Points of Sale in the Tri-City Area (Poland). J. Food Prot. 2021, 84, 1911–1914. [Google Scholar] [CrossRef]
- Murugadas, V.; Joseph, T.C.; Reshmi, K.; Lalitha, K.V. Prevalence of Methicillin Resistant Staphylococcus aureus in Selected Seafood Markets and Aquaculture Farms in Kerala, South-West Coast of India. Indian J. Fish. 2016, 63, 150–153. [Google Scholar] [CrossRef]
- Pimenta, L.K.L.; Rodrigues, C.A.; Filho, A.R.G.; Coelho, C.J.; Goes, V.; Estrela, M.; de Souza, P.; Avelino, M.A.G.; Vieira, J.D.G.; Carneiro, L. Staphylococcus spp. Causatives of Infections and Carrier of BlaZ, FemA, and MecA Genes Associated with Resistance. Antibiotics 2023, 12, 671. [Google Scholar] [CrossRef]
- Siciliano, V.; Passerotto, R.A.; Chiuchiarelli, M.; Leanza, G.M.; Ojetti, V. Difficult-to-Treat Pathogens: A Review on the Management of Multidrug-Resistant Staphylococcus Epidermidis. Life 2023, 13, 1126. [Google Scholar] [CrossRef]
- Zheng, Y.; Qin, C.; Zhang, X.; Zhu, Y.; Li, A.; Wang, M.; Tang, Y.; Kreiswirth, B.N.; Chen, L.; Zhang, H.; et al. The Tst Gene Associated Staphylococcus aureus Pathogenicity Island Facilitates Its Pathogenesis by Promoting the Secretion of Inflammatory Cytokines and Inducing Immune Suppression. Microb. Pathog. 2020, 138, 103797. [Google Scholar] [CrossRef]
- Cafini, F.; Nguyen, L.T.T.; Higashide, M.; Román, F.; Prieto, J.; Morikawa, K. Horizontal Gene Transmission of Thecfrgene to MRSA and Enterococcus: Role of Staphylococcus epidermidis as a Reservoir and Alternative Pathway for the Spread of Linezolid Resistance. J. Antimicrob. Chemother. 2016, 71, 587–592. [Google Scholar] [CrossRef]
- Speziale, P.; Pietrocola, G. The Multivalent Role of Fibronectin-Binding Proteins A and B (FnBPA and FnBPB) of Staphylococcus aureus in Host Infections. Front. Microbiol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Darboe, S.; Dobreniecki, S.; Jarju, S.; Jallow, M.; Mohammed, N.I.; Wathuo, M.; Ceesay, B.; Tweed, S.; Basu Roy, R.; Okomo, U.; et al. Prevalence of Panton-Valentine Leukocidin (PVL) and Antimicrobial Resistance in Community-Acquired Clinical Staphylococcus aureus in an Urban Gambian Hospital: A 11-Year Period Retrospective Pilot Study. Front. Cell. Infect. Microbiol. 2019, 9, 170. [Google Scholar] [CrossRef]
- Raghu, A.; Velayudhannair, K. Phytochemical Analysis and Antibacterial Potential of Stevia Rebaudiana (Bertoni, 1899) Leaf Extracts against Aeromonas Species: Influence of Extraction Methods and Solvents in Aquaculture Applications. J. Pure Appl. Microbiol. 2023, 17, 2352–2366. [Google Scholar] [CrossRef]
- Aranda-Ledesma, N.E.; González-Hernández, M.D.; Rojas, R.; Paz-González, A.D.; Rivera, G.; Luna-Sosa, B.; Martínez-Ávila, G.C.G. Essential Oil and Polyphenolic Compounds of Flourensia Cernua Leaves: Chemical Profiling and Functional Properties. Agronomy 2022, 12, 2274. [Google Scholar] [CrossRef]
- Singh, T.A.; Sharma, A.; Tejwan, N.; Ghosh, N.; Das, J.; Sil, P.C. A State of the Art Review on the Synthesis, Antibacterial, Antioxidant, Antidiabetic and Tissue Regeneration Activities of Zinc Oxide Nanoparticles. Adv. Colloid Interface Sci. 2021, 295, 102495. [Google Scholar] [CrossRef]
- Volkov, D.; Rogova, O.; Proskurnin, M. Temperature Dependences of IR Spectra of Humic Substances of Brown Coal. Agronomy 2021, 11, 1822. [Google Scholar] [CrossRef]
- Luo, L.; Lv, J.; Chen, Z. Synchrotron Infrared Microspectroscopy Reveals the Roles of Aliphatic and Aromatic Moieties in Sorption of Nitroaromatic Compounds to Soils. Sci. Total Environ. 2018, 624, 210–214. [Google Scholar] [CrossRef]
- Yadav, S.; Nadar, T.; Lakkakula, J.; Wagh, N.S. Biogenic Synthesis of Nanomaterials: Bioactive Compounds as Reducing, and Capping Agents. In Biogenic Nanomaterials for Environmental Sustainability: Principles, Practices, and Opportunities; Springer: Berlin/Heidelberg, Germany, 2024; pp. 147–188. [Google Scholar]
- Ali, A.; Nawaz, H.; Irfan Majeed, M.; Ghamkhar, M. Quantitative Analysis of Solid Dosage Forms of Atenolol by Raman Spectroscopy. Drug Dev. Ind. Pharm. 2024, 50, 619–627. [Google Scholar] [CrossRef]
- Ansari, N.; Basree; Tripathi, A.; Ameen, S.; Shaheer Akhtar, M.; Jabeen, F.; Rahman Khan, A.; Luqman, M.; Rahman, Q.I. Green Synthesis of Nanomaterials: Properties and Their Potential Applications. Sci. Adv. Mater. 2024, 16, 837–854. [Google Scholar] [CrossRef]
- de Matos, Y.M.L.S.; Vasconcelos, D.L.M.; Barreto, A.C.H.; Rocha, J.E.; de Araújo-Neto, J.B.; Campina, F.F.; da Silva, M.M.C.; Al Yafawi, T.T.; Sobral-Souza, C.E.; Pinheiro, J.C.A.; et al. Protection against the Phytotoxic Effect of Mercury Chloride by Catechin and Quercetin. J. Chem. 2022, 2022, 1–7. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Mizaikoff, B. Recent Advances on the Characterization of Nanoparticles Using Infrared Spectroscopy. TrAC Trends Anal. Chem. 2016, 84, 97–106. [Google Scholar] [CrossRef]
- Elrefaey, A.; El-Gamal, A.; Hamed, S.; El-belely, E. Algae-Mediated Biosynthesis of Zinc Oxide Nanoparticles from Cystoseira crinite (Fucales; Sargassaceae) and It’s Antimicrobial and Antioxidant Activities. Egypt. J. Chem. 2021, 65, 231–240. [Google Scholar] [CrossRef]
- Chuntonov, L.; Kumar, R.; Kuroda, D.G. Non-Linear Infrared Spectroscopy of the Water Bending Mode: Direct Experimental Evidence of Hydration Shell Reorganization? Phys. Chem. Chem. Phys. 2014, 16, 13172–13181. [Google Scholar] [CrossRef]
- Seki, T.; Chiang, K.-Y.; Yu, C.-C.; Yu, X.; Okuno, M.; Hunger, J.; Nagata, Y.; Bonn, M. The Bending Mode of Water: A Powerful Probe for Hydrogen Bond Structure of Aqueous Systems. J. Phys. Chem. Lett. 2020, 11, 8459–8469. [Google Scholar] [CrossRef]
- Amarasinghe, P.M.; Katti, K.S.; Katti, D.R. Nature of Organic Fluid–montmorillonite Interactions: An FTIR Spectroscopic Study. J. Colloid Interface Sci. 2009, 337, 97–105. [Google Scholar] [CrossRef]
- Zaidan, U.H.; Mohamad Zen, N.I.; Amran, N.A.; Shamsi, S.; Gani, S.S.A. Biochemical Evaluation of Phenolic Compounds and Steviol Glycoside from Stevia rebaudiana Extracts Associated with in Vitro Antidiabetic Potential. Biocatal. Agric. Biotechnol. 2019, 18, 101049. [Google Scholar] [CrossRef]
- Basnet, P.; Inakhunbi Chanu, T.; Samanta, D.; Chatterjee, S. A Review on Bio-Synthesized Zinc Oxide Nanoparticles Using Plant Extracts as Reductants and Stabilizing Agents. J. Photochem. Photobiol. B Biol. 2018, 183, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Devi, S.; Paul, A.K. Synthesis and Characterization of Zinc Oxide Nanoparticles for Ethanol Detection. In Physics of Semiconductor Devices; Jain, V., Verma, A., Eds.; Environmental Science and Engineering; Springer: Cham, Switzerland, 2014; pp. 707–708. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Facile Green Synthesis of Zinc Oxide Nanoparticles with Potential Synergistic Activity with Common Antifungal Agents against Multidrug-Resistant Candidal Strains. Crystals 2022, 12, 774. [Google Scholar] [CrossRef]
- El-Masry, R.M.; Talat, D.; Hassoubah, S.A.; Zabermawi, N.M.; Eleiwa, N.Z.; Sherif, R.M.; Abourehab, M.A.S.; Abdel-Sattar, R.M.; Gamal, M.; Ibrahim, M.S.; et al. Evaluation of the Antimicrobial Activity of ZnO Nanoparticles against Enterotoxigenic Staphylococcus aureus. Life 2022, 12, 1662. [Google Scholar] [CrossRef]
- Ahmad, I.; Alshahrani, M.Y.; Wahab, S.; Al-Harbi, A.I.; Nisar, N.; Alraey, Y.; Alqahtani, A.; Mir, M.A.; Irfan, S.; Saeed, M. Zinc Oxide Nanoparticle: An Effective Antibacterial Agent against Pathogenic Bacterial Isolates. J. King Saud. Univ. Sci. 2022, 34, 102110. [Google Scholar] [CrossRef]
- Husain, F.M.; Qais, F.A.; Ahmad, I.; Hakeem, M.J.; Baig, M.H.; Masood Khan, J.; Al-Shabib, N.A. Biosynthesized Zinc Oxide Nanoparticles Disrupt Established Biofilms of Pathogenic Bacteria. Appl. Sci. 2022, 12, 710. [Google Scholar] [CrossRef]
- Lahiri, D.; Ray, R.R.; Sarkar, T.; Upadhye, V.J.; Ghosh, S.; Pandit, S.; Pati, S.; Edinur, H.A.; Abdul Kari, Z.; Nag, M.; et al. Anti-Biofilm Efficacy of Green-Synthesized ZnO Nanoparticles on Oral Biofilm: In Vitro and in Silico Study. Front. Microbiol. 2022, 13, 939390. [Google Scholar] [CrossRef]
- Rosenberg, M.; Visnapuu, M.; Vija, H.; Kisand, V.; Kasemets, K.; Kahru, A.; Ivask, A. Selective Antibiofilm Properties and Biocompatibility of Nano-ZnO and Nano-ZnO/Ag Coated Surfaces. Sci. Rep. 2020, 10, 13478. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Nano-Food Packaging: An Overview of Market, Migration Research, and Safety Regulations. J. Food Sci. 2015, 80, R910–R923. [Google Scholar] [CrossRef]
- Aristizabal-Gil, M.V.; Santiago-Toro, S.; Sanchez, L.T.; Pinzon, M.I.; Gutierrez, J.A.; Villa, C.C. ZnO and ZnO/CaO Nanoparticles in Alginate Films. Synthesis, Mechanical Characterization, Barrier Properties and Release Kinetics. LWT 2019, 112, 108217. [Google Scholar] [CrossRef]
- Heydari-Majd, M.; Ghanbarzadeh, B.; Shahidi-Noghabi, M.; Najafi, M.A.; Hosseini, M. A New Active Nanocomposite Film Based on PLA/ZnO Nanoparticle/Essential Oils for the Preservation of Refrigerated Otolithes Ruber Fillets. Food Packag. Shelf Life 2019, 19, 94–103. [Google Scholar] [CrossRef]
- Abdelraheem, W.M.; Khairy, R.M.M.; Zaki, A.I.; Zaki, S.H. Effect of ZnO Nanoparticles on Methicillin, Vancomycin, Linezolid Resistance and Biofilm Formation in Staphylococcus aureus Isolates. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 54. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Li, Z.; Huang, J.; Wu, J.; Zhang, Y.; Ge, H.; Zhao, Y. Antibacterial Effect of Low-Concentration ZnO Nanoparticles on Sulfate-Reducing Bacteria under Visible Light. Nanomaterials 2023, 13, 2033. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.d.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial Action and Target Mechanisms of Zinc Oxide Nanoparticles against Bacterial Pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Metryka, O.; Wasilkowski, D.; Adamczyk-Habrajska, M.; Mrozik, A. Undesirable Consequences of the Metallic Nanoparticles Action on the Properties and Functioning of Escherichia Coli, Bacillus cereus and Staphylococcus epidermidis Membranes. J. Hazard. Mater. 2023, 446, 130728. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Recent Advances in Zinc Oxide Nanostructures with Antimicrobial Activities. Int. J. Mol. Sci. 2020, 21, 8836. [Google Scholar] [CrossRef]
- Shobha, B.; Ashwini, B.S.; Ghazwani, M.; Hani, U.; Atwah, B.; Alhumaidi, M.S.; Basavaraju, S.; Chowdappa, S.; Ravikiran, T.; Wahab, S.; et al. Trichoderma-Mediated ZnO Nanoparticles and Their Antibiofilm and Antibacterial Activities. J. Fungi 2023, 9, 133. [Google Scholar] [CrossRef]
- Valadbeigi, H.; Sadeghifard, N.; Kaviar, V.H.; Haddadi, M.H.; Ghafourian, S.; Maleki, A. Effect of ZnO Nanoparticles on Biofilm Formation and Gene Expression of the Toxin-Antitoxin System in Clinical Isolates of Pseudomonas aeruginosa. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 89. [Google Scholar] [CrossRef]


| Isolate Code | Biofilm Inhibition (%) | |||||
|---|---|---|---|---|---|---|
| 0 µg | 50 µg | 100 µg | 150 µg | 200 µg | 250 µg | |
| MFSE-M-1 | 0.0 ± 0.0 a* | 21.2 ± 1.3 a | 40.3 ± 2.1 abc | 53.5 ± 1.3 abc | 80.3 ± 1.2 ac | 89.4 ± 1.1 a |
| MFSE-NT-21 | 0.0 ± 0.0 a | 27.1 ± 0.9 b | 37.7 ± 1.5 b | 50.3 ± 1.6 b | 74.4 ± 1.5 bd | 82.1 ± 2.5 b |
| MFSE-S-49 | 0.0 ± 0.0 a | 31.8 ± 1.4 c | 44.3 ± 2.2 c | 56.6 ± 1.8 c | 77.3 ± 2.4 ab | 85.5 ± 2.3 abc |
| MFSE-S-11 | 0.0 ± 0.0 a | 16.3 ± 1.5 d | 22.3 ± 2.5 d | 42.5 ± 2.4 d | 64.7 ± 1.5 e | 72.2 ± 1.2 d |
| MFSE-S-4 | 0.0 ± 0.0 a | 45.1 ± 0.9 e | 54.3 ± 3.2 e | 63.8 ± 1.0 e | 80.9 ± 1.2 ac | 90.7 ± 2.2 a |
| MFSE-S-20 | 0.0 ± 0.0 a | 19.3 ± 2.3 ad | 27.5 ± 2.3 f | 46.9 ± 1.9 b | 70.9 ± 0.6 df | 78.8 ± 1.1 b |
| MFSE-M-8 | 0.0 ± 0.0 a | 36.5 ± 2.2 f | 46.7 ± 1.5 c | 56.4 ± 1.4 c | 78.9 ± 1.0 a | 88.9 ± 2.0 a |
| MFSE-M-3 | 0.0 ± 0.0 a | 40.7 ± 2.5 f | 55.2 ± 3.0 e | 64.7 ± 1.6 e | 84.6 ± 1.4 c | 92.1 ± 1.7 a |
| MFSE-NT-35 | 0.0 ± 0.0 a | 27.3 ± 1.2 b | 35.7 ± 2.5 b | 49.9 ± 1.3 b | 73.2 ± 1.9 bf | 81.5 ± 1.4 bc |
| p-value | NA | 0.975 | 0.957 | 0.974 | 0.923 | 0.925 |
| Isolate Code | Biofilm Inhibition (%) | |||||
|---|---|---|---|---|---|---|
| 0 µg | 50 µg | 100 µg | 150 µg | 200 µg | 250 µg | |
| MFSE-M-1 | 0.0 ± 0.0 a* | 21.2 ± 1.3 a | 40.3 ± 2.1 abc | 53.5 ± 1.3 abc | 80.3 ± 1.2 ac | 89.4 ± 1.1 a |
| MFSE-NT-21 | 0.0 ± 0.0 a | 27.1 ± 0.9 b | 37.7 ± 1.5 b | 50.3 ± 1.6 b | 74.4 ± 1.5 bd | 82.1 ± 2.5 b |
| MFSE-S-49 | 0.0 ± 0.0 a | 31.8 ± 1.4 c | 44.3 ± 2.2 c | 56.6 ± 1.8 c | 77.3 ± 2.4 ab | 85.5 ± 2.3 abc |
| MFSE-S-11 | 0.0 ± 0.0 a | 16.3 ± 1.5 d | 22.3 ± 2.5 d | 42.5 ± 2.4 d | 64.7 ± 1.5 e | 72.2 ± 1.2 d |
| MFSE-S-4 | 0.0 ± 0.0 a | 45.1 ± 0.9 e | 54.3 ± 3.2 e | 63.8 ± 1.0 e | 80.9 ± 1.2 ac | 90.7 ± 2.2 a |
| MFSE-S-20 | 0.0 ± 0.0 a | 19.3 ± 2.3 ad | 27.5 ± 2.3 f | 46.9 ± 1.9 b | 70.9 ± 0.6 df | 78.8 ± 1.1 b |
| MFSE-M-8 | 0.0 ± 0.0 a | 36.5 ± 2.2 f | 46.7 ± 1.5 c | 56.4 ± 1.4 c | 78.9 ± 1.0 a | 88.9 ± 2.0 a |
| MFSE-M-3 | 0.0 ± 0.0 a | 40.7 ± 2.5 f | 55.2 ± 3.0 e | 64.7 ± 1.6 e | 84.6 ± 1.4 c | 92.1 ± 1.7 a |
| MFSE-NT-35 | 0.0 ± 0.0 a | 27.3 ± 1.2 b | 35.7 ± 2.5 b | 49.9 ± 1.3 b | 73.2 ± 1.9 bf | 81.5 ± 1.4 bc |
| p-value | NA | 0.975 | 0.957 | 0.974 | 0.923 | 0.925 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elabbasy, M.T.; El Bayomi, R.M.; Abdelkarim, E.A.; Hafez, A.E.-S.E.; Othman, M.S.; Ghoniem, M.E.; Samak, M.A.; Alshammari, M.H.; Almarshadi, F.A.; Elsamahy, T.; et al. Harnessing Stevia rebaudiana for Zinc Oxide Nanoparticle Green Synthesis: A Sustainable Solution to Combat Multidrug-Resistant Bacterial Pathogens. Nanomaterials 2025, 15, 369. https://doi.org/10.3390/nano15050369
Elabbasy MT, El Bayomi RM, Abdelkarim EA, Hafez AE-SE, Othman MS, Ghoniem ME, Samak MA, Alshammari MH, Almarshadi FA, Elsamahy T, et al. Harnessing Stevia rebaudiana for Zinc Oxide Nanoparticle Green Synthesis: A Sustainable Solution to Combat Multidrug-Resistant Bacterial Pathogens. Nanomaterials. 2025; 15(5):369. https://doi.org/10.3390/nano15050369
Chicago/Turabian StyleElabbasy, Mohamed Tharwat, Rasha M. El Bayomi, Esraa A. Abdelkarim, Abd El-Salam E. Hafez, Mohamed S. Othman, Mohamed E. Ghoniem, Mai A. Samak, Muteb H. Alshammari, Fahad Awwadh Almarshadi, Tamer Elsamahy, and et al. 2025. "Harnessing Stevia rebaudiana for Zinc Oxide Nanoparticle Green Synthesis: A Sustainable Solution to Combat Multidrug-Resistant Bacterial Pathogens" Nanomaterials 15, no. 5: 369. https://doi.org/10.3390/nano15050369
APA StyleElabbasy, M. T., El Bayomi, R. M., Abdelkarim, E. A., Hafez, A. E.-S. E., Othman, M. S., Ghoniem, M. E., Samak, M. A., Alshammari, M. H., Almarshadi, F. A., Elsamahy, T., & Hussein, M. A. (2025). Harnessing Stevia rebaudiana for Zinc Oxide Nanoparticle Green Synthesis: A Sustainable Solution to Combat Multidrug-Resistant Bacterial Pathogens. Nanomaterials, 15(5), 369. https://doi.org/10.3390/nano15050369






